Expanding the Repertoire of Dielectric Fractional Models: A Comprehensive Development and Functional Applications to Predict Metabolic Alterations in Experimentally-Inaccessible Cells or Tissues
Abstract
:1. Introduction
1.1. General Considerations
1.2. Biological Tissues
1.3. Synthetic Description of the Interaction between Electric Field and Biological Tissues
1.4. Interaction of Electrical Field with Biological Tissue
2. Importance of Phenomenological and State-Equations
3. Remarks on Linear Response Theory
Dielectric Relaxation Phenomena
4. Remarks on the Dielectric Kluitenberg-Model
4.1. Phenomenological Dielectric Equations
4.2. Linear Approximation State Equations
5. Classical Fractional Model
6. Fractional Model
7. Deduction of the Cole–Cole Model
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Ciancio, V.; Farsaci, F.; di Marco, G. A method for experimental evaluation of phenomenological coefficients in media with dielectric relaxation. Phys. B Condens. Matter 2007, 387, 130–135. [Google Scholar] [CrossRef]
- Ciancio, V.; Farsaci, F.; Rogolino, P. Phenomenological approach on wave propagation in dielectric media with two relaxation times. Phys. B Condens. Matter 2009, 404, 320–324. [Google Scholar] [CrossRef]
- Ciancio, V.; Farsaci, F.; Rogolino, P. Mathematical approach to the relaxation phenomena. Appl. Sci. 2009, 11, 48–59. [Google Scholar]
- Ciancio, V.; Farsaci, F.; Rogolino, P. On a thermodynamical model for dielectric relaxation phenomena. Phys. B Condens. Matter 2010, 405, 175–179. [Google Scholar] [CrossRef]
- Farsaci, F.; Ciancio, V.; Rogolino, P. Mechanical model for relaxation phenomena in viscoanelastic media of order one. Phys. B Condens. Matter 2010, 405, 3208–3212. [Google Scholar] [CrossRef]
- Farsaci, F.; Rogolino, P. An alternative dielectric model for low and high frequencies: A non-equilibrium thermodynamic approach. J. Non-Equilib. Thermodyn. 2012, 37, 27–41. [Google Scholar] [CrossRef]
- Kluitenberg, G.A. On dielectric and magnetic relaxation phenomena and non-equilibrium thermodynamics. Physica 1973, 68, 75–92. [Google Scholar] [CrossRef]
- Kluitenberg, G.A. On dielectric and magnetic relaxation phenomena and vectorial internal degrees. Phys. A Stat. Mech. Its Appl. 1977, 87, 302–330. [Google Scholar] [CrossRef]
- Kluitenberg, G.A. On vectorial internal variables and dielectric and magnetic relaxation phenomena. Phys. A Stat. Mech. Its Appl. 1981, 109, 91–122. [Google Scholar] [CrossRef]
- Farsaci, F.; Russo, A.; Ficarra, S.; Tellone, E. Dielectric properties of human normal and malignant liver tissue: A non-equilibrium thermodynamics approach. Open Access Libr. J. 2015, 2, e1395. [Google Scholar] [CrossRef]
- Peggy, C.L. Quick & Easy Medical Terminology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ongole, R.; Praveen, B.N. Textbook of Oral Medicine, Oral Diagnosis and Oral Radiology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Ing, V.W. The Etiology and Management of Leukopenia. Can. Fam. Phys. 1984, 30, 1835–1839. [Google Scholar]
