Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions
Abstract
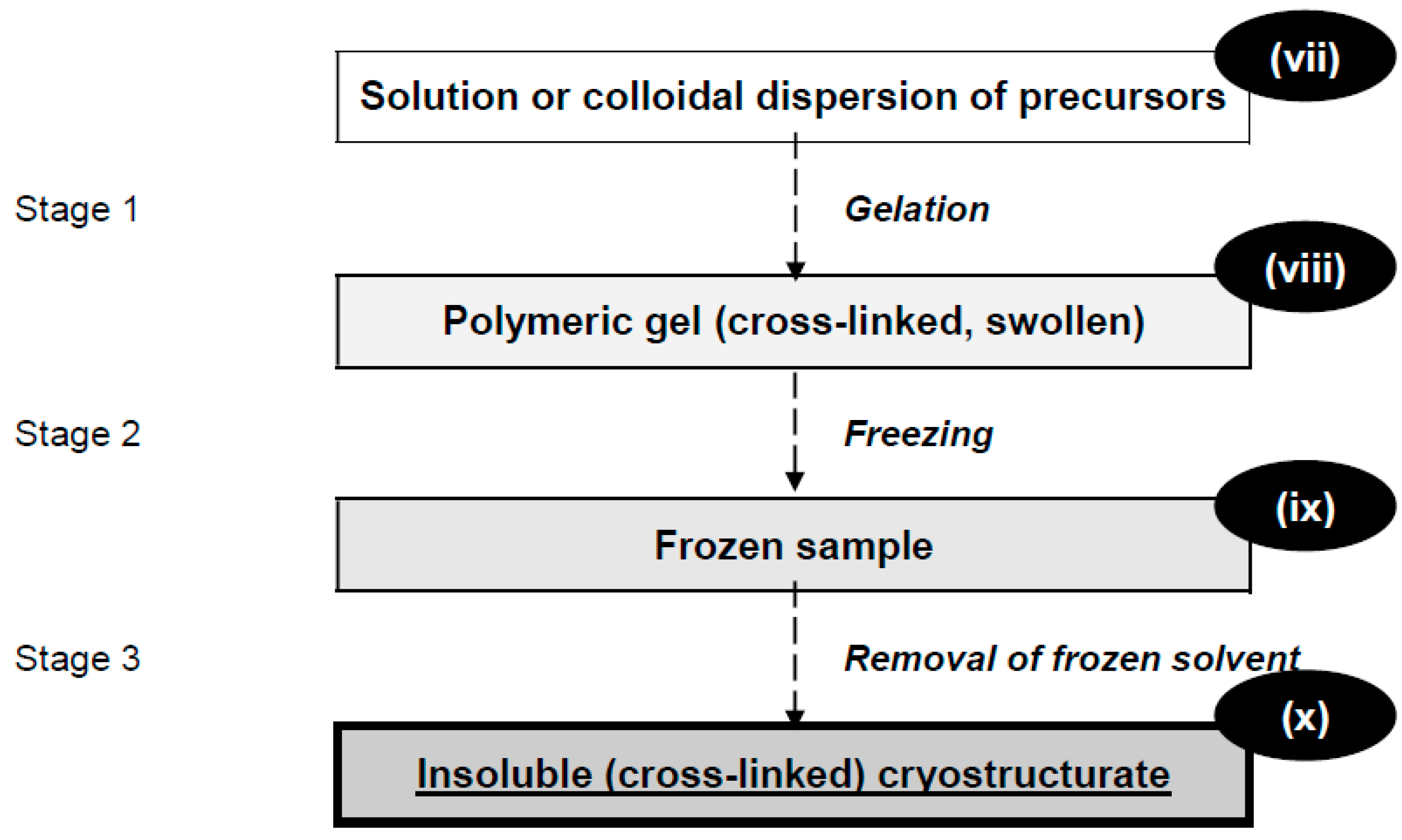
:1. Cryogels and Cryostructurates
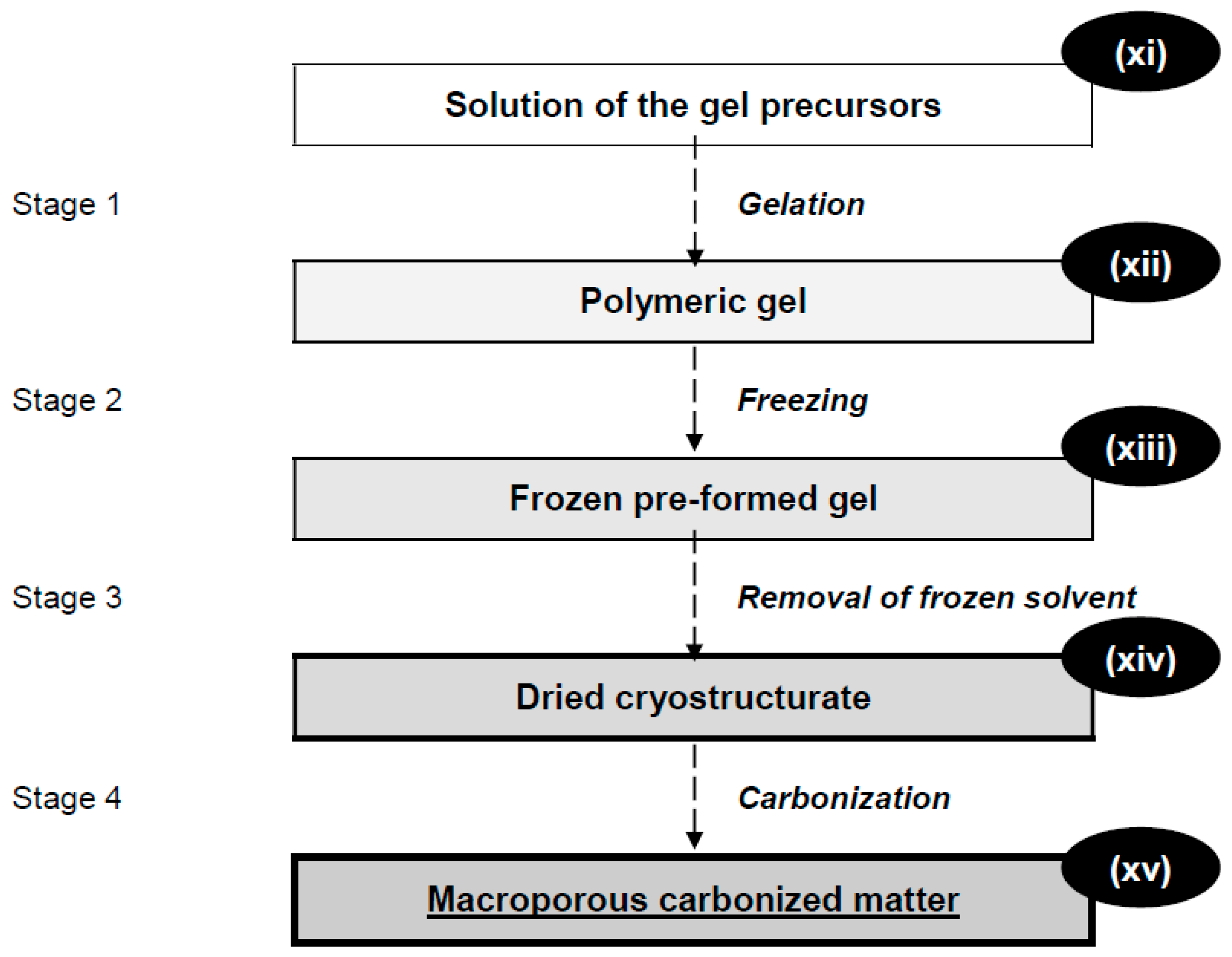
2. Cryotropic Gel-Formation and Cryostructuring
Unfortunately, terminological imprecision which distorts the essence of the considered problems are often met in the literature concerning the wide-pore cryogels. For instance, the term ‘monoliths’ is acquired popularity in the recent time, although the monolith (from the Greek word μονολίτης (monolithis)—a single stone, whole stone lump) can rather be referred to a block of the well-known homophase poly(acrylamide) gel, which is widely used as a medium for the electrophoretic separation of substances, rather than to the supermacroporous heterophase cryogel. The use of the term ‘monolith’ with respect to a soft spongy material, the liquid from which can simply be pressed out under a small mechanical load, seems quite unsubstantiated. If the pressed-out sample, which has lost its primary shape due to this, is again placed in the original solvent, then the cryogel very rapidly swells absorbing the liquid through the system of interconnected capillaries, and recovers the shape completely. Therefore, its elastic properties in the swollen state are mainly determined not by the rigidity of the polymeric framework, as some authors surprisingly believe, but by the swelling pressure and capillary forces, which have been shown for the non-covalent poly(galactomannan) and chemically cross-linked poly(isobutylene) cryogels.
