RNA Biomarkers: Frontier of Precision Medicine for Cancer
Abstract
:1. Introduction
2. Comparison of Different Types of Biomarkers
3. Different Types of RNA Biomarkers in Cancer
4. Category of Extracellular RNAs
5. Biogenesis of exRNAs
6. Clinical Relevance of exRNAs in Cancer
7. Extracellular RNA Biomarkers
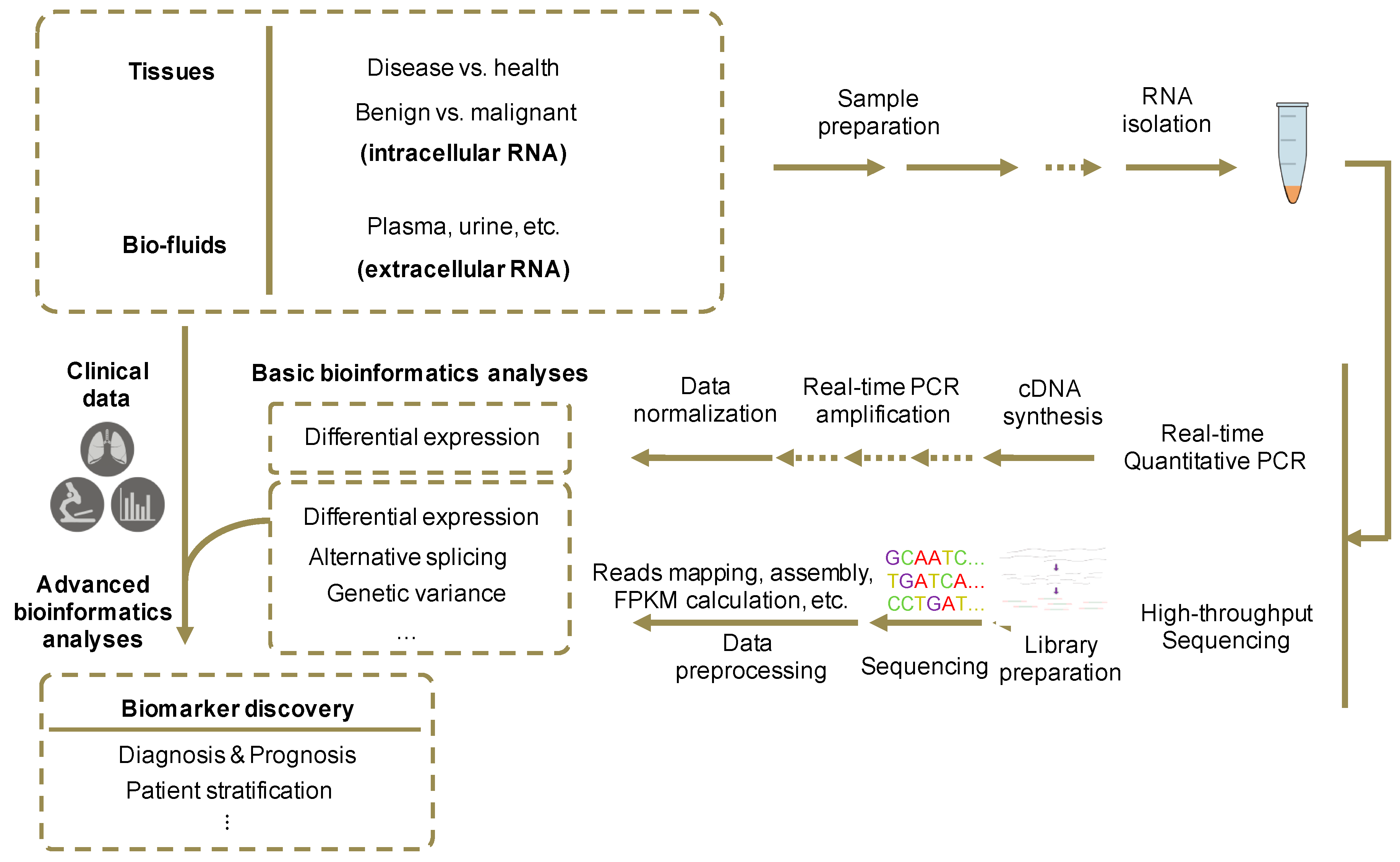
8. Identification of Novel Extracellular RNA Biomarkers
9. Published Databases of RNA Biomarkers
10. Future Perspectives and Challenges
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, K.L.; Sanda, M.G.; Moreno, C.S. RNA biomarkers to facilitate the identification of aggressive prostate cancer. Mol. Asp. Med. 2015, 45, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Shelling, A.; Muthukaruppan, A.; Lasham, A.; Blenkiron, C.; Laking, G. Predictive and prognostic molecular markers for cancer medicine. Ther. Adv. Med. Oncol. 2010, 2, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mariani, T.J. Array of hope: Expression profiling identifies disease biomarkers and mechanism. Biochem. Soc. Trans. 2009, 37, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.T.; Di, C.; Hu, B.; Zhou, M.; Liu, Y.; Song, N.; Li, Y.; Umetsu, J.; Lu, Z.J. CLIPdb: A CLIP-seq database for protein-RNA interactions. BMC Genom. 2015, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, Y.-C.T.; Huang, Y.; Zhu, Y.; Lu, Z.J. POSTAR: A platform for exploring post-transcriptional regulation coordinated by RNA-binding proteins. Nucleic Acids Res. 2017, 45, D104–D114. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.P.; Cruceanu, C.; Fiori, L.M.; Laboissiere, S.; Guillet, I.; Fontaine, J.; Ragoussis, J.; Benes, V.; Turecki, G.; Ernst, C. Biomarker discovery: Quantification of microRNAs and other small non-coding RNAs using next generation sequencing. BMC Med. Genom. 2015, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016, 17, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Russo, I.H. Techniques and Methodological Approaches in Breast Cancer Research; Springer: New York, NY, USA, 2014. [Google Scholar]
- Finka, A.; Goloubinoff, P. Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones 2013, 18, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta Rev. Cancer 2007, 1775, 181–232. [Google Scholar] [CrossRef] [PubMed]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Di, C.; Kai, M.; Yang, Y.-C.T.; Li, Y.; Qiu, Y.; Hu, X.; Yip, K.Y.; Zhang, M.Q.; Lu, Z.J. A common set of distinct features that characterize noncoding RNAs across multiple species. Nucleic Acids Res. 2015, 43, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, Z.; Hu, B.; Lu, Z.J. COME: A robust coding potential calculation tool for lncRNA identification and characterization based on multiple features. Nucleic Acids Res. 2017, 45, e2. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.