- Tellone, E.; Galtieri, A.; Russo, A.; Giardina, B.; Ficarra, S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2015, 2015, 392169. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Galtieri, A.; Russo, A.; Ficarra, S. How does Resveratrol influence the genesis of some neurodegenerative diseases? Neural Regen. Res. 2016, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Galtieri, A.; Russo, A.; Ficarra, S. Protective effects of the caffeine against neurodegenerative diseases. Curr. Med. Chem. 2017, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Talarek, S.; Listos, J.; Barreca, D.; Tellone, E.; Sureda, A.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Neuroprotective effects of honokiol: From chemistry to medicine. Biofactors 2017. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Currò, M.; Bellocco, E.; Ficarra, S.; Laganà, G.; Tellone, E.; Giunta, L.; Visalli, G.; Caccamo, D.; Galtieri, A.; et al. Neuroprotective effects of phloretin and its glycosylated derivative on rotenone-induced toxicity in human SH-SY5Y neuronal-like cells. Biofactors 2017, 43, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Farsaci, F.; Tellone, E.; Cavallaro, M.; Russo, A.; Ficarra, S. Low frequency dielectric characteristics of human blood: A non-equilibrium thermodynamic approach. J. Mol. Liq. 2013, 188, 113–119. [Google Scholar] [CrossRef]
- Farsaci, F.; Ficarra, S.; Russo, A.; Galtieri, A.; Tellone, E. Dielectric properties of human diabetic blood: Thermodynamic characterization and new prospective for alternative diagnostic techniques. J. Adv. Dielectr. 2015, 5, 1550021. [Google Scholar] [CrossRef]
- Farsaci, F.; Tellone, E.; Galtieri, A.; Russo, A.; Ficarra, S. Evaluation of the human blood entropy production: A new thermodynamic approach. J. Ultrasound 2016, 19, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Farsaci, F.; Tellone, E.; Russo, A.; Galtieri, A.; Ficarra, S. Rheological properties of human blood in the network of non-equilibrium thermodynamic with internal variables by means of ultrasound wave perturbation. J. Mol. Liquids 2017, 231, 206–212. [Google Scholar] [CrossRef]
- Farsaci, F.; Ficarra, S.; Russo, A.; Galtieri, A.; Tellone, E. On evaluation of electric conductivity by mean of non equilibrium thermodynamic approach with internal variables. An application to human erythrocyte suspension for metabolic characterizations. J. Mol. Liq. 2016, 224, 1181–1188. [Google Scholar] [CrossRef]
- Scala, A.; Ficarra, S.; Russo, A.; Barreca, D.; Giunta, E.; Galtieri, A.; Grassi, G.; Tellone, E. Alterations in red blood cell functionality induced by an indole scaffold containing a y-iminodiketo moiety: Potential antiproliferative conditions. Oxid. Med. Cell Longev. 2016, 2016, 2104247. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, S.; Russo, A.; Barreca, D.; Giunta, E.; Galtieri, A.; Tellone, E. Short-term effects of chlorpromazine on oxidative stress in erythrocyte functionality: Activation of metabolism and membrane perturbation. Oxid. Med. Cell Longev. 2016, 2016, 2394130. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; de Rosa, M.C.; Pirolli, D.; Russo, A.; Giardina, B.; Galtieri, A.; Ficarra, S. Molecular interactions of hemoglobin with resveratrol: Potential protective antioxidant role and metabolic adaptations of the erythrocyte. Biol. Chem. 2014, 395, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, S.; Misiti, F.; Russo, A.; Carelli-Alinovi, C.; Bellocco, E.; Barreca, D.; Laganà, G.; Leuzzi, U.; Toscano, G.; Giardina, B.; et al. Antiepileptic carbamazepine drug treatment induces alteration of membrane in red blood cells: Possible positive effects on metabolism and oxidative stress. Biochimie 2013, 95, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Ficarra, S.; Russo, A.; Bellocco, E.; Barreca, D.; Laganà, G.; Leuzzi, U.; Pirolli, D.; de Rosa, M.C.; Giardina, B.; Galtieri, A. Caffeine inhibits erythrocyte membrane derangement by antioxidant activity and by blocking caspase 3 activation. Biochimie 2012, 94, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Galtieri, A.; Tellone, E.; Ficarra, S.; Russo, A.; Bellocco, E.; Barreca, D.; Scatena, R.; Laganà, G.; Leuzzi, U.; Giardina, B. Resveratrol treatment induces redox stress in red blood cells: A possible role of caspase 3 in metabolism and anion transport. Biol. Chem. 2010, 391, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Misiti, F.; Orsini, F.; Clementi, M.E.; Masala, D.; Tellone, E.; Galtieri, A.; Giardina, B. Amyloid peptide inhibits ATP release from human erythrocytes. Biochem. Cell Biol. 2008, 86, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, F. Fractional Calculus and Waves in Linear Viscoelasticity: An Introduction to Mathematical Models; Imperial College Press: London, UK, 2010. [Google Scholar]
- Magin, R.L. Fractional calculus models of complex dynamics in biological tissues. Comput. Math. Appl. 2010, 59, 1586–1593. [Google Scholar] [CrossRef]
- Rosa, C.F.; de Oliveira, E.C. Relaxation Equations: Fractional Models. arXiv, 2015; arXiv:1510.01681. [Google Scholar]
- Khamzin, A.A.; Nigmatullin, R.R.; Popov, I.I. Log-periodic corrections to the Cole–Cole expression in dielectric relaxation. Phys. A Stat. Mech. Its Appl. 2013, 392, 136–148. [Google Scholar] [CrossRef]
- Khamzin, A.A.; Nigmatullin, R.R.; Popov, I.I. Microscopic model of a non-debye dielectric relaxation: The Cole—Cole law and its generalization. Theor. Math. Phys. 2012, 173, 1604–1619. [Google Scholar] [CrossRef]
- Farsaci, F.; Ficarra, S.; Galtieri, A.; Tellone, E. A New Non-Equilibrium Thermodynamic Fractional Visco-Inelastic Model to Predict Experimentally Inaccessible Processes and Investigate Pathophysiological Cellular Structures. Fluids 2017, 2, 59. [Google Scholar] [CrossRef]
- Trimble, W.S.; Grinstein, S. Barriers to the free diffusion of proteins and lipids in the plasma membrane. J. Cell Biol. 2015, 208, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Munson, J.M.; Shieh, A.C. Interstitial fluid flow in cancer: Implications for disease progression and treatment. Cancer Manag. Res. 2014, 6, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.S.; Tan, W.H.K.; Lee, T.; Wang, C.H. Transient interstitial fluid flow in brain tumors: Effect on drug delivery. Chem. Eng. Sci. 2005, 60, 4803–4821. [Google Scholar] [CrossRef]
- Abdalla, S.; Al-ameer, S.S.; Al-Magaishi, S.H. Electrical properties with relaxation through human blood. Biomicrofluidics 2010, 4, 034101. [Google Scholar] [CrossRef] [PubMed]
