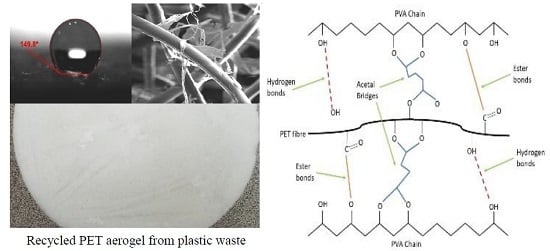
Advanced Recycled Polyethylene Terephthalate Aerogels from Plastic Waste for Acoustic and Thermal Insulation Applications
Abstract
:1. Introduction
2. Results and Discussion
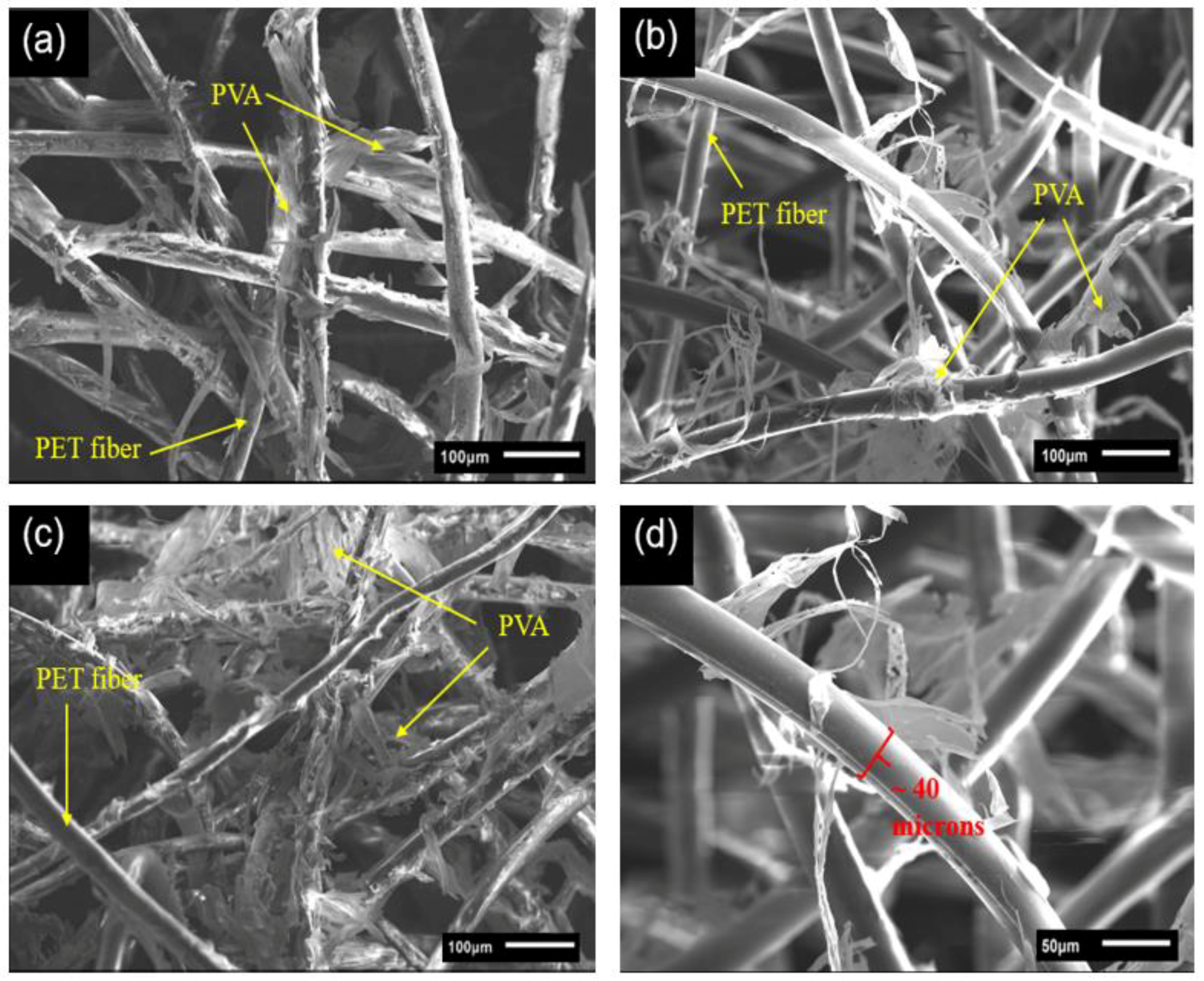
2.1. Morphologies and Structures of the rPET Aerogels
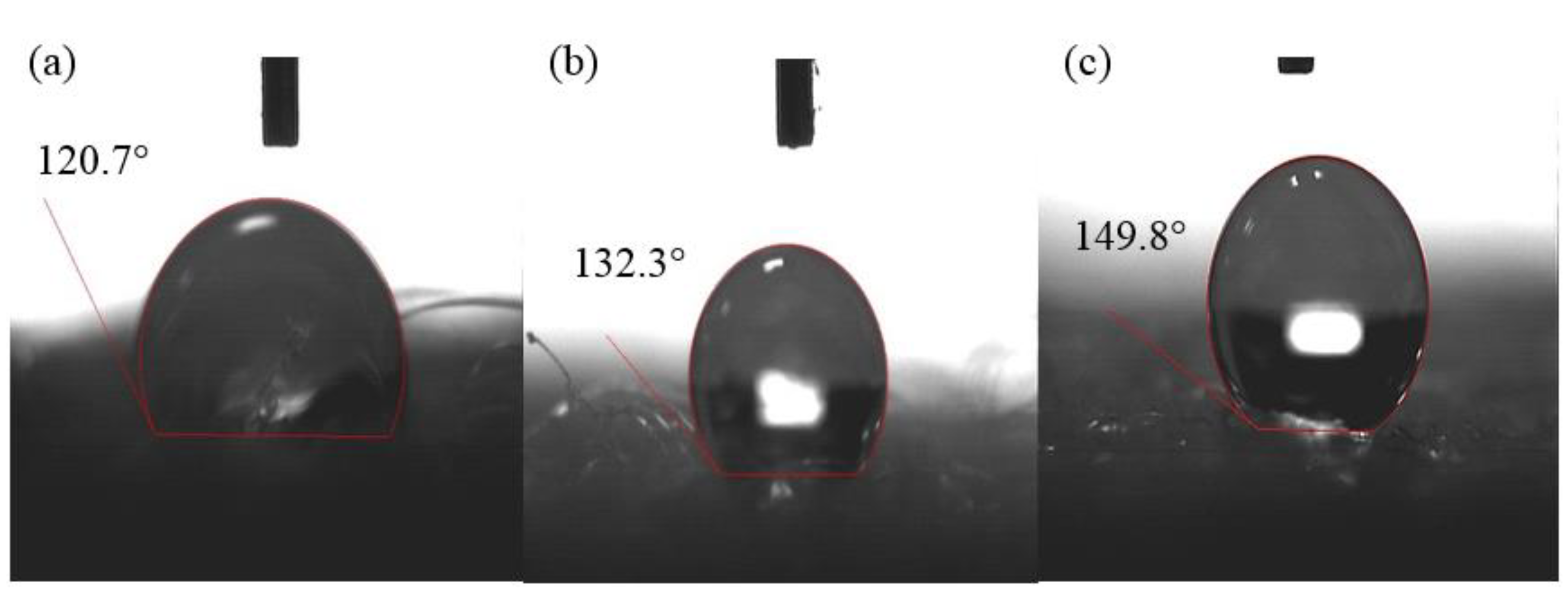
2.2. Super-Hydrophobicity Properties of the rPET Aerogels
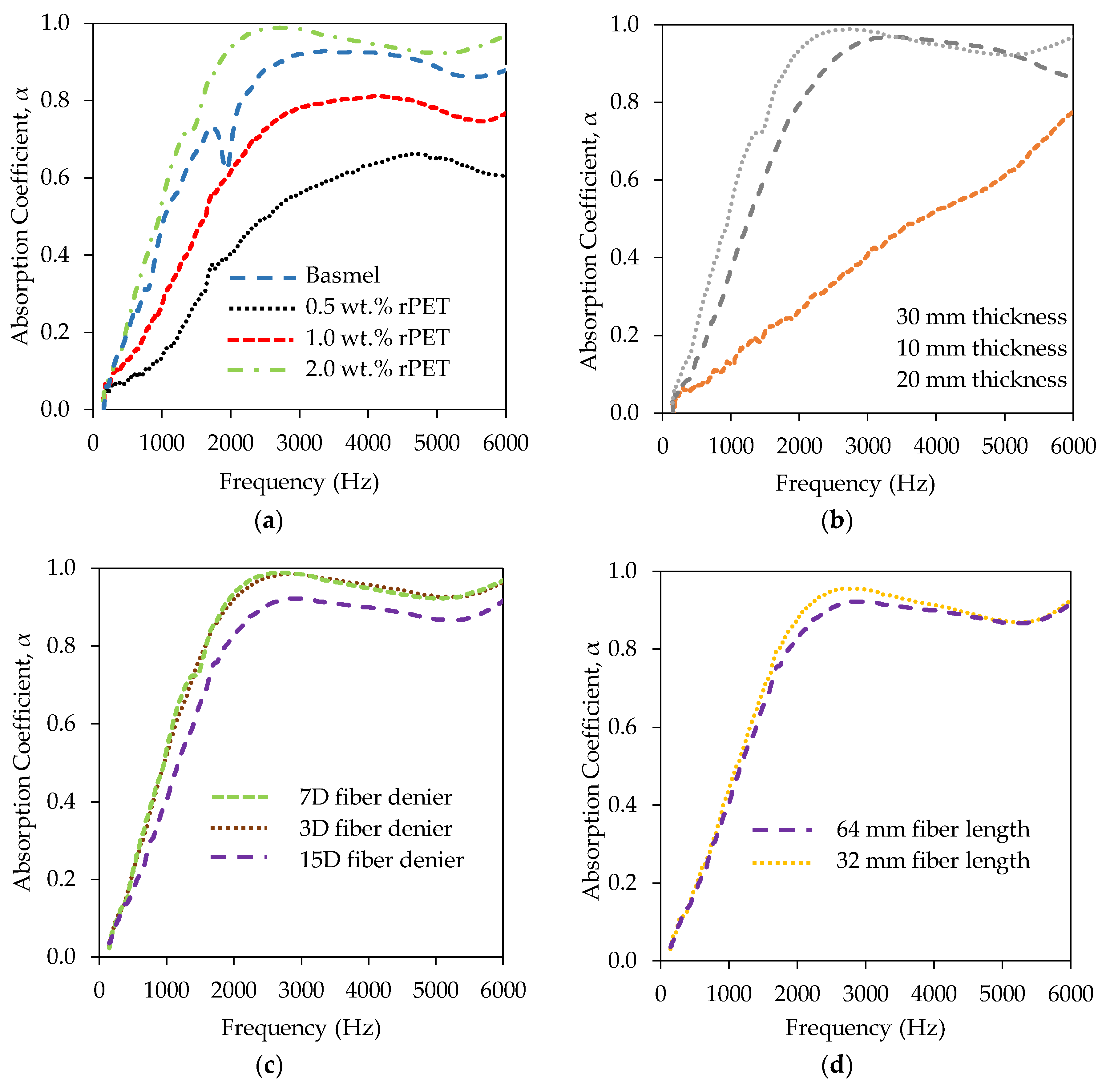
2.3. Acoustic Insulation Properties of the rPET Aerogels
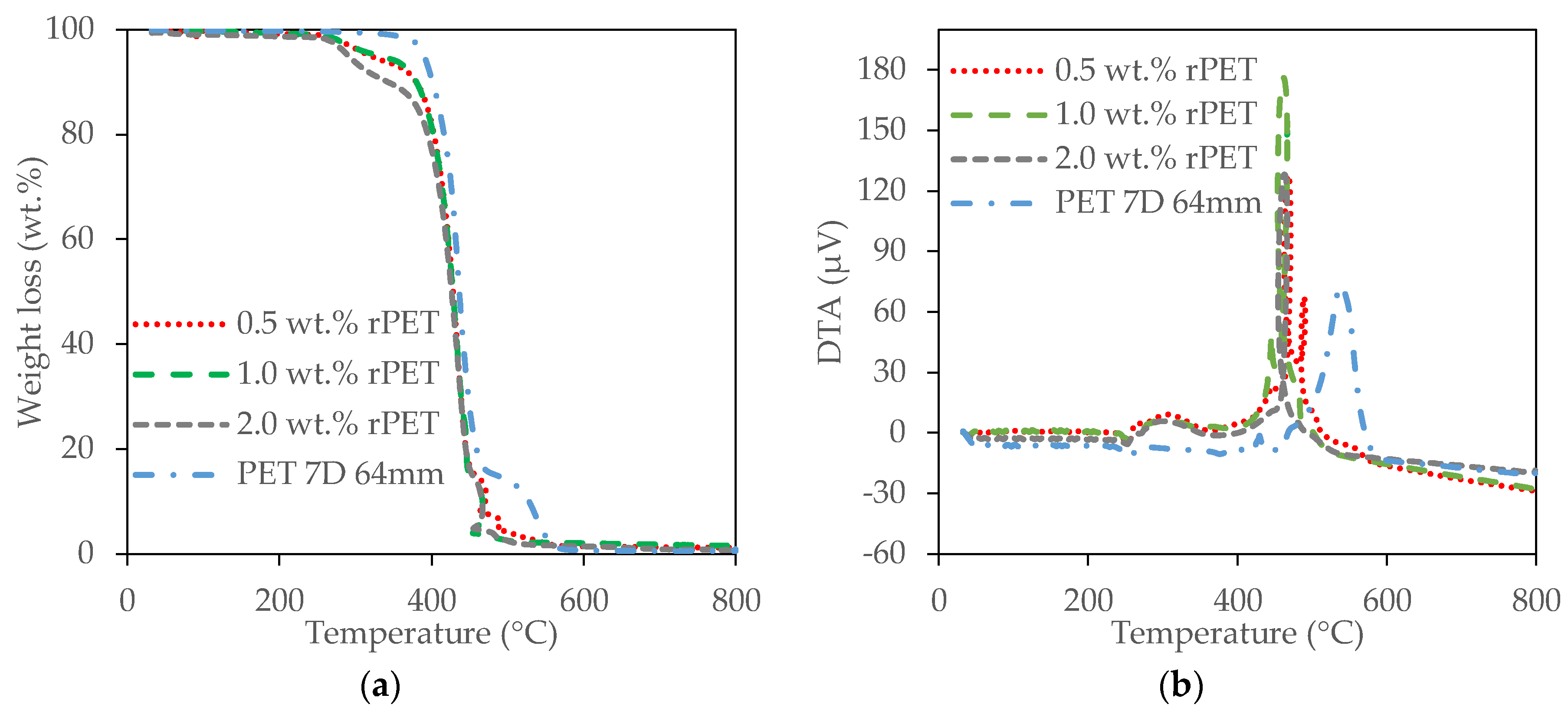
2.4. Thermal Properties of the rPET Aerogels
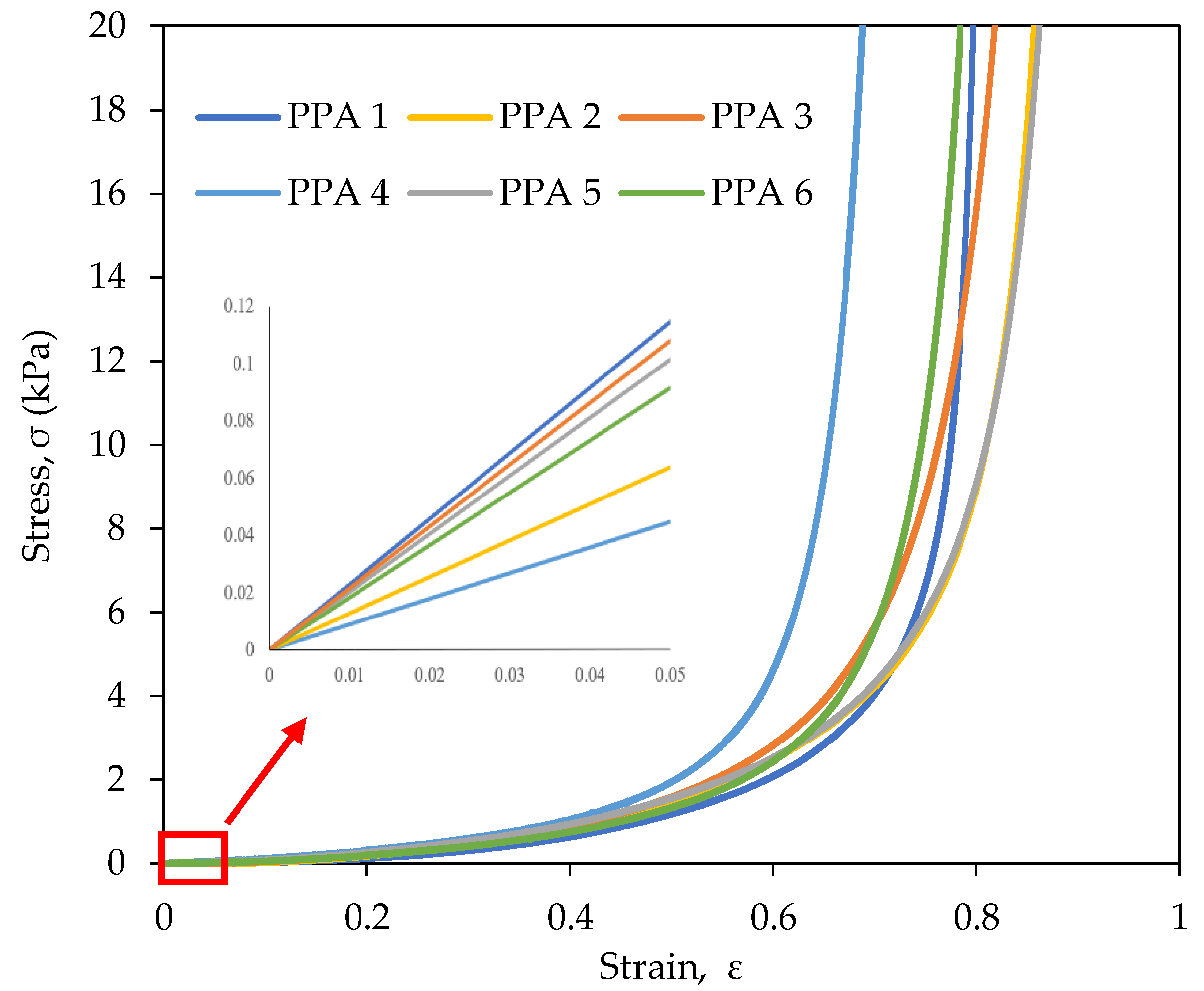
2.5. Mechanical Properties of the rPET Aerogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of the rPET Aerogels
4.3. Characterizations of the rPET Aerogels
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Du, E.I. Pont de Nemours and Co. Br. Patent 784,248, 9 October 1957. [Google Scholar]