Funding
Conflicts of Interest
References
- Lawrence, R.; Consolacion, F.; Jelen, P. Formation of structured protein foods by freeze texturization. Food Technol. 1986, 40, 77–90. [Google Scholar]
- Lozinsky, V.I.; Vakula, A.V.; Zubov, A.L. Application of poly(vinyl alcohol) cryogels in biotechnology. IV. Literature data overview. Sov. Biotechnol. 1992, 4, 4–17. [Google Scholar]
- Chu, K.C.; Rutt, B.K. Poly(vinyl alcohol) cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Altunina, L.K.; Kuvshinov, V.A.; Dolgikh, S.N. Cryogels—A promising material for underground works in permafrost. In Advances in Geological Storage of Carbon Dioxide; Lombardi, S., Altunina, L.K., Beaubien, S.E., Eds.; NATO Science Series IV; Springer: Heidelberg, Germany, 2006; Volume 65, pp. 103–110. ISBN 978-1-4020-4469-4. [Google Scholar]
- Daniak, M.B.; Galaev, I.Y.; Kumar, A.; Plieva, F.M.; Mattiasson, B. Chromatography of living cells using supermacroporous hydrogels, cryogels. Adv. Biochem. Eng. Biotechnol. 2007, 106, 101–127. [Google Scholar] [CrossRef]
- Lozinsky, V.I. New generation of macroporous and supermacroporous materials of biotechnological interest—Polymeric cryogels. Russ. Chem. Bull. 2008, 57, 1015–1032. [Google Scholar] [CrossRef]
- Hoskins, P.R. Simulation and validation of arterial ultrasound imagining and blood flow. Ultrasound Med. Biol. 2008, 34, 693–717. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwatz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Geidobler, R.; Winter, G. Controlled ice nucleation in the field of freeze-drying: Fundamentals and technology review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Okay, O. (Ed.) Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Springer: Cham, Switzerland, 2014; 330p, ISBN 978-3-319-05845-0. [Google Scholar]
- Liu, C.; Tong, G.; Chen, C.; Tan, Z.; Quan, C.; Zhang, C. Polymeric cryogel: Preparation, properties and biomedical applications. Progr. Chem. 2014, 26, 1190–1201. [Google Scholar] [CrossRef]
- Altunina, L.K.; Fufaeva, M.S.; Filatov, D.A.; Scarovskaya, L.I.; Rozhdestvenskii, E.A.; Gan-Erdene, T. Effect of cryogel on soil properties. Euroasian Soil Sci. 2014, 47, 425–431. [Google Scholar] [CrossRef]
- Vasiliev, N.K.; Pronk, A.D.C.; Shatalina, I.N.; Janssen, F.H.M.E.; Houben, R.W.G. A review on the development of reinforced ice for use as a building material in cold regions. Cold Reg. Sci. Technol. 2015, 115, 56–63. [Google Scholar] [CrossRef]
- Choudhury, S.; Connolly, D.; White, B. Supermacroporous polyHIPE and cryogel monolithic materials as stationary phases in separation science: A review. Anal. Methods 2015, 7, 6967–6982. [Google Scholar] [CrossRef]
- Kumar, A. (Ed.) Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2016; 480p, ISBN 978-1-4822-281-6. [Google Scholar]
- Jiang, S.; Agarwal, S.; Greiner, A. Low-density open cellular sponges as functional materials. Angew. Chem. Int. Ed. 2017, 56, 15520–15538. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Privar, Y.; Malakhova, I.; Pestov, A.; Fedorets, A.; Azarova, Y.; Schwarz, S.; Bratskaya, S. Polyethyleneimine cryogels for metal ions sorption. Chem. Eng. J. 2018, 334, 1392–1398. [Google Scholar] [CrossRef]
- Nadgorny, M.; Collins, J.; Xiao, Z.; Scales, P.J.; Connal, L.A. 3D-printing of dynamic self-healing cryogels with tunable properties. Polym. Chem. 2018, 9, 1684–1692. [Google Scholar] [CrossRef]
- Lozinsky, V.I. A breif history of polymeric cryogels. Adv. Polym. Sci. 2014, 263, 1–48. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–102. [Google Scholar] [CrossRef]
- Kuhn, B.; Peterli, E.; Majer, H. Freezing point depression of gels produced by high polymer networks. J. Polym. Sci. 1955, 16, 539–548. [Google Scholar] [CrossRef]
- Oikawa, H.; Murakami, K. Relationship between swollen network structure of rabber vulcanizates and mechanism of freezing point depression of swelling solvent. J. Macromol. Sci. Phys. 1989, 28, 187–216. [Google Scholar] [CrossRef]
- Henisch, H.K. Crystal Growth in Gels; Dover Publications: New York, NY, USA, 1996; 112p, ISBN 0486689158. [Google Scholar]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Preparation of mesoporous carbon by freeze drying. Carbon 1999, 37, 2049–2055. [Google Scholar] [CrossRef]
- Job, N.; Thery, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.N.; Beguin, F.; Pirard, J.P. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2494. [Google Scholar] [CrossRef]
- Cryogel®. Available online: http://www.gelatin.com/en/cold-solubles/product-range/cryogel (accessed on 9 September 2018).