- Schwan, H.P. Electrical properties of blood and its constitutents: Alternating current spectroscopy. Ann. Hematol. 1983, 46, 185–197. [Google Scholar] [CrossRef]
- Chelidze, T. Dielectric spectroscopy of blood. J. Non-Cryst. Solids 2002, 305, 285–294. [Google Scholar] [CrossRef]
- O’Rourke, A.P.; Lazebnik, M.; Bertram, J.M.; Converse, M.C.; Hagness, S.C.; Webster, J.G.; Mahvi, D.M. Dielectric properties of human normal, malignant and cirrhotic liver tissue: In vivo and ex vivo measurements from 0.5 to 20 ghz using a precision open-ended coaxial probe. Phys. Med. Biol. 2007, 52, 4707. [Google Scholar] [CrossRef] [PubMed]
- Stańczyk, M.; Zegadło, A.; Zwierowicz, T.; Zak, D.; Bogusławska, R.; Maruszyński, M. Microwave Ablation of Liver Tumors as a New Instrument for Minimally Invasive Liver Surgery. Polski Merkur. Lek. 2009, 26, 545–549. [Google Scholar]
- Abdalla, S. Complex permittivity of blood cells and E. coli suspensions. J. Mol. Liq. 2011, 160, 130–135. [Google Scholar] [CrossRef]
- Abdalla, S. Low frequency dielectric properties of human blood. IEEE Trans. Nanobiosci. 2011, 10, 113–120. [Google Scholar] [CrossRef] [PubMed]
- De Groot, S.R.; Mazur, P. Non-Equilibrium Thermodynamics; Dover Publication: New York, NY, USA, 1984. [Google Scholar]
- Champeney, D.C.; Taylor, C.A. Fourier Transforms and Their Physical Applications; Academic Press: London, UK; New York, NY, USA, 1974. [Google Scholar]
- McCrum, N.G.; Read, B.E.; Williams, G. An Elastic and Dielectric Effects in Polymeric Solids; John Wiley and Sons Ltd.: London, UK, 1967. [Google Scholar]
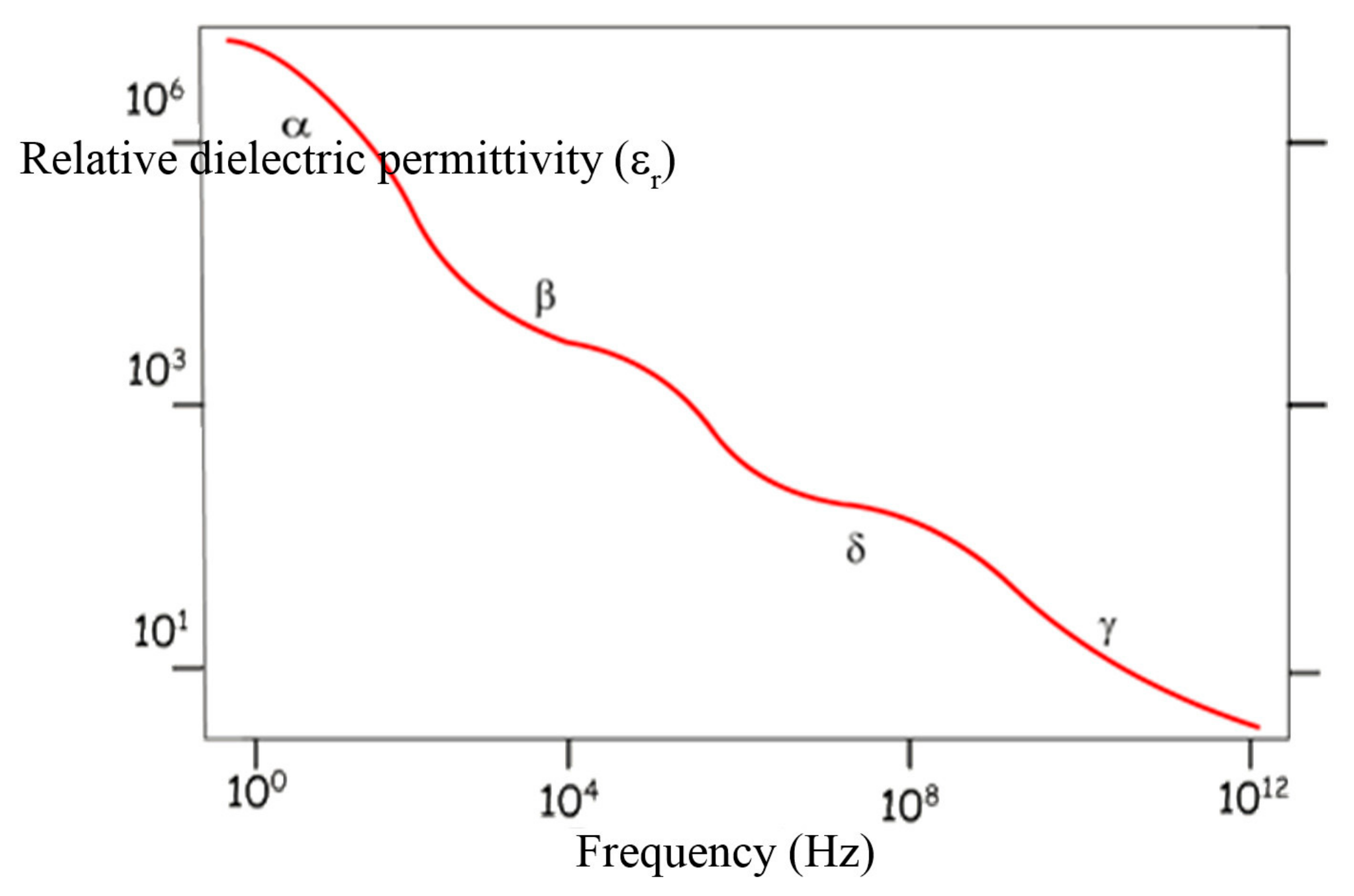

| Polarization | Relaxation Time (s) | Frequency (Hz) | Wave |
|---|---|---|---|
| Electronic deformation | 10−16 | 1015 | UV |
| Atomic deformation | 10−14–10−13 | 1013–1012 | IR |
| Dipolar Orientation | 10−11–10−3 | 1010–102 | Microwave |
| Interfacial | 10−3–10−1 | 102–100 | Low-frequency |
| Frequency (Hz) | Method |
|---|---|
| 10−4 to 10−1 | d.c. Transient measures |
| 10−2 to 102 | Ultra-low frequency bridge |
| 10 to 107 | Schering bridge |
| Transformer bridge | |
| 105 to 108 | Resonance circuits |
| 108 to 109 | Coaxial line |
| Re-entrant cavity | |
| 109 to 3 × 1010 | H01n cavity resonator |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farsaci, F.; Tellone, E.; Galtieri, A.; Ficarra, S. Expanding the Repertoire of Dielectric Fractional Models: A Comprehensive Development and Functional Applications to Predict Metabolic Alterations in Experimentally-Inaccessible Cells or Tissues. Fluids 2018, 3, 9. https://doi.org/10.3390/fluids3010009
Farsaci F, Tellone E, Galtieri A, Ficarra S. Expanding the Repertoire of Dielectric Fractional Models: A Comprehensive Development and Functional Applications to Predict Metabolic Alterations in Experimentally-Inaccessible Cells or Tissues. Fluids. 2018; 3(1):9. https://doi.org/10.3390/fluids3010009
Chicago/Turabian StyleFarsaci, Francesco, Ester Tellone, Antonio Galtieri, and Silvana Ficarra. 2018. "Expanding the Repertoire of Dielectric Fractional Models: A Comprehensive Development and Functional Applications to Predict Metabolic Alterations in Experimentally-Inaccessible Cells or Tissues" Fluids 3, no. 1: 9. https://doi.org/10.3390/fluids3010009