- Achilias, D.; Bikiaris, D. Synthesis and biodegradation of three poly(alkylene succinate)s: Mathematical modelling of the esterification reaction and the enzymatic hydrolysis. Environ. Toxicol. 2006, 1, 309–318. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philosophical transactions. Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, L.; Stassen, F.; Sheppard, R.; Gilman, T. The New Plastics Economy: Rethinking the Future of Plastics; World Economic Forum: Cologne, Switzerland, 2016. [Google Scholar]
- Nema, P.; Nema, S.; Roy, P. An overview of global climate changing in current scenario and mitigation action. Renew. Sustain. Energy Rev. 2012, 16, 2329–2336. [Google Scholar] [CrossRef]
- ASHRAE. Chapter 25, Heat, Air, and Moisture Control in Building Assemblies—Fundamentals. In Handbook of Fundamentals; American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.: Atlanta, GA, USA, 2009. [Google Scholar]
- ASHRAE. Chapter 23, Thermal and Moisture control in Insulated Assemblies-Fundamentals. In Handbook of Fundamentals; American Society of Heating, Refrigerating and Air Conditioning Engineers, Inc.: Atlanta, GA, USA, 2001. [Google Scholar]
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Seidman, M.D.; Standring, R.T. Noise and quality of life. Int. J. Environ. Res. Public Health 2010, 7, 3730–3738. [Google Scholar] [CrossRef] [PubMed]
- Goines, L.; Hagler, L. Noise pollution: A modern plague. South. Med. J. 2007, 100, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Tzivian, L.; Winkler, A.; Dlugaj, M.; Schikowski, T.; Vossoughi, M.; Fuks, K.; Weinmayr, G.; Hoffmann, B. Effect of long-term outdoor air pollution and noise on cognitive and psychological functions in adults. Int. J. Environ. Res. Public Health 2015, 218, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tzivian, L.; Jokisch, M.; Winkler, A.; Weimar, C.; Hennig, F.; Sugiri, D.; Soppa, V.J.; Dragano, N.; Erbel, R.; Jöckel, K.-H. Associations of long-term exposure to air pollution and road traffic noise with cognitive function—An analysis of effect measure modification. Environ. Int. 2017, 103, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A