- Cryogel® Z. Available online: https://www.aerogel.com/products-and-solutions/cryogel-z (accessed on 9 September 2018).
- Miyamoto, K.; Tokita, K.; Miashita, K.; Sakashita, E. Cryogelation in vitro. Int. J. Biol. Macromol. 2001, 28, 183–189. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Korotaeva, G.F.; Rogozhin, S.V. Study of cryostructurization of polymer systems. III. Cryostructurization in organic media. Coll. Polym. Sci. 1984, 262, 617–622. [Google Scholar] [CrossRef]
- Web of Science. Available online: http://apps.webofknowledge.com/CitationReport.do?product=WOS&search_mode=CitationReport&SID=F2nYMNzOJrWECQJdDi5&page=1&cr_pqid=4&viewType=summary&colName=WOS (accessed on 10 September 2018).
- Okay, O.; Lozinsky, V.I. Synthesis, structure-property relationships of cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar] [CrossRef]
- Sergeev, G.B.; Batyuk, V.A. Reactions in frozen multicomponent systems. Russ. Chem. Revs. 1976, 45, 391–408. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Shabatina, T.I.; Lozinsky, V.I. Cryostructuring of polymer systems. 44. Freeze-dried and then chemically cross-linked wide porous cryostructurates based on serum albumin. e-Polymers 2017, 17, 263–274. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kulakova, V.K.; Ivanov, R.V.; Petrenko, A.Y.; Rogulska, O.Y.; Petrenko, Y.A. Cryostructuring of polymer systems. 47. Preparation of wide porous gelatin-based cryostructurates in sterilizing organic media and assessment of the suitability of thus formed matrices as spongy scaffolds for 3D cell culturing. e-Polymers 2018, 18, 175–186. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Ivanov, R.V.; Petrenko, A.Y.; Lozinsky, V.I. Coupling of gelatin to inner surfaces of pore walls in spongy alginate-based scaffolds facilitates the adhesion, growth and differentiation of human bone marrow mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2011, 22, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Brovko, O.S.; Palamarchuk, I.A.; Val’chuk, N.A.; Chukhchin, D.G.; Bogolitsyn, K.G.; Boitsova, T.A. Gels of sodium alginate—Chitosan interpolyelectrolyte complexes. Russ. J. Phys. Chem. Ser. A 2017, 91, 1580–1585. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryotropic gelation of poly(vinyl alcohol) solutions. Russ. Chem. Rev. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Willcox, P.J.; Howie, D.W.; Schmidt-Rohr, K.; Hoagland, D.A.; Gido, S.P.; Pudjijanto, S.; Kleiner, W.; Venkatraman, S. Microstructure of poly(vinyl alcohol) hydrogels produced by freeze/thaw cycling. J. Polym. Sci. B Polym. Phys. 1999, 37, 3438–3454. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Uno, J.; Yamada, C.; Kishi, A.; Hatakeyama, H. Gel-sol transition of poly(vinyl alcohol) hydrogels formed by freezing and thawing. Thermochim. Acta 2005, 431, 144–148. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Shaskol’skii, B.L.; Babushkina, T.A.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 27. Physicochemical properties of poly(vinyl alcohol) cryogels and features of their macroporous morphology. Colloid J. 2007, 69, 747–764. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 28. Physicochemical and morphological properties of poly(vinyl alcohol) cryogels formed via multiple freezing-thawing technique. Colloid J. 2008, 70, 189–198. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Progr. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Tanthapanichakoon, W.; Tamon, H.; Nakagawa, K.; Charinpanitkul, T. Synthesis of porous materials and their microstructural control through ice templating. Eng. J. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-lonked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Girolamo, R.D. Kinetic analysis of cryotropic gelation of poly(vinyl alcohol)/water solutions by small-angle neutron scattering. Adv. Polym. Sci. 2014, 263, 159–197. [Google Scholar] [CrossRef]