-C.T.; Yuan, J.; Lu, Z.J.; Li, J.J. Large-scale mapping of mammalian transcriptomes identifies conserved genes associated with different cell states. Nucleic Acids Res. 2017, 45, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ledesma, E.; Verhaak, R.G.; Treviño, V. Identification of a multi-cancer gene expression biomarker for cancer clinical outcomes using a network-based algorithm. Sci. Rep. 2015, 5, 11966. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; Brothman, A.R.; Berney, D.M.; Reid, J.E.; Mesher, D.; Speights, V.; Stankiewicz, E.; Foster, C.S. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef]
- Glavac, D.; Hrasovec, S. MicroRNAs as novel biomarkers in colorectal cancer. Front. Genet. 2012, 3, 180. [Google Scholar]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Voortman, J.; Giovannetti, E.; Steinberg, S.M.; Leon, L.G.; Kim, Y.-T.; Funel, N.; Park, J.K.; Kim, M.A.; Kang, G.H. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS ONE 2010, 5, e10630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Gao, W.; Feng, J. Long noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancer. Med. Oncol. 2014, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, M.; Salavaty, A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biol. Med. 2015, 12, 1–9. [Google Scholar] [PubMed]
- Cordeiro, A.; Navarro, A.; Gaya, A.; Díaz-Beyá, M.; Gonzalez-Farré, B.; Castellano, J.J.; Fuster, D.; Martínez, C.; Martínez, A.; Monzó, M. PiwiRNA-651 as marker of treatment response and survival in classical Hodgkin lymphoma. Oncotarget 2016, 7, 46002–46013. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yu, L.; Mei, Y.; Guarnera, M.; Shen, J.; Li, R.; Liu, Z.; Jiang, F. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Mol. Cancer 2010, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, S.; Chen, H.; Mo, X.; Li, T.; Shao, Y.; Xiao, B.; Guo, J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin. Chim. Acta 2015, 444, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Clark, D.; Mao, L. Novel dimensions of piRNAs in cancer. Cancer Lett. 2013, 336, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, G.A.; Filippova, J.A.; Komissarov, A.B.; Kuligina, E.V.; Richter, V.A.; Semenov, D.V. Regulatory role of small nucleolar RNAs in human diseases. BioMed Res. Int. 2015, 2015, 206849. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.; Stamm, S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science 2006, 311, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Ender, C.; Krek, A.; Friedländer, M.R.; Beitzinger, M.; Weinmann, L.; Chen, W.; Pfeffer, S.; Rajewsky, N.; Meister, G. A human snoRNA with microRNA-like functions. Mol. Cell 2008, 32, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Liu, X.; Qu, S.; Song, E.; Zou, H.; Gong, C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013, 104, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ying, D.; Lau, Y.-L. In-depth cDNA library sequencing provides quantitative gene expression profiling in cancer biomarker discovery. Genom. Proteom. Bioinform. 2009, 7, 1–12. [Google Scholar] [CrossRef]