review of unconventional sustainable building insulation materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Moretti, E.; Belloni, E.; Agosti, F. Innovative mineral fiber insulation panels for buildings: Thermal and acoustic characterization. Appl. Energy 2016, 169, 421–432. [Google Scholar] [CrossRef]
- Pedroso, M.; de Brito, J.; Silvestre, J. Characterization of eco-efficient acoustic insulation materials (traditional and innovative). Constr. Build. Mater. 2017, 140, 221–228. [Google Scholar] [CrossRef]
- Oh, K.W.; Kim, D.K.; Kim, S.H. Ultra-porous flexible pet/aerogel blanket for sound absorption and thermal insulation. Fibers Polym. 2009, 10, 731–737. [Google Scholar] [CrossRef]
- Cotana, F.; Pisello, A.L.; Moretti, E.; Buratti, C. Multipurpose characterization of glazing systems with silica aerogel: In-field experimental analysis of thermal-energy, lighting and acoustic performance. Build. Environ. 2014, 81, 92–102. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Schmidt, M.; Schwertfeger, F. Applications for silica aerogel products. J. Non Cryst. Solids 1998, 225, 364–368. [Google Scholar] [CrossRef]
- Feng, J.; Le, D.; Nguyen, S.T.; Tan Chin Nien, V.; Jewell, D.; Duong, H.M. Silica-cellulose hybrid aerogels for thermal and acoustic insulation applications. Coll. Surf. A 2016, 506, 298–305. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.; Le, A.T.T.; Hoang, N.; Tan, V.B.C.; Duong, H.M. Cellulose aerogel from paper waste for crude oil spill cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Berardi, U.; Nosrati, R.H. Long-term thermal conductivity of aerogel-enhanced insulating materials under different laboratory aging conditions. Energy 2018, 147, 1188–1202. [Google Scholar] [CrossRef]
- Nosrati, R.H.; Berardi, U. Hygrothermal characteristics of aerogel-enhanced insulating materials under different humidity and temperature conditions. Energy Build. 2018, 158, 698–711. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Ng, S.K.; Wong, J.P.W.; Tan, V.B.C.; Duong, H.M. Advanced thermal insulation and absorption properties of recycled cellulose aerogels. Coll. Surf. A 2014, 445, 128–134. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose-silica nanocomposite aerogels by in situ formation of silica in cellulose gel. Angew. Chem. 2012, 124, 2118–2121. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Hunt, A.J.; Jantzen, C.A.; Cao, W. Aerogel—A high performance insulating material at 0.1 bar. In Insulation Materials: Testing and Applications; ASTM International: West Conshohocken, PA, USA, 1991. [Google Scholar]
- Kamiuto, K.; Miyamoto, T.; Saitoh, S. Thermal characteristics of a solar tank with aerogel surface insulation. Appl. Energy 1999, 62, 113–123. [Google Scholar] [CrossRef]
- Gibiat, V.; Lefeuvre, O.; Woignier, T.; Pelous, J.; Phalippou, J. Acoustic properties and potential applications of silica aerogels. J. Non Cryst. Solids 1995, 186, 244–255. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Sipyagina, N.A.; Malkova, A.N.; Baranchikov, A.E.; Erov, K.E.; Petukhov, D.I.; Ivanov, V.K. Methyltrimethoxysilane-based