Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution
Abstract
:1. Introduction



2. Results and Discussion
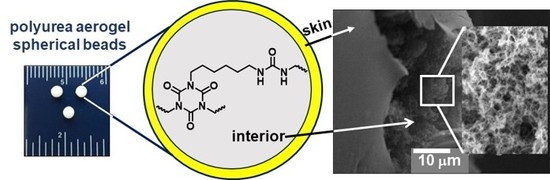
2.1. Synthesis of Spherical PUA Aerogel Beads
2.2. General Material Properties of Spherical PUA Aerogel Beads
2.3. Chemical Characterization of Spherical PUA Aerogel Beads
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Polyurea (PUA) Aerogel Beads
4.2. Synthesis of Polyurea (PUA) Monoliths
- (a)
- for PUA-A monoliths: Desmodur N3300 (9.4 mL, 11 g, 21.8 mmol), ethylenediamine (2.0 mL, 1.8 g, 30.2 mmol) and propylene carbonate (94 mL).
- (b)
- for PUA-B monoliths: Desmodur N3300 (9.4 mL, 11 g, 21.8 mmol), water (1.2 mL, 1.2 g, 66.7 mmol), Et3N (0.4 mL, 0.3 g, 2.9 mmol) and propylene carbonate (94 mL).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer Science & Business Media: New York, NY, USA, 2011; ISBN 978-1-4419-7589-8. [Google Scholar]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click synthesis of monolithic silicon carbide aerogels from polyacrylonitrile-coated 3D silica networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, J.L.; Arachchige, I.U.; Brock, S.L. Porous semiconductor chalcogenide aerogels. Science 2005, 307, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Rewatkar, P.M.; Taghvaee, T.; Saeed, A.M.; Donthula, S.; Mandal, C.; Chandrasekaran, N.; Leventis, T.; Shruthi, T.K.; Sotiriou-Leventis, C.; Leventis, N. Sturdy, monolithic SiC and Si3N4 aerogels from compressed polymer-cross-linked silica xerogel powders. Chem. Mater. 2018, 30, 1635–1647. [Google Scholar] [CrossRef]
- Leventis, N.; Vassilaras, P.; Fabrizio, E.F.; Dass, A. Polymer nanoencapsulated rare earth aerogels: Chemically complex but stoichiometrically similar core-shell superstructures with skeletal properties of pure compounds. J. Mater. Chem. 2007, 17, 1502–1508. [Google Scholar] [CrossRef]
- Schäfer, H.; Milow, B.; Ratke, L. Synthesis of inorganic aerogels via rapid gelation using chloride precursors. RSC Adv. 2013, 3, 15263–15272. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogels in chemical engineering: Strategies toward tailor-made aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the road to biopolymer aerogels—Dealing with the solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Donthula, S.; Mandal, C.; Leventis, T.; Schisler, J.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Shape memory superelastic poly (isocyanurate-urethane) aerogels (PIR-PUR) for deployable panels and biomimetic applications. Chem. Mater. 2017, 29, 4461–4477. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Agnello, M.; McCorkle, L.; Vivod, S.L.; Wilmoth, N. Moisture-resistant polyimide aerogels containing propylene oxide links in the backbone. ACS Appl. Mater. Interfaces 2016, 8, 29073–29079. [Google Scholar] [CrossRef] [PubMed]
- Kanellou, A.; Anyfantis, G.C.; Chriti, D.; Raptopoulos, G.; Pitsikalis, M.; Paraskevopoulou, P. Poly (urethane-norbornene) aerogels via ring opening metathesis polymerization of dendritic urethane-norbornene monomers: Structure-property relationships as a function of an aliphatic versus an aromatic core and the number of peripheral norbornene moieties. Molecules 2018, 23, 1007. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Larimore, Z.J.; Lu, H.; Churu, G.; Mang, J.T. Multifunctional polyurea aerogels from isocyanates and water. A structure−property case study. Chem. Mater. 2010, 22, 6692–6710. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Sotiriou-Leventis, C.; Lu, H. One-pot synthesis of interpenetrating inorganic/organic networks of CuO/resorcinol-formaldehyde aerogels: Nanostructured energetic materials. J. Am. Chem. Soc. 2009, 131, 4576–4577. [Google Scholar] [CrossRef] [PubMed]