- Shen, S.; Wang, Y.; Wang, C.; Wu, Y.N.; Xing, Y. SURVIV for survival analysis of mRNA isoform variation. Nat. Commun. 2016, 7, 11548. [Google Scholar] [CrossRef] [PubMed]
- Asmann, Y.W.; Necela, B.M.; Kalari, K.R.; Hossain, A.; Baker, T.R.; Carr, J.M.; Davis, C.; Getz, J.E.; Hostetter, G.; Li, X. Detection of redundant fusion transcripts as biomarkers or disease-specific therapeutic targets in breast cancer. Cancer Res. 2012, 72, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Clark, J.; Ambroisine, L.; Fisher, G.; Kovacs, G.; Flohr, P.; Berney, D.; Foster, C.; Fletcher, A.; Gerald, W. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 2008, 27, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.C.; Chang, T.T.; Young, K.C.; Lin, X.Z.; Lin, C.Y.; Wu, H.L. Detection of HCV RNA in saliva, urine, seminal fluid, and ascites. J. Med. Virol. 1992, 37, 197–202. [Google Scholar] [PubMed]
- Wieczorek, A.J.; Rhyner, C.; Block, L.H. Isolation and characterization of an RNA-proteolipid complex associated with the malignant state in humans. Proc. Natl. Acad. Sci. USA 1985, 82, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Kolodny, G. Evidence for transfer of macromolecular RNA between mammalian cells in culture. Exp. Cell Res. 1971, 65, 313–324. [Google Scholar] [CrossRef]
- Dinger, M.E.; Mercer, T.R.; Mattick, J.S. RNAs as extracellular signaling molecules. J. Mol. Endocrinol. 2008, 40, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tsui, N.B.; Ng, E.K.; Lo, Y.D. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin. Chem. 2002, 48, 1647–1653. [Google Scholar] [PubMed]
- Freedman, J.E.; Gerstein, M.; Mick, E.; Rozowsky, J.; Levy, D.; Kitchen, R.; Das, S.; Shah, R.; Danielson, K.; Beaulieu, L. Diverse human extracellular RNAs are widely detected in human plasma. Nat. Commun. 2016, 7, 11106. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Halicka, H.D.; Bedner, E.; Darzynkiewicz, Z. Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis. Exp. Cell Res. 2000, 260, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.D.; Silva, A.M.; Kanlikilicer-Unaldi, P.; Filant, J.; Rashed, M.H.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. Exosomal non-coding RNAs: Diagnostic, prognostic and therapeutic applications in cancer. Non-Coding RNA 2015, 1, 53–68. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Nishida, N.; Calin, G.A.; Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T.; Yan, I.K.; Lin, W.-L.; Patel, T. Extracellular Vesicle–Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer 2013, 4, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T.; Lin, W.L.; Yan, I.K.; Braconi, C.; Patel, T. Intercellular nanovesicle-mediated microRNA transfer: A mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 2011, 54, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Filipazzi, P.; Bürdek, M.; Villa, A.; Rivoltini, L.; Huber, V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin. Cancer Biol. 2012, 22, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Quesenberry, P.J.; Aliotta, J.; Camussi, G.; Abdel-Mageed, A.B.; Wen, S.; Goldberg, L.; Zhang, H.