2.1. Microgel Preparation
Microgels were prepared by heating Blg solutions at different pH values (MG
59, MG
58, MG
57), while a portion of MG
58 were subsequently treated with glutaraldehyde to reduce their deformability (MG
X). The average hydrodynamic diameters of all resulting Blg microgels were between 150 and 300 nm (
Table 1), significantly larger than the hydrodynamic diameter of 2.8 nm for unheated Blg. Increased pH of the solution during heat treatment led to microgels with a significantly reduced particle size so that MG
59 were the smallest and MG
57 were the largest, which was consistent with previous experiments [
13]. The polydispersity indices for all microgel samples were <0.05, indicating highly monodisperse size distributions that matched previous observations of Blg microgels isolated from residual unaggregated protein [
17]. Glutaraldehyde treatment (MG
X) did not significantly alter the average particle size when compared to the original MG
58. A similar lack of size change following treatment was noted in a prior study, wherein glutaraldehyde created covalent cross-links within the microgel interior [
17]. Transglutaminase treatment was also used in a prior study to create cross-links within microgels, and could be considered as an alternative to glutaraldehyde treatment, yet it was found to be less effective [
17].
2.2. Emulsion Properties Following Homogenization
Dilute, oil-in-water emulsions were prepared with the isolated microgels as a model for a typical beverage emulsion product. All emulsion samples rapidly separated to form a significant cream layer and a relatively less turbid lower phase quickly after homogenization, yet the cream layers were easily redispersed by vortexing the samples prior to subsequent characterization. Such relative ease in redispersing the cream layer was comparable to prior findings with emulsions stabilized with Blg microgels, where stabilized emulsions were prone to reversible flocculation yet resistant to coalescence [
15].
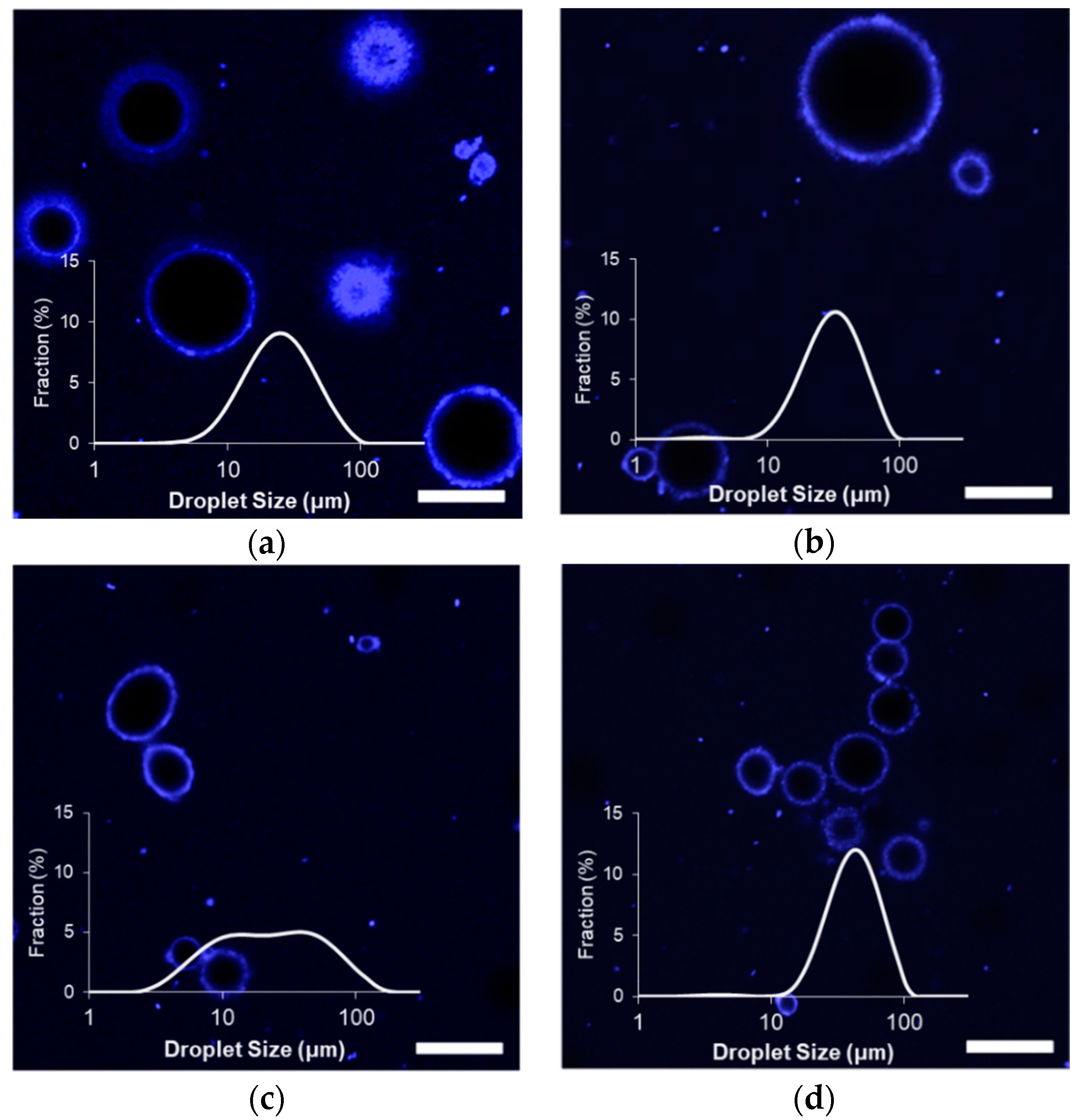
The size distributions of the stabilized limonene/corn oil droplets fell between 5 and 100 um, with the largest average droplet sizes observed when stabilized with MG
X (
Figure 1). Apart from the MG
X-stabilized emulsions, D
43 values of all microgel-stabilized emulsions were similar to those prepared with the unheated Blg (D
43 = 34.6 μm), which can be attributed to a limitation in the bench-top homogenizer used for these experiments (
Table 2). However, homogenization techniques utilizing greater shear energies may disrupt microgels [
15] and were avoided in these tests. Furthermore, similar emulsions prepared with whey protein aggregates and higher intensity emulsification techniques did not achieve droplet sizes any smaller than the D
32 values observed in this study (
Table 2) [
18], indicating that the lower intensity homogenization was sufficient. Because large particles require a reduced droplet curvature in order to adsorb effectively at the interface, the minimum stable droplet size must be relatively large, and greater intensity during homogenization would be largely ineffective.