- Mohite, D.P.; Larimore, Z.J.; Lu, H.; Mang, J.T.; Sotiriou-Leventis, C.; Leventis, N. Monolithic hierarchical fractal assemblies of silica nanoparticles cross-linked with polynorbornene via ROMP: A structure–property correlation from molecular to bulk through Nano. Chem. Mater. 2012, 24, 3434–3448. [Google Scholar] [CrossRef]
- Leventis, N. Three-dimensional core-shell superstructures: Mechanically strong aerogels. Acc. Chem. Res. 2007, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, P.; Gurikov, P.; Raptopoulos, G.; Chriti, D.; Papastergiou, M.; Kypritidou, Z.; Skounakis, V.; Argyraki, A. Strategies toward catalytic biopolymers: Incorporation of tungsten in alginate aerogels. Polyhedron 2019. [Google Scholar] [CrossRef]
- Chen, H.-B.; Schiraldi, D.A. Flammability of polymer/clay aerogel composites: An overview. Polym. Rev. 2018, 1–24. [Google Scholar] [CrossRef]
- De Vos, R.; Biesmans, G.L.J.G. Organic Aerogels. U.S. Patent 5,484,818, 16 January 1996. [Google Scholar]
- Moon, S.-Y.; Jeon, E.; Bae, J.-S.; Byeon, M.; Park, J.-W. Polyurea networks via organic sol–gel crosslinking polymerization of tetrafunctional amines and diisocyanates and their selective adsorption and filtration of carbon dioxide. Polym. Chem. 2014, 5, 1124. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, X.; Zhu, X.; Kong, X.Z. A facile pathway to polyurea nanofiber fabrication and polymer morphology control in copolymerization of oxydianiline and toluene diisocyanate in acetone. RSC Adv 2015, 5, 7426–7432. [Google Scholar] [CrossRef]
- Leventis, N.; Chidambareswarapattar, C.; Bang, A.; Sotiriou-Leventis, C. Cocoon-in-web-like superhydrophobic aerogels from hydrophilic polyurea and use in environmental remediation. ACS Appl. Mater. Interfaces 2014, 6, 6872–6882. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Chidambareswarapattar, C.; Sadekar, A.; Mohite, D.; Mahadik, S.S.; Larimore, Z.J.; Lu, H.; et al. Isocyanate-derived organic aerogels: Polyureas, polyimides, polyamides. MRS Online Proc. Libr. Arch. 2011, 1306. [Google Scholar] [CrossRef]
- Jones, F.N.; Nichols, M.E.; Pappas, S.P. Organic Coatings: Science and Technology; John Wiley & Sons: New York, NY, USA, 2017; ISBN 978-1-119-33715-7. [Google Scholar]









| PUA Sample | Bulk Density ρb (g cm–3) | Skeletal Density ρs (g cm–3) | Porosity a II (% v/v) | BET Surf. Area b σ (m2 g–1) | VTotalc (V1.7–300nm) d (cm3 g−1) | av. pore diam. e (4VTotal/σ) (nm) | Particle Size f r (nm) |
|---|---|---|---|---|---|---|---|
| PUA-A beads | 0.166 ± 0.001 | 1.246 ± 0.005 | 87 | 197 | 5.2 (1.2) | 24 (105) | 12 |
| PUA-B monolith-propylene carbonate | 0.110 ± 0.001 | 1.236 ± 0.006 | 91 | 73 | 8.3 (0.2) | 13 (455) | 33 |
| PUA-B monolith-acetone g | 0.126 ± 0.001 | 1.265 ± 0.006 | 90 | 169 | 7.1 (1.1) | 42 (169) | 14 |
| PUA Sample | Start (°C) | End (°C) | 1st Peak (°C) | 2nd Peak (°C) | 3rd Peak (°C) | 4th Peak (°C) |
|---|---|---|---|---|---|---|
| PUA-A beads | 179.7 | 510.5 | 246.7 | 321.3 | 381.0 | 463.0 |
| PUA-A monolith | 179.9 | 510.5 | 238.4 | 320.4 | 382.3 | 465.8 |
| PUA-B monolith | 217.1 | 508.6 | - | - | 381.3 | 464.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chriti, D.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P. Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution. Gels 2018, 4, 66. https://doi.org/10.3390/gels4030066
Chriti D, Raptopoulos G, Papastergiou M, Paraskevopoulou P. Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution. Gels. 2018; 4(3):66. https://doi.org/10.3390/gels4030066
Chicago/Turabian StyleChriti, Despoina, Grigorios Raptopoulos, Maria Papastergiou, and Patrina Paraskevopoulou. 2018. "Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution" Gels 4, no. 3: 66. https://doi.org/10.3390/gels4030066