- Suzuki, A.; Sasaki, S. Swelling and mechanical properties of physically crosslinked poly(vinyl alcohol) hydrogels. J. Eng. Med. 2015, 229, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, C.R.T.; Norton, I.T. Study of cryostructuration of polymer systems. XVIII. Freeze-thaw-influence on water-solubilized artificial mixtures of amylopectin and amylose. J. Appl. Polym. Sci. 2000, 78, 371–381. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, C.R.T.; Norton, I.T. Study of cryostructuring of polymer systems. XIX. On the nature of intermolecular links in the cryogels of locust bean gum. Polym. Int. 2000, 49, 1434–1443. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. IV. Cryostructurization of the system: Solvent—Vinyl monomer—Divinyl monomer—Initiator of polymerization. Coll. Polym. Sci. 1984, 262, 769–774. [Google Scholar] [CrossRef]
- Belavtseva, E.M.; Titova, E.F.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Study of cryostructurization of polymer systems. V. Electron microscopic studies of cross-linked polyacrylamide cryogels. Coll. Polym. Sci. 1984, 262, 775–779. [Google Scholar] [CrossRef]
- Gusev, D.G.; Lozinsky, V.I.; Bakhmutov, V.I. Study of cryostructurization of polymer systems. X. 1H- and 2H-NMR studies of the formation of crosslinked polyacrylamide cryogels. Eur. Polym. J. 1993, 29, 49–56. [Google Scholar] [CrossRef]
- Plieva, F.M.; Karlsson, M.; Aguilar, M.-R.; Gomez, D.; Mikhalovsky, S.; Galaev, I.Y. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter 2005, 1, 303–309. [Google Scholar] [CrossRef]
- Ivanov, R.V.; Lozinsky, V.I.; Noh, S.K.; Han, S.S.; Lyoo, W.S. Preparation and characterization of polyacrylamide cryogels produced from a high-molecular weight precursor. I. Influence of the reaction temperature and concentration of the cross-linking agent. J. Appl. Polym. Sci. 2007, 106, 1470–1475. [Google Scholar] [CrossRef]
- Ivanov, R.V.; Lozinsky, V.I.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Lyoo, W.S. Preparation and characterization of polyacrylamide cryogels produced from a high-molecular-weight precursor. II. The influence of the molecular weight of the polymeric precursor. J. Appl. Polym. Sci. 2008, 107, 382–390. [Google Scholar] [CrossRef]
- Carvalho, B.M.A.; Da Silva, S.L.; Da Silva, L.H.M.; Minim, V.P.R.; Da Silva, M.C.H.; Carvalho, L.M.; Minim, L.A. Cryogel poly(acrylamide): Synthesis, structure and applications. Sep. Purif. Rev. 2014, 43, 241–262. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kalinina, E.V.; Grinberg, V.Y.; Grinberg, N.V.; Chupov, V.A.; Plate, N.A. Thermoresponsive cryogels based on cross-linked poly(N,N-diethylacrylamide). Polym. Sci. Ser. A 1997, 39, 1300–1305. [Google Scholar]
- Zhang, X.-Z.; Zhuo, R.-X. A novel method to prepare a fast responsive, temperature-sensitive poly(N-isopropylacrylamide) hydrogel. Macromol. Rapid Commun. 1999, 20, 229–231. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Zhuo, R.-X. Preparation of fast responsive, temperature-sensitive poly(N-isopropylacrylamide) hydrogel. Macromol. Chem. Phys. 1999, 200, 2602–2605. [Google Scholar] [CrossRef]
- Srivastava, A.; Jain, E.; Kumar, A. The physical characterization of supermacroporous poly(N-isopropylacrylamide) cryogel: Mechanical strength and swelling/de-swellining kinetics. Mater. Sci. Eng. A 2007, 464, 93–100. [Google Scholar] [CrossRef]
- Strachotova, B.; Strachota, A.; Uchman, M.; Šlouf, M.; Brus, J.; Pleštil, L.; Matĕjka, L. Super porous organic-inorganic poly(N-isopropylacrylamide)-based hydrogel with a very fast temperature response. Polymer 2007, 48, 1471–1482. [Google Scholar] [CrossRef]
- Chalal, M.; Ehrburger-Dolle, F.; Morfin, I.; Vial, J.C.; de Armas, M.R.A.; Roman, J.S.; Bülgen, N.; Pişkin, E.; Ziane, O.; Caslegno, R. Imaging the structure of macroporous hydrogels by two-proton fluorescence microscopy. Macromolecules 2009, 42, 2749–2755. [Google Scholar] [CrossRef]
- Sahiner, N. Super macroporous poly(N-isopropyl acrylamide) cryogel for separation purposes. Polym. Adv. Technol. 2018, 29, 2184–2191. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, A. Thermoresposive poly(N-vinylcaprolactam) cryogels: Synthesis and its biophysical evaluation for tissue engineering applications. J. Mater. Sci. Mater. Med. 2010, 21, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Kaetsu, I. Radiation synthesis of polymeric materials for biomedical and biochemical applications. Adv. Polym. Sci. 1993, 105, 81–97. [Google Scholar]