The microgel diameter had a significant impact on the initial droplet sizes of the emulsions (
Figure 1 and
Table 2). Emulsions prepared with the relatively small MG
59 possessed the smallest D
32 of the tested microgel stabilizers and were only slightly larger than emulsions prepared with unheated Blg (D
32 = 8.4 μm). The relatively broad size distribution of these emulsions indicated that the sub-population of smaller droplets was dominant with a minor fraction of large droplets. Determined D
32 values were larger when prepared with MG
57 and MG
58 and larger still when prepared with MG
X, indicating that larger stabilizers produced emulsions with a larger average droplet size. Previous findings showed that larger microgels diffuse slower to the interface [
16]; this would lead to lower interfacial concentrations directly after homogenization, permitting droplet size growth via coalescence before sufficient microgels could adsorb and stabilize the droplet interface.
Impacts of the different microgels on the initial properties of formed emulsions were further clarified by confocal laser scanning microscopy (CLSM), which are also shown in
Figure 1. All emulsion samples possessed a strongly fluorescing interfacial layer, possibly indicating the presence of a thick layer of microgels at the interface to provide steric-stabilization against droplet growth [
19]. Images of the emulsions formed with the small MG
59 confirmed the presence of relatively small diameter droplets, some of which were flocculated within clusters. As no droplets in MG
59 emulsions were observed with a diameter larger than 25 micrometers, the sub-population of larger objects detected by light scattering could be attributed to these clusters. Images of MG
X emulsions showed similar clusters of smaller droplets, and some of the larger particles indicated by light scattering (
Table 2) could again be attributed to significant flocculation. The flocculated droplets were not observed among MG
57 and MG
58 emulsions; rather, CLSM images showed droplets that were relatively larger than MG
59 emulsion droplets. It was also possible to see a small population of droplets and protein aggregates of less than a few micrometers in diameter within the background of all samples. These much smaller droplets would not have contributed significantly to light scattering due to their much smaller volume in the suspensions, which would explain their minimal contribution to detected size distributions (
Figure 1).
Flocculation among particle-stabilized emulsions has been previously attributed to the simultaneous adsorption of particles to two neighboring droplets following a collision (i.e., bridging) [
20]. Such a scenario would occur if the droplet surfaces possessed few adhered particles, so that there would be little chance of particle-particle repulsions during droplet collisions. The concentration of whey protein microgels at the point of contact between droplets has been previously observed among emulsions with low interfacial loads [
21]. Coalescence is inhibited after minimal particle adsorption to emulsion droplets [
20], and so the large yet unflocculated droplets observed among the MG
58 and MG
57 emulsions implied negligible surface coverage by the larger microgels at early times following homogenization. Interestingly, the similarity in initial droplet morphology between MG
X and MG
59 emulsions indicated that MG
X also adsorbed quickly to the droplet interfaces despite the microgels being of a comparable size to MG
57 and MG
58. It was speculated that glutaraldehyde-treatment, by reducing microgel deformability, facilitated early stabilization of the interfaces by accelerating adsorption (reduced viscous drag in the aqueous phase) or by reducing interfacial spreading.