-G.; Tetta, C.; Franklin, J.; Coffey, R.J. Potential functional applications of extracellular vesicles: A report by the NIH Common Fund Extracellular RNA Communication Consortium. J. Extracell. Vesicles 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Redzic, J.S.; Balaj, L.; van der Vos, K.E.; Breakefield, X.O. Extracellular RNA mediates and marks cancer progression. Semin. Cancer Biol. 2014, 28, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Khvalevsky, E.Z.; Gabai, R.; Rachmut, I.H.; Horwitz, E.; Brunschwig, Z.; Orbach, A.; Shemi, A.; Golan, T.; Domb, A.J.; Yavin, E. Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20723–20728. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Jung, E.-J.; Shah, M.Y.; Lu, C.; Spizzo, R.; Shimizu, M.; Han, H.D.; Ivan, C.; Rossi, S.; Zhang, X. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013, 3, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Huang, X.; Woodcock, M.; Du, M.; Dittmar, R.; Wang, Y.; Tsai, S.; Kohli, M.; Boardman, L.; Patel, T. Plasma extracellular RNA profiles in healthy and cancer patients. Sci. Rep. 2016, 6, 19413. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Patel, T.; Wong, D.; Das, S.; Freedman, J.E.; Laurent, L.C.; Carter, B.S.; Hochberg, F.; Van Keuren-Jensen, K.; Huentelman, M. Extracellular RNAs: Development as biomarkers of human disease. J. Extracell. Vesicles 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Kopreski, M.S.; Benko, F.A.; Gocke, C.D. Circulating RNA as a Tumor Marker. Ann. N. Y. Acad. Sci. 2001, 945, 172–178. [Google Scholar] [CrossRef] [PubMed]
- March-Villalba, J.A.; Martínez-Jabaloyas, J.M.; Herrero, M.J.; Santamaria, J.; Alino, S.F.; Dasí, F. Cell-free circulating plasma hTERT mRNA is a useful marker for prostate cancer diagnosis and is associated with poor prognosis tumor characteristics. PLoS ONE 2012, 7, e43470. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O'Briant, K.C.; Allen, A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Bussemakers, M.J.; van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.; Schalken, J.A.; Debruyne, F.M.; Ru, N.; Isaacs, W.B. DD3: A New Prostate-specific Gene, Highly Overexpressed in Prostate Cancer. Cancer Res. 1999, 59, 5975–5979. [Google Scholar] [PubMed]
- Hessels, D.; Gunnewiek, J.M.K.; van Oort, I.; Karthaus, H.F.; van Leenders, G.J.; van Balken, B.; Kiemeney, L.A.; Witjes, J.A.; Schalken, J.A. DD3 PCA3-based molecular urine analysis for the diagnosis of prostate cancer. Eur. Urol. 2003, 44, 8–16. [Google Scholar] [CrossRef]