- Kumakura, M. Preparation method of porous polymer materials by radiation technique and its application. Polym. Adv. Technol. 2001, 12, 415–421. [Google Scholar] [CrossRef]
- Pavlova, L.A.; Kastelyanos-Dominges, O.M. Preparation of cryogels by polymerization of 2-hydroxyethyl methacrylate in the presence of sodium persulfate-N,N,N′,N′-tetramethylethylenediamine initiating system. Russ. J. Appl. Chem. 1996, 69, 1396–1400. [Google Scholar]
- Perçin, I.; Saglar, E.; Yavuz, H.; Aksoz, E.; Denizli, A. Poly(hydroxyethyl methacrylate) based affinity cryogel for plasmid DNA purification. Int. J. Biol. Macromol. 2011, 48, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Elowsson, L.; Kirsebom, H.; Carmignac, V.; Durbeej, M.; Mattiasson, B. Porous protein-based scaffolds prepared through freezing as potential scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Lozinsky, V.I. Cryostructuring of polymeric systems. 40. Proteinaceous wide-pore cryogels generated by the action of denaturant/reductant mixtures on bovine serum albumin in moderately-frozen aqueous media. Soft Matter 2015, 11, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S. Crosslinking strategies for porous gelatin scaffolds. J. Mater. Sci. 2016, 51, 4349–4357. [Google Scholar] [CrossRef]
- Ni, N.; Duquette, D.; Dumont, M.-J. Synthesis and characterization of zein-based cryogels and their potential as diesel fuel absorbent. Eur. Polym. J. 2017, 91, 420–428. [Google Scholar] [CrossRef]
- Wu, Q.; Lindh, V.H.; Johansson, E.; Olsson, R.T.; Hedenqvist, M.S. Freeze-dried wheat gluten biofoams: Scaling up with water welding. Ind. Crops Prod. 2017, 97, 184–190. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Study of cryostructurization of polymer systems. II. The influence of freezing of reacting mass on the properties of products in the preparation of covalently cross-linked gels. Coll. Polym. Sci. 1982, 260, 776–780. [Google Scholar] [CrossRef]
- Kirsebom, H.; Aguilar, M.R.; San Roman, J.; Fernandez, M.; Prieto, M.A.; Bondar, B. Macroporous scaffolds based on chitosan and bioactive molecules. J. Bioact. Compat. Polym. 2007, 22, 621–636. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Bloch, K.O.; Vardi, P.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y. Cryostructuring of polymer systems. XXIX. Preparation and characterization of supermacroporous (spongy) agarose-based cryogels used as three-dimensional scaffolds for culturing insulin-producing cell aggregates. J. Appl. Polym. Sci. 2008, 108, 3046–3062. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous polysaccharide gels. In Macroporous Polymers: Production, Properties and Biotechnological/Biomedical Applications; Mattiasson, B., Kumar, A., Galaev, I.Y., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010; pp. 131–154. ISBN 978-1-4200-8561-0. [Google Scholar]
- Reichelt, S.; Becher, J.; Weisser, J.; Prager, A.; Decker, U.; Möller, S.; Berg, A.; Schnabelrauch, M. Biocompatible polysaccharide-based cryogels. Mater. Sci. Eng. C 2014, 35, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, W.; Chen, Z.; Wu, T. Freeze-thaw-induced gelation of alginates. Carbohydr. Polym. 2016, 148, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.L.; Trzebicka, B.; Kostova, B.; Petrov, P.D. Super-macroporous dextran cryogels via UV-induced crosslinking: Synthesis and characterization. Polym. Int. 2017, 66, 1306–1311. [Google Scholar] [CrossRef]
- Okay, O. DNA Hydrogels: New Functional Soft Materials. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 551–556. [Google Scholar] [CrossRef]
- Karacan, P.; Okay, O. Ethidium bromide binding to DNA cryogels. React. Funct. Polym. 2013, 73, 442–450. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Golovina, T.O.; Gusev, D.G. Study of cryostructuration of polymer systems. XIII. Some characteristic features of the behaviour of macromolecular thiols in frozen aqueous solutions. Polymer 2000, 41, 35–47. [Google Scholar] [CrossRef]
- Ceylan, D.; Okay, O. Macroporous polyisobutylene gels: A novel tough organogel with superfast responsibility. Macromolecules 2007, 40, 8742–8749. [Google Scholar] [CrossRef]