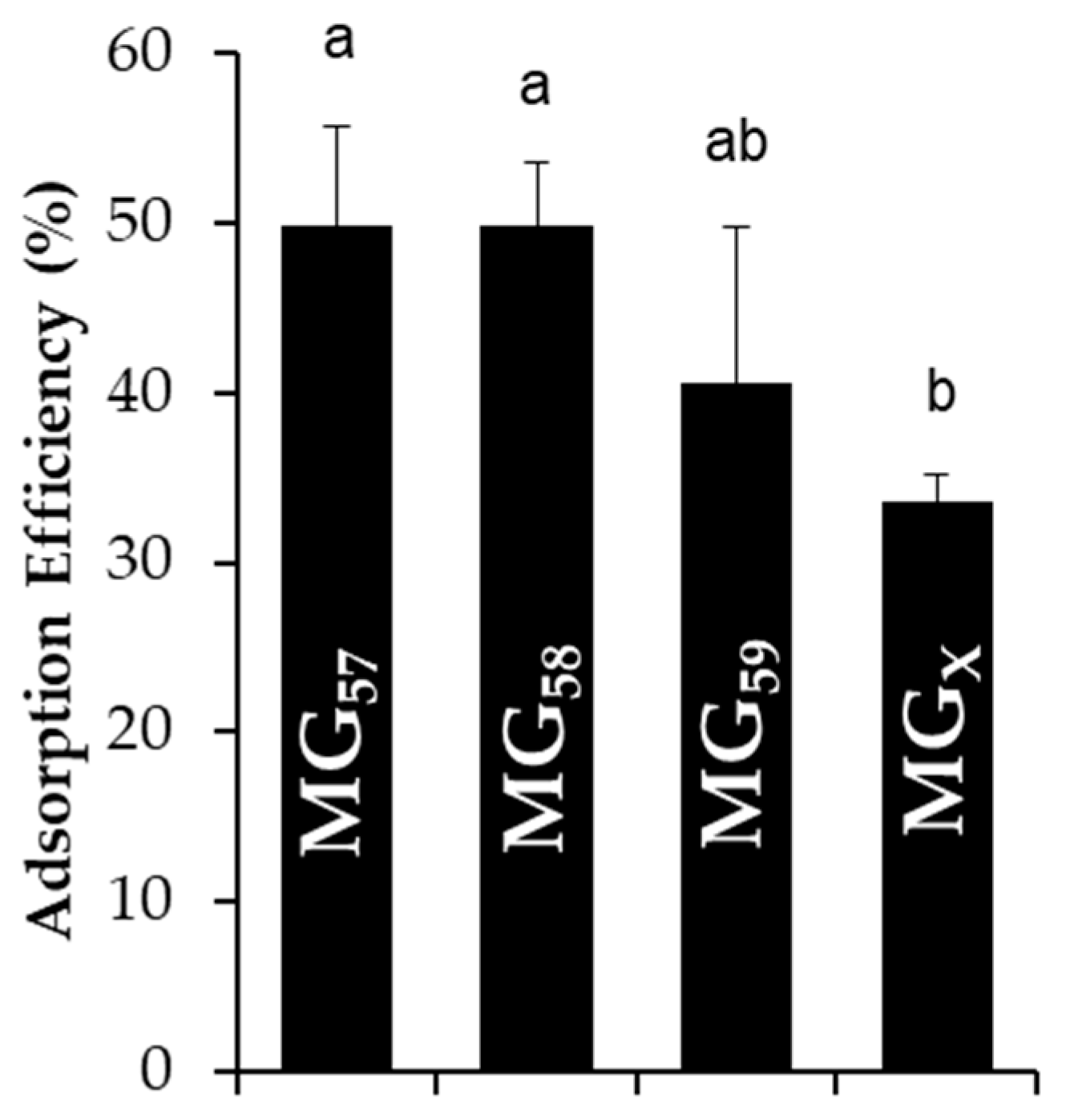
To estimate the final surface coverage of microgels on stabilized emulsion droplets, the adsorption efficiency was quantified by the content of non-adsorbed microgels remaining in the continuous phase. The adsorption efficiency of all microgel types was approximately 40–60% with only MG
X possessing a slightly lower adsorption efficiency in relation to the others (
Figure 2). These values were comparable to the observations for emulsions stabilized by whey protein aggregates [
18]. The amount of microgels adsorbed to droplet interfaces could also be predicted by considering the total surface area of emulsion droplets derived from light scattering measurements, the number of particles that could pack onto the droplet surfaces (assuming an interfacial contact angle of 45° for whey protein microgels [
18]), and the total number of microgels available in the suspensions [
15]. Even in a conservative estimate that assumed microgels did not swell/deform at the interface, less than 15% of available microgels would have been required to saturate the droplet interfaces. Since the experimental adsorption efficiency was significantly greater than 15%, there were sufficient microgels to saturate the droplet interfaces and even potentially form multiple, deposited layers, which has been previously observed among the emulsions stabilized by whey protein particles [
18]. In such a scenario, the microgels would have first adsorbed to all available droplet interfaces and subsequently deposited as increasingly thick layers, as the interfacial adsorption of microgels is energetically preferred to their aggregation [
22].
2.3. Effect of Storage on Emulsion Droplet Stability
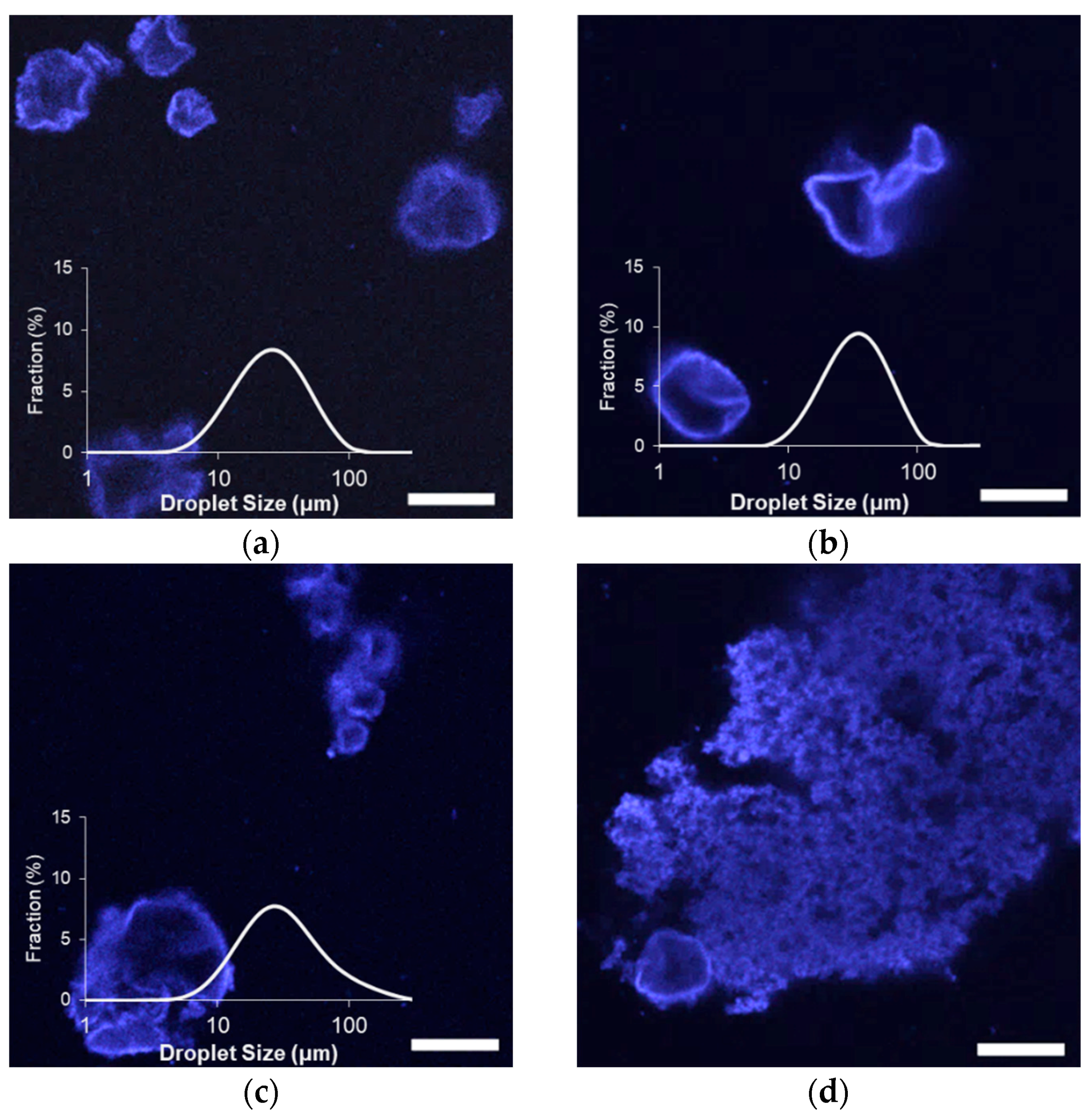
After three and six weeks of storage at 25 °C, all emulsion droplets exhibited non-spherical morphologies, attributed to droplet shrinkage that was associated with the gradual loss of limonene (
Figure 3 and
Figure S1). Limonene, like other terpenes, has a low water solubility with a strong tendency to partition in hydrophobic phases or the air [
23]. Any limonene diffusing out into the aqueous phase would then quickly diffuse to the headspace above the emulsions, which represented a significant volume in each sample vessel.
A loss of limonene would be expected for emulsions stabilized by standard surfactants, given the high content of limonene in the dispersed phase, but significant losses were not expected for a particle-stabilized emulsion [
4]. Limonene represented 90% of the oil phase volume in the emulsions, and its loss necessitated a reduction in droplet surface area to maintain the spheroidal shape, which is accommodated in traditional emulsions by the desorption of small-molecule surfactants. Particle stabilizers have a significantly greater desorption energy, and so the droplets had to adopt non-spherical shapes in order to reduce volume while maintaining the surface area taken up by the particles. A similar formation of non-spherical droplets has been observed in Pickering emulsions when forced to undergo partial coalescence [
24] or when negative pressures were applied to the oil phase [
16,
25]. This can also occur for droplet interfaces saturated with strongly interactive biopolymers possessing signficant compression and shear elasticity [
26].
Despite the partial loss of volume via limonene diffusion among all tested emulsions, light scattering measurements indicated either an increase in size or minimal changes during storage (
Figure 3). There were negligible increases in average diameter among MG
57 and MG
58 emulsions, to significant increases in size for MG
59 and MG
X emulsions (
Table 2). A comparison of the size distributions also showed that the relative fraction of small (<10 μm) droplets in MG
59 emulsions decreased considerably between zero and six weeks (
Figure 1 and
Figure 3). The growth in detected size, despite volume losses from limonene loss, could be attributed to an increase in droplet flocculation, which was observed in the microscopy images. Flocculation was so extensive in MG
X emulsions that small aggregates were visible to the naked eye.