- Burgos, K.L.; Javaherian, A.; Bomprezzi, R.; Ghaffari, L.; Rhodes, S.; Courtright, A.; Tembe, W.; Kim, S.; Metpally, R.; Van Keuren-Jensen, K. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA 2013, 19, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lou, Y.; Zhang, X.; Zhou, H.; Deng, H.; Song, H.; Yu, X.; Xiao, B.; Wang, W.; Guo, J. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clin. Biochem. 2011, 44, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; John, M.A.S.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.-H. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Matse, J.H.; Yoshizawa, J.; Wang, X.; Elashoff, D.; Bolscher, J.G.; Veerman, E.C.; Bloemena, E.; Wong, D.T. Discovery and prevalidation of salivary extracellular microRNA biomarkers panel for the noninvasive detection of benign and malignant parotid gland tumors. Clin. Cancer Res. 2013, 19, 3032–3038. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, G.; Zhang, X.; Li, D.; Huang, J.; Yang, C.; Zhang, P.; Qin, Y.; Duan, Y.; Gong, B. Salivary microRNAs as promising biomarkers for detection of esophageal cancer. PLoS ONE 2013, 8, e57502. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yin, X.; Gong, B.; Nie, W.; Wu, B.; Zhang, X.; Huang, J.; Zhang, P.; Zhou, Z.; Li, Z. Salivary microRNAs show potential as a noninvasive biomarker for detecting resectable pancreatic cancer. Cancer Prev. Res. 2015, 8, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Park, J.W.; Guo, M.; Lin, G.; Crandall, L.; Compton, T.; Wang, X.; Li, X.J.; Chen, F.P.; Xu, R.H. Lack of ABCG2 expression and side population properties in human pluripotent stem cells. Stem Cells 2009, 27, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Bahn, J.H.; Zhang, Q.; Li, F.; Chan, T.-M.; Lin, X.; Kim, Y.; Wong, D.T.; Xiao, X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 2015, 61, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, F.E.; Molenaar, R.J.; Leenstra, S. Recent advances in the molecular understanding of glioblastoma. J. Neuro-Oncol. 2012, 108, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teplyuk, N.M.; Mollenhauer, B.; Gabriely, G.; Giese, A.; Kim, E.; Smolsky, M.; Kim, R.Y.; Saria, M.G.; Pastorino, S.; Kesari, S. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro-Oncology 2012, 14, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): A platform for glioblastoma biomarker development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Truini, A.; Nakata, A.; Lin, J.; Reddy, R.M.; Chang, A.C.; Ramnath, N.; Gotoh, N.; Beer, D.G.; Chen, G. A novel serum 4-microRNA signature for lung cancer detection. Sci. Rep. 2015, 5, 12464. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Kim, J.; Kang, S.H.; Yang, S.R.; Cho, J.-Y.; Cho, H.C.; Shim, S.G.; Paik, Y.-H. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp. Mol. Med. 2015, 47, e184. [Google Scholar] [CrossRef] [PubMed]
- Baraniskin, A.; Nöpel-Dünnebacke, S.; Ahrens, M.; Jensen, S.G.; Zöllner, H.; Maghnouj, A.; Wos, A.; Mayerle, J.; Munding, J.; Kost, D. Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for pancreatic and colorectal adenocarcinoma. Int. J. Cancer 2013, 132, E48–E57. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, J.D.; Wimberger, P.; Wilsch, K.; Fluck, M.; Suter, L.; Brunner, G. Increased level of circulating U2 small nuclear RNA fragments indicates metastasis in melanoma patients. Clin. Chem. Lab. Med. (CCLM) 2015, 53, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzas, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seashols-Williams, S.; Lewis, C.; Calloway, C.; Peace, N.; Harrison, A.; Hayes-Nash, C.; Fleming, S.; Wu, Q.; Zehner, Z.E. High-throughput miRNA sequencing and identification of biomarkers for forensically relevant biological fluids. Electrophoresis 2016, 37, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, D. Flow cytometry and RNA studies. Biol. Cell 1993, 78, 27–30. [Google Scholar] [CrossRef]
- Chu, B. Dynamic Light Scattering, in Soft Matter Characterization; Springer: Dordrecht, The Netherlands, 2008; pp. 335–372. [Google Scholar]
- Jönsson, U.; Fägerstam, L.; Löfas, S.; Stenberg, E.; Karlsson, R.; Frostell, A.; Markey, F.; Schindler, F. Introducing a biosensor based technology for real-time biospecific interaction analysis. Ann. Biol. Clin. 1992, 51, 19–26. [Google Scholar]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Witten, D.M.; Johnstone, I.M.; Tibshirani, R. Normalization, testing, and false discovery rate estimation for RNA-sequencing data. Biostatistics 2012, 13, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Poos, K.; Smida, J.; Nathrath, M.; Maugg, D.; Baumhoer, D.; Neumann, A.; Korsching, E. Structuring osteosarcoma knowledge: An osteosarcoma-gene association database based on literature mining and manual annotation. Database 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Kumar, B.; Jayadev, M.; Raghav, D.; Singh, A. CoReCG: A comprehensive database of genes associated with colon-rectal cancer. Database 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Dancik, G.M. An online tool for evaluating diagnostic and prognostic gene expression biomarkers in bladder cancer. BMC Urol. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Bravo, À.; Cases, M.; Queralt-Rosinach, N.; Sanz, F.; Furlong, L.I. A knowledge-driven approach to extract disease-related biomarkers from the literature. BioMed Res. Int. 2014, 2014, 253128. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.; Knight, R.; Melino, G.; Barlev, N.; Tsvetkov, P. MIRUMIR: An online tool to test microRNAs as biomarkers to predict survival in cancer using multiple clinical data sets. Cell Death Differ. 2013, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.F.; Tran, J.J.; Crichton, D.J.; Anton, K.; Kincaid, H.; Kelly, S.C.; Hughes, J.S.; Mattmann, C. An Extensible Biomarker Curation Approach and Software Infrastructure for the Early Detection of Cancer. HEALTHINF 2009, 387–392. [Google Scholar]
- Ainsztein, A.M.; Brooks, P.J.; Dugan, V.G.; Ganguly, A.; Guo, M.; Howcroft, T.K.; Kelley, C.A.; Kuo, L.S.; Labosky, P.A.; Lenzi, R. The NIH Extracellular RNA Communication Consortium. J. Extracell. Vesicles 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Di Bella, S.; Nigita, G.; Macca, V.; Lagana, A.; Giugno, R.; Pulvirenti, A.; Ferro, A. miRandola: Extracellular circulating microRNAs database. PLoS ONE 2012, 7, e47786. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.-M.; Xu, Y.-M.; Huang, L.-F.; Wang, X.-Z. Exosomes: Novel biomarkers for clinical diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Available online: http://commonfund.nih.gov/Exrna (accessed on 17 February 2017).