- Oztoprak, Z.; Hekimoglu, T.; Karaturuk, I.; Tunkaboylu, D.C.; Okay, O. Porous rubber cryogels: Effect of the gel preparation temperature. Polym. Bull. 2014, 71, 1983–1999. [Google Scholar] [CrossRef]
- Karakutuk, I.; Okay, O. Macroporous rabber gels as reusable sorbents for the removal of oil from surface waters. React. Funct. Polym. 2010, 70, 585–595. [Google Scholar] [CrossRef]
- Petrov, P.; Utrata-Wesołek, A.; Trzebicka, B.; Tsvetanov, C.B.; Dworak, A.; Anioł, J.; Sieroń, A. Biocompatible cryogels of thermosensitive polyglycidol derivatives with ultra-rapid swelling properties. Eur. Polym. J. 2011, 47, 981–988. [Google Scholar] [CrossRef]
- Konstantinova, N.R.; Lozinsky, V.I. Cryotropic gelation of ovalbumin solutions. Food Hydrocoll. 1997, 11, 113–123. [Google Scholar] [CrossRef]
- Giannouli, P.; Morris, E.R. Cryogelation of xantan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Zeira, A.; Nussinovitch, A. Mechhanical properties of weak locust bean gum (LBG) gels under controlled rapid freeze-thawing. J. Texture Stud. 2004, 34, 561–573. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze-thaw technique. React. Func. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Muthukumar, T.; Aravinthan, A.; Sharmila, J.; Kim, N.S.; Kim, J.-H. Collagen/chitosan porous bone tissue engineering composite scaffold incorporated with Ginseng compound K. Carbohydr. Polym. 2016, 12, 566–574. [Google Scholar] [CrossRef] [PubMed]
- De la Portilla, F.; Pereira, S.; Molero, M.; De Marco, F.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Microstructural, mechanical, and histological evaluation of modified alginate-based scaffolds. J. Biomed. Mater. Res. A 2016, 104, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Vernaya, O.V.; Shabatin, V.P.; Nuzhdina, A.V.; Zvukova, N.D.; Khvatov, D.I.; Semenov, A.M.; Lozinsky, V.I.; Shabatina, T.I.; Mel’nikov, M.Y. Cryochemical synthesis and antibacterial activity of hybrid nanocomposites of dioxydine with Ag and Cu nanoparticles entrapped in biopolymeric cryostructurates. Russ. Chem. Bull. 2017, 66, 2152–2156. [Google Scholar] [CrossRef]
- Cassanelli, M.; Norton, I.; Mills, T. Role of gellan gum microstructure in freeze drying and rehydration mechanisms. Food Hydrocoll. 2018, 75, 51–61. [Google Scholar] [CrossRef]
- Sazhnev, N.A.; Drozdova, M.G.; Rodionov, I.A.; Kil’deeva, N.R.; Balabanova, T.V.; Markvicheva, E.A.; Lozinsky, V.I. Preparation of chitosan cryostructurates with controlled porous morphology and their use as 3D-scaffolds for the cultivation of animal cells. Appl. Biochem. Microbiol. 2018, 54, 459–467. [Google Scholar] [CrossRef]
- Varfolomeev, S.D.; Rainina, E.I.; Lozinsky, V.I. Cryoimmobilized enzymes and cells in organic synthesis. Pure Appl. Chem. 1992, 64, 1193–1196. [Google Scholar] [CrossRef]
- Lazzeri, L. Progress in bioartificial polymeric materials. Trends Polym. Sci. 1996, 4, 249–252. [Google Scholar]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzyme Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Surry, K.J.M.; Austin, H.J.B.; Fenster, A.; Peters, T.M. Poly(vinyl alcohol) cryogel phantoms for use in ultrasound and MR imaging. Phys. Med. Biol. 2004, 49, 5529–5546. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous gels prepared at subzero temperatures as novel materials foe chromatography of particulate-containing fluids and cell culture applications. J. Sep. Sci. 2007, 30, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhardwaj, A. Methods in cell separation for biomedical application: Cryogels as a new tool. Biomed. Mater. 2008, 3, 034008. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Ice-templated materials: Sophisticated structures exhibiting nhanced functionalities obtained after unidirectional freezing and ice-segregation-induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Alves, M.H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Coll. Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; J’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef]