2.4. Effect of pH, Ionic Strength, and Surfactants on Initial Emulsion Droplet Stability
The above tests demonstrated that emulsions prepared with MG
58 were relatively stable during storage when compared to those prepared with smaller or cross-linked microgels. To further test the stabilizing capabilities of MG
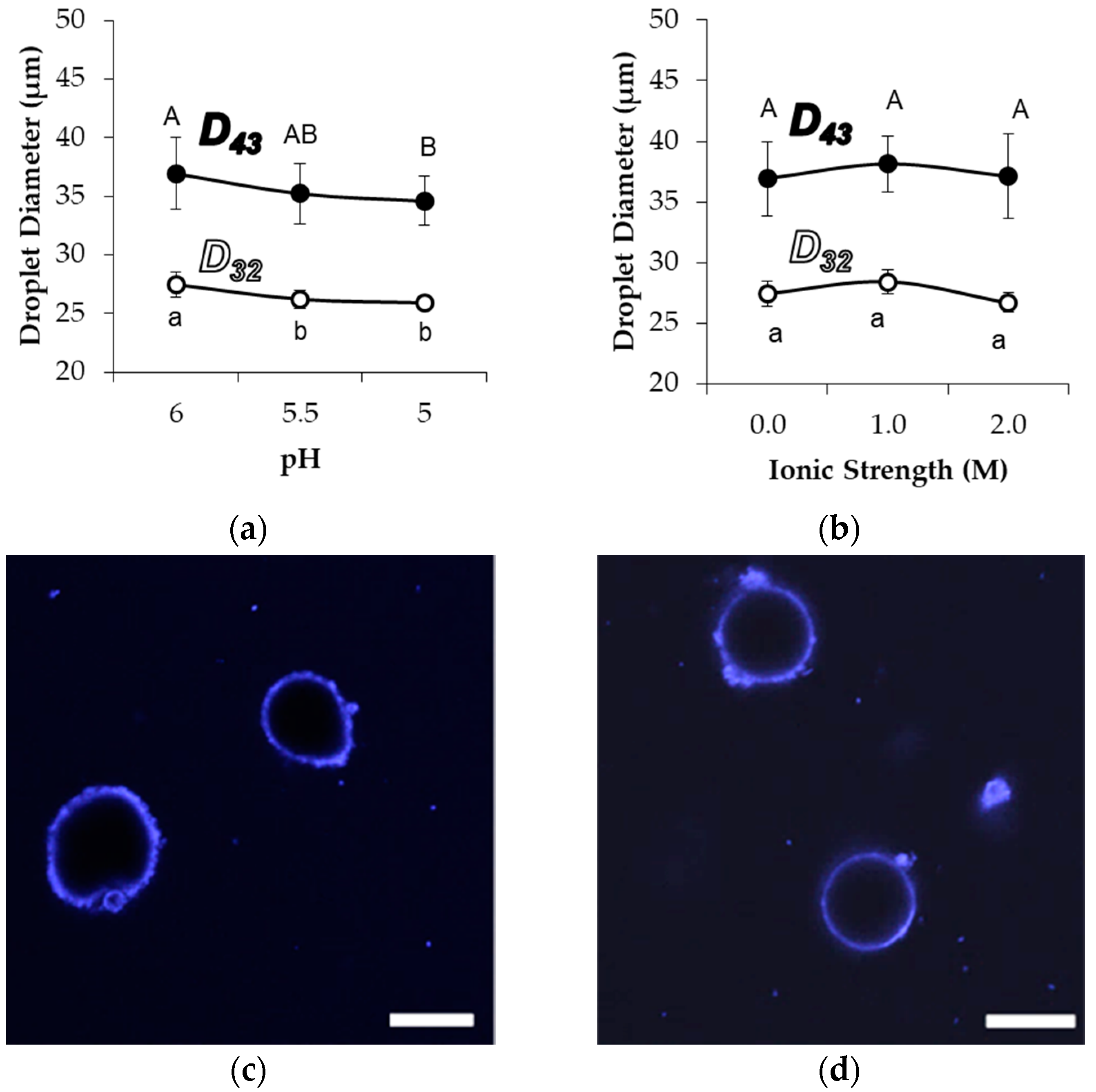
58, droplet size and morphology were observed after exposure to acid, increased ion concentration, or common surfactants. The acidification of emulsions to pH 5.0, as well as an increase in the sodium chloride concentration up to 2.0 M, were expected to diminish effective electrostatic repulsions among the stabilized droplets and cause destabilization based on prior observations of aggregation stability among Blg microgels [
17,
27], yet the emulsion droplet size did not increase (
Figure 4). The average detected droplet diameters did not increase with increased ionic strength, while acidification actually resulted in a small but significant decrease in the average diameter of detected droplets during light scattering. There was no associated change in the droplet size distribution about the mean, indicating that the entire population of droplets was similarly affected by the pH change (not shown). Further, CLSM images did not indicate any major changes in the interfacial coverage or aggregation of droplets under different pH or ionic strength conditions (
Figure 4c,d). However, a limited level of non-sphericity was observed with samples at pH 5.0, implying an accelerated droplet shrinkage and limonene migration. It is plausible that such accelerated shrinkage was caused by an increased pressure exerted on the droplet interface because of the tendency for compaction within and among microgels at the lower pH.