- Shah, R.; Tanriverdi, K.; Levy, D.; Larson, M.; Gerstein, M.; Mick, E.; Rozowsky, J.; Kitchen, R.; Murthy, V.; Mikalev, E. Discordant Expression of Circulating microRNA from Cellular and Extracellular Sources. PLoS ONE 2016, 11, e0153691. [Google Scholar] [CrossRef] [PubMed]

| RNA | DNA | Protein | |
|---|---|---|---|
| Biological function | Transmitter of genetic information, regulatory factor | Carrier of genetic information | Catalyst, regulatory factor, structural component |
| Amount per human cell | 10~30 pg [12] | ~6 pg [12] | 130~150 pg [13] |
| Amount in 1 mL plasma | 1~1000 ng [14,15] | 1~1000 ng [14,15] | ~6 × 104 ng [16] |
| Biological stability | Not stable in alkaline conditions; reactive | Stable in alkaline conditions; less reactive than RNAs | Usually more stable than nucleic acids |
| Current stage as biomarker | Under preliminary research; used for clinical counseling | Under preliminary research; used for clinical counseling | Widely used in routine diagnosis |
| Features as biomarker | Expression profile | Mutation, epigenetic modification | Abundance; proteomics profile |
| Accuracy and Specificity | Relatively higher | Relatively higher | Relatively lower (mostly rely on good antibody being found) |
| Detection methods | RT-qPCR, RNA-seq, microarray | qPCR, DNA-seq, microarray | Mass spectrometry, immunoassay, electrophoresis |
| Example | PAM50 (Breast cancer) | BRCA1 mutation (Breast cancer) | AFP (Liver cancer) |
| Biomarker Name | RNA Type | Cancer Type | Up/Down | Value | Reference |
|---|---|---|---|---|---|
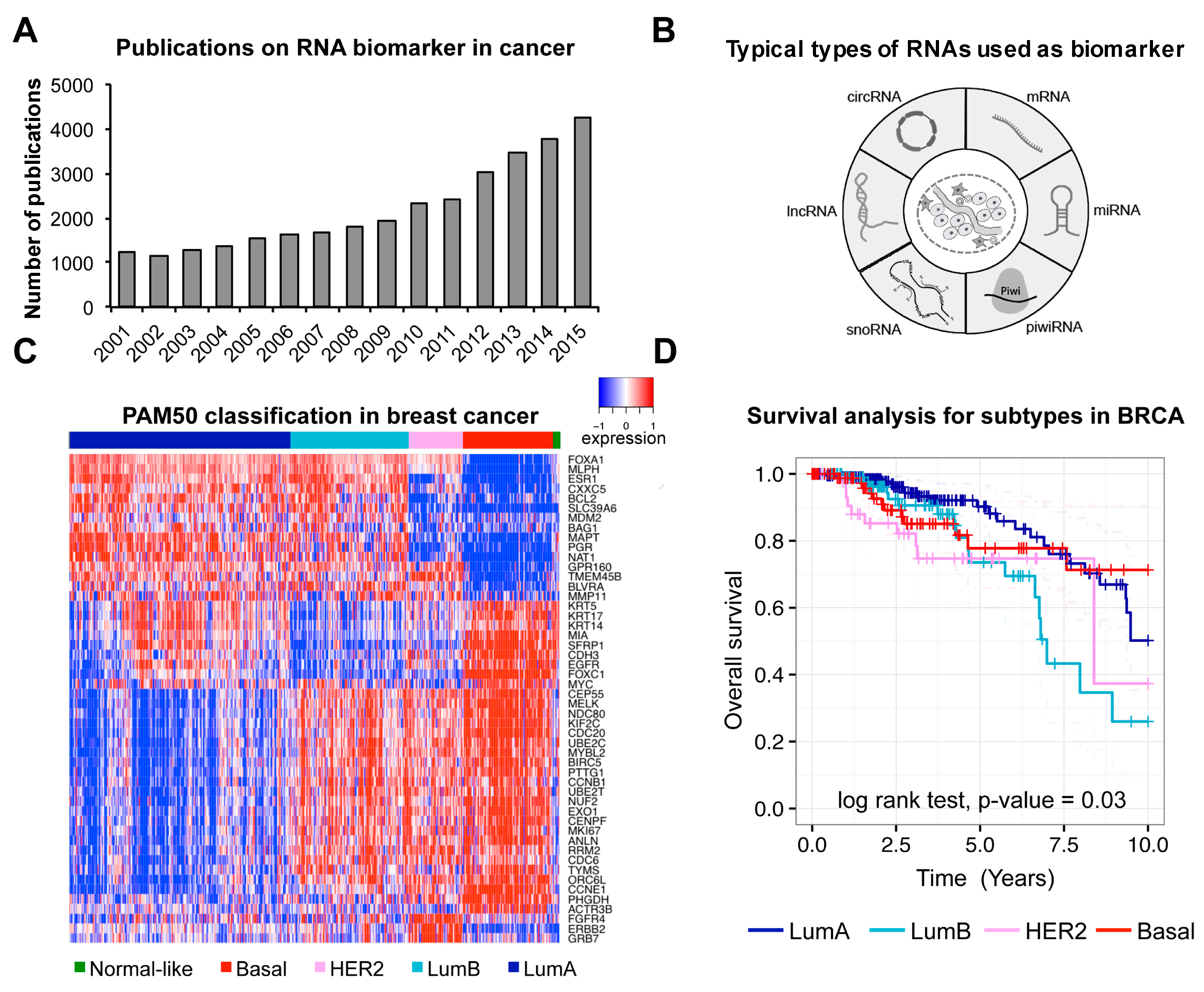
| PAM50 | mRNA | Breast cancer | - | Diagnosis/Prognosis | [2] |
| Cell Cycle Progression mRNA panel | mRNA | Prostate cancer | Up | Prognosis | [22] |
| PCA3 | lncRNA | Prostate cancer | Up | Diagnosis | [3] |
| HULC | lncRNA | Pancreatic cancer | Up | Prognosis | [26] |
| HOTAIR | lncRNA | Nasopharyngeal cancer | Up | Prognosis | [27] |
| miR-21 | miRNA | Pancreatic cancer | Down | Clinical outcome prediction, potential therapy target | [25] |
| piR-651 | piwiRNA | Lymphoma | Down | Prognosis | [28] |
| SNORD33, SNORD66, SNORD76 | snoRNA | Non-small-cell lung cancer | Up | Diagnosis | [29] |
| Hsa_circ_002059 | circRNA | Gastric cancer | Down | Diagnosis | [30] |
| Cancers by Body System | Cancer Type | Biomarker Name | RNA Type | Up/Down | Value | Source | Reference |
|---|---|---|---|---|---|---|---|
| Genitourinary system | Prostate cancer | hTERT | mRNA | Up | Diagnosis | Peripheral blood | [71] |
| Genitourinary system | Prostate cancer | PCA3 | lncRNA | Up | Diagnosis | Urine | [3] |
| Digestive system | Gastric cancer | piR-651 | piwiRNA | Down | Diagnosis | Peripheral blood | [76] |