- Mattiasson, B. Cryogels for biotechnological applications. Adv. Polym. Sci. 2014, 263, 245–282. [Google Scholar] [CrossRef]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(vinyl alcohol) cryogels for biomedical application. Adv. Polym. Sci. 2014, 263, 283–321. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, C.; Tomsia, A.P. Freeze casting for assembling bioinspired structural materials. Adv. Mater. 2017, 29, 1703155. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, S. Introduction to macroporous cryogels. Methods Mol. Biol. 2015, 1286, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Yan, X.; Huang, C.; Melerzanov, A.; Du, Y. Biomaterials as carrier and reactor for cell-based regenerative medicine. Protein Cell 2015, 6, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Sedlačik, T.; Okar, O.K.; Studenovská, H.; Kotelnikov, I.; Kučka, J.; Konečná, Z.; Zikmund, T.; Kaiser, J.; Kose, G.T.; Rypáček, F. Chondrogenik potential of macroporous biodegradable cryogels based on synthetic poly(α-amino acids). Soft Matter 2018, 14, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Lyagin, I.V.; Lozinsky, V.I. Enzymatic biocatalysts immobilized on/in the cryogel-type carriers. In Supermacroporous Cryogels: Biomedical and Biotechnological Applications; Kumar, A., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2016; pp. 301–324. ISBN 978-1-4822-281-6. [Google Scholar]
- Andaç, M.; Galaev, I.Y.; Denizli, A. Affinity based and molecularly imprinted cryogels: Applications in biomacromolecule purification. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1021, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Grenho, L.; Fernandez, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, biocompatibility, and osteoconduction evaluation of collagen-nanohydroxyapatite cryogels for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2016, 104, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhat, S.; Chaudhari, B.P.; Gupta, K.C.; Tägil, M.; Zheng, M.H.; Kumar, A.; Lidgren, L. Cell factory-derived bioactive molecules with polymeric cryogel scaffold enhance the repair of subchoronal cartilage defect in rabbits. J. Tissue Eng. Regen. Med. 2017, 11, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.R.; Kandalam, U. Three-dimensional macroporous materials for tissue engineering of craniofacial bone. Br. J. Oral Maxillofac. Surg. 2017, 55, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thaw synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Çorman, M.E. Poly-l-lysin mofified cryogels for efficient bilirubin removal from human plasma. Colloids Surfaces B Biointerfaces 2018, 167, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopolous, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 59. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, S.S.; Bae, S.; Lee, H.; Hwang, N.S. Heparin functionalized injectable cryogel with rapid shape recovery property for neovascularization. Biomacromolecules 2018, 19, 2257–2269. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, P.A.; Singh, A.; Kumar, A. Oxygen-releasing antioxidant cryogel scaffolds with sustained oxygen delivery for tissue engineering applications. ACS Appl. Mater. Interfaces 2018, 10, 18458–18469. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Seab, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Svec, F. Porous polymer monoliths: Amazingly wide variety of techniques enabling their preparation. J. Chromatogr. A 2010, 1217, 902–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquah, C.; Moy, C.K.S.; Danquah, M.K.; Ongkudon, C.M. Developments and characteristics of polymer monoliths for advanced LC bioscreening applications: A review. J. Chromatogr. B 2016, 1015–1016, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rodriguez, E.; Azaria, S.; Pekarek, A.; Hage, D.S. Affinity monolith chromatography: A review of general principles and applications. Electrophoresis 2017, 38, 2837–2850. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. https://doi.org/10.3390/gels4030077
Lozinsky VI. Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels. 2018; 4(3):77. https://doi.org/10.3390/gels4030077
Chicago/Turabian StyleLozinsky, Vladimir I. 2018. "Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions" Gels 4, no. 3: 77. https://doi.org/10.3390/gels4030077