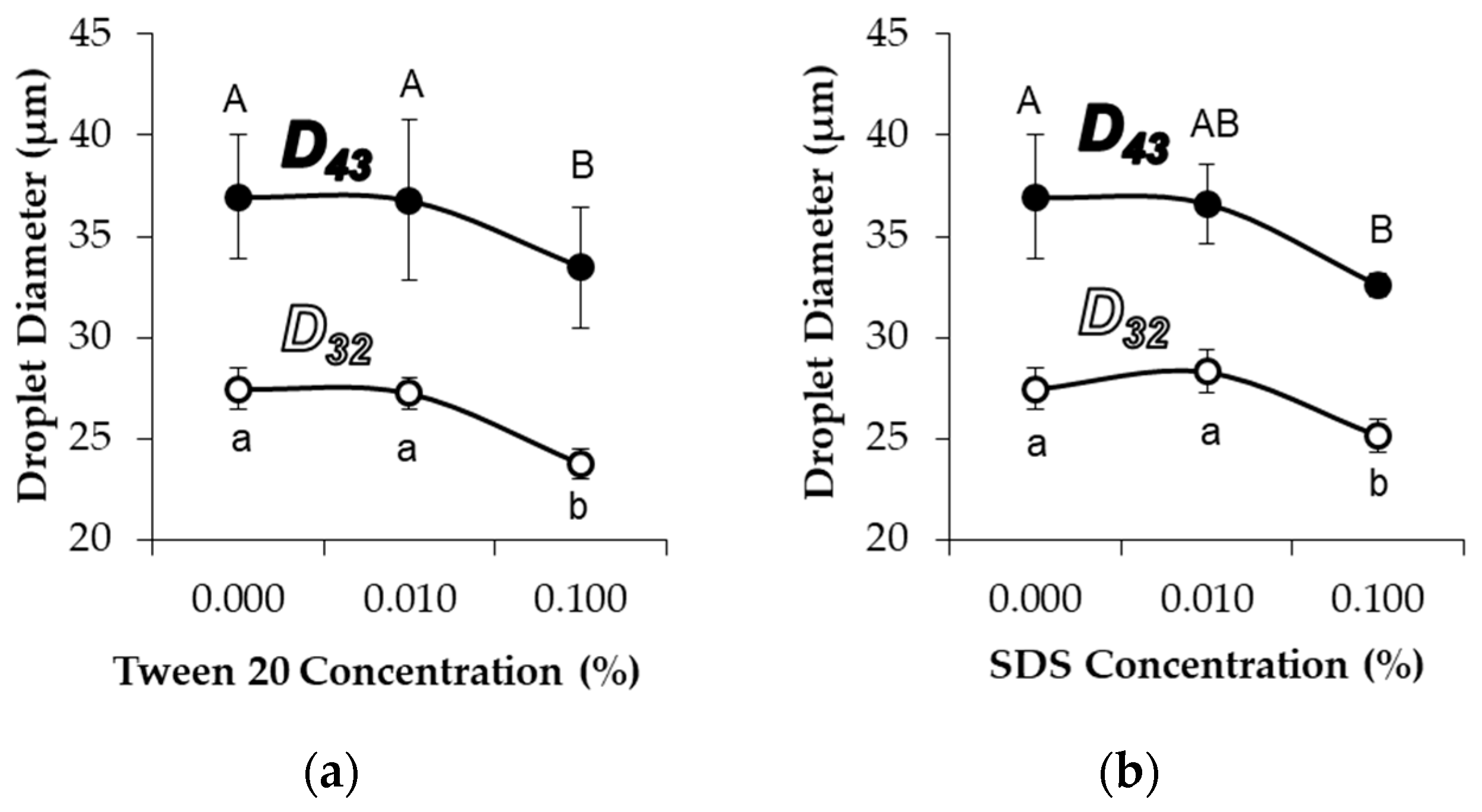
The addition of either 0.1% Tween 20 (non-ionic surfactant) or 0.1% SDS (anionic surfactant) significantly decreased the average diameters of MG
58 emulsion droplets (
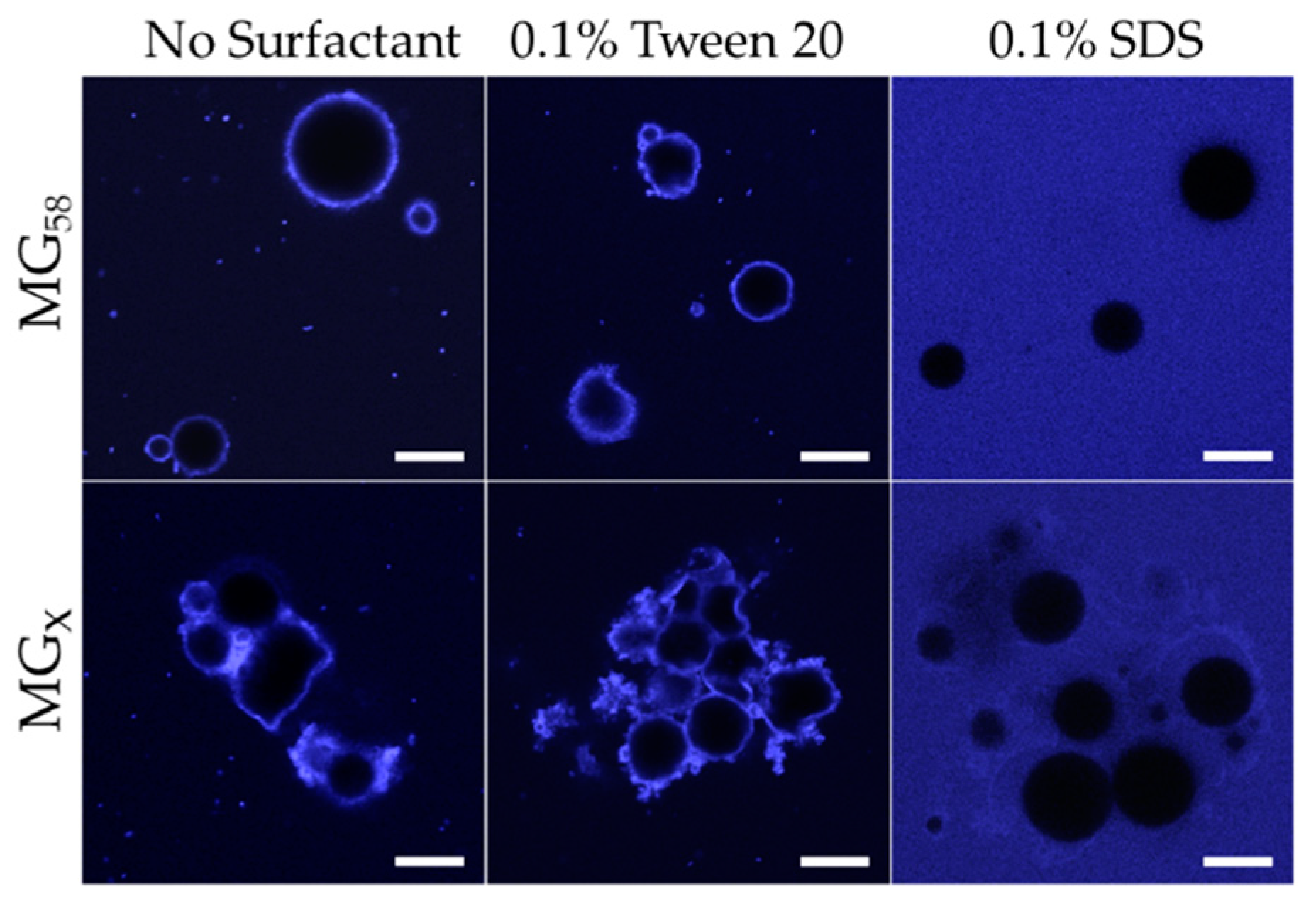
Figure 5). As with pH adjustment, the droplet size distribution indicated a general shift in the droplet size, as opposed to the formation of a separate population of smaller droplets (not shown). CLSM images showed that the addition of 0.1% Tween 20 to either MG
58 emulsions or MG
X emulsions caused the droplets to become non-spherical, but the strong fluorescence at the interface indicated that the quantity of protein in the interfacial layer was not substantially reduced (
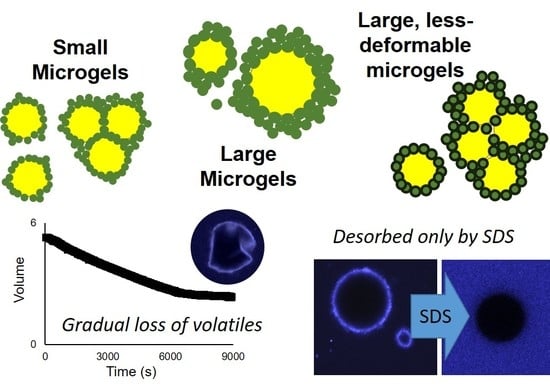
Figure 6). The extent of non-sphericity observed was significantly greater when compared to the emulsions without added surfactant during early storage, including those adjusted to pH 5.0. In contrast, the addition of 0.1% SDS appeared to fully displace microgels from the interface, resulting in smaller, highly spherical droplets (
Figure 6). Among MGX emulsions, SDS was less effective at disrupting flocculated clusters.
The displacement of microgels at the interface or faster adoption of non-spherical morphologies could both be attributed to the decreased interfacial tensions associated with surfactant adsorption. In the case of Tween 20, adsorption of surfactant between microgels at the interface lowered the interfacial tension and reduced the energy for interfacial deformation. Unlike Tween 20, adsorption of SDS at the bare spots of the interface was able to supplant the adsorbed microgels, and the resulting SDS-stabilized droplets subsequently behaved as a typical flavor emulsion system with a reversibly adsorbing/desorbing surfactant. The persistence of flocculated droplets in MG
X emulsions after the addition of SDS could be attributed to the inability of SDS to remove the cross-linked microgels that were bridging between two neighboring droplets (
Figure 6, lower right). In all scenarios, the speculated outcome for surfactant addition, based on the observed acceleration of interfacial deformation or microgel displacement, would be an increased loss rate of limonene.