| Digestive system | Gastric cancer | piR-823 | piwiRNA | Down | Diagnosis | Peripheral blood | [76] |
| Digestive system | Oral cancer | miR-125a | miRNA | Down | Diagnosis | Saliva | [81] |
| Digestive system | Oral cancer | miR-200a | miRNA | Down | Diagnosis | Saliva | [81] |
| Digestive system | Liver cancer | miR-18a | miRNA | Up | Diagnosis | Serum exosome | [87] |
| Digestive system | Liver cancer | miR-221 | miRNA | Up | Diagnosis | Serum exosome | [87] |
| Digestive system | Liver cancer | miR-222 | miRNA | Up | Diagnosis | Serum exosome | [87] |
| Digestive system | Liver cancer | miR-224 | miRNA | Up | Diagnosis | Serum exosome | [87] |
| Respiratory system | Non-small-cell lung cancer | miR-193b, miR-301, miR-141, miR-200b | miRNA | - | Diagnosis | Serum | [86] |
| Respiratory system | Non-small-cell lung cancer | SNORD33, SNORD66, SNORD76 | snoRNA | Up | Diagnosis | Plasma | [29] |
| Nervous system | Glioblastoma and brain metastasis | miR-10b | miRNA | Up | Diagnosis | Cerebrospinal fluid | [84] |
| Nervous system | Glioblastoma and brain metastasis | miR-21 | miRNA | Up | Diagnosis | Cerebrospinal fluid | [84] |
| Name | Biomarker Type | Dysfunction Type | Website |
|---|---|---|---|
| HMDD v2.0 | miRNA | miRNA expression level, miRNA gene copy number alteration, miRNA mutation, miRNA binding site altertaion, genetic varation in miRNA binding-site | http://cmbi.bjmu.edu.cn/hmdd |
| Osteosarcoma Database | miRNA | miRNA expression level | http://osteosarcoma-db.uni-muenster.de |
| CoReCG | RNA and DNA | gene expression level, gene mutation, gene polymorphism | lms.snu.edu.in/corecg |
| BC-BET | RNA | gene expression level | http://bioinformatics.easternct.edu/BCBET2/ |
| Database of disease-related biomarkers | RNA | gene expression level | http://ibi.imim.es/biomarkers/ |
| MIRUMIR | miRNA | miRNA expression level | http://www.bioprofiling.de/MIRUMIR |
| BMDB | RNA, DNA, and protein | gene encoding protein assotiated with disease, gene copy number alteration, alternative splicing etc. | https://edrn.nci.nih.gov/biomarkers |
| exRNA Atlas | exRNA * | small RNA sequencing and RT-qPCR-derived exRNA profiles | http://exrna-atlas.org/ |
| miRandola | exRNA | expression level | http://mirandola.iit.cnr.it/ |
| ExoCarta | exosomal proteins, RNA and lipids | expression level | http://www.exocarta.org/ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, X.; Li, T.; Huang, Y.; Sun, J.; Zhu, Y.; Yang, Y.; Lu, Z.J. RNA Biomarkers: Frontier of Precision Medicine for Cancer. Non-Coding RNA 2017, 3, 9. https://doi.org/10.3390/ncrna3010009
Xi X, Li T, Huang Y, Sun J, Zhu Y, Yang Y, Lu ZJ. RNA Biomarkers: Frontier of Precision Medicine for Cancer. Non-Coding RNA. 2017; 3(1):9. https://doi.org/10.3390/ncrna3010009
Chicago/Turabian StyleXi, Xiaochen, Tianxiao Li, Yiming Huang, Jiahui Sun, Yumin Zhu, Yang Yang, and Zhi John Lu. 2017. "RNA Biomarkers: Frontier of Precision Medicine for Cancer" Non-Coding RNA 3, no. 1: 9. https://doi.org/10.3390/ncrna3010009