2.5. Flavor Release
In order to determine the capacity of adsorbed microgels to diminish the release of flavor compounds, the volume of pendant droplets containing the volatile molecule hexanol (50%
v/
v) was monitored over time with or without stabilizing microgels (
Figure S2), and the ability of the microgels to prevent droplet shrinkage or slow the rate of shrinkage is described in
Figure 7. Droplet interfacial tension decreased initially (not shown) in a manner similar to previous droplet tensiometry measurements with Blg microgels [
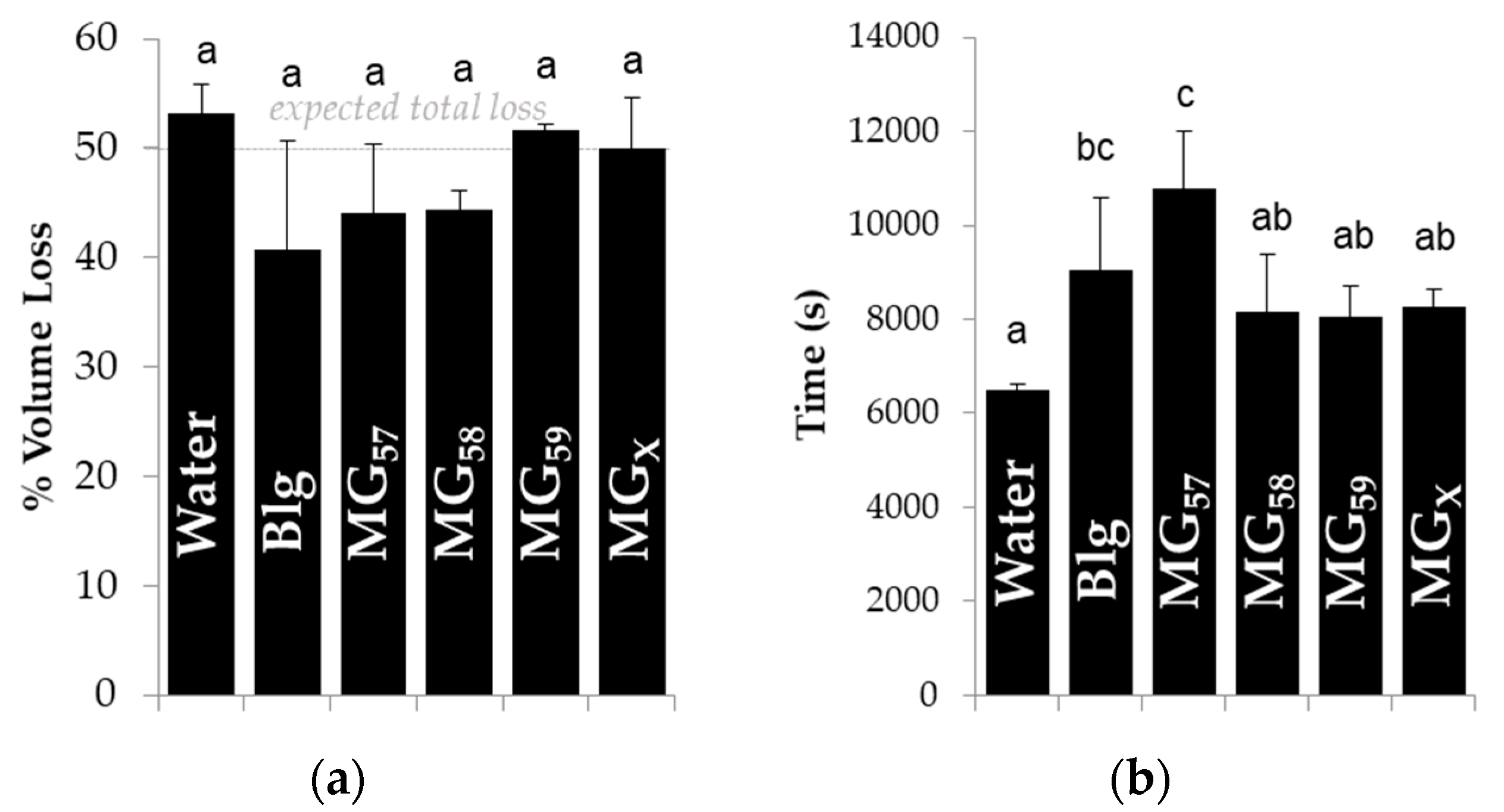
16], which confirmed the adsorption of microgels to the oil-water interface. None of the tested stabilizers significantly reduced the total volume loss (
Figure 7a), which reflected the strong affinity of hexanol for the aqueous phase and the inability of microgels or protein to impede the migration of entrapped volatiles from the droplets, as already described for bulk emulsions after storage in
Figure 3. When compared to bare interfaces (pure water) or other microgels, the larger MG
57 significantly increased the time required for droplets to shrink (
Figure 7b), indicating that these larger microgels at the interface inhibited flavor migration. The smaller MG
58, MG
59, or MG
X did not significantly increase the time for shrinkage, yet unheated Blg did increase the time for shrinkage and was not statistically different from MG
57.
Larger microgels were expected to form a thicker overall interfacial layer, which may have increased the average path length required for the hexanol molecules to diffuse away from the droplet, and also increased the time for complete hexanol release. A lesser effect was expected for the droplets stabilized by MG
58 and MG
59, but any such difference may have been below the detection threshold of this experiment. Strong evidence has linked changes in droplet size during shrinkage to the initial droplet size [
26], but this factor was discounted because the initial volume was controlled in these tests. Droplet shrinkage and associated buckling have also been linked to the ratio of interfacial thickness to initial droplet size [
28], which would again point to the thicker interfacial layer of larger MG
57 as the cause of a slowed shrinkage rate. The long release times observed with Blg-stabilized droplets could be attributed to the greater capacity of individual Blg molecules to adsorb, unfold, and spread at the interface, giving an advantage over the larger microgels at earlier shrinkage times. Future studies require an improved experimental technique that allows measurement of volume losses from droplets with a fully saturated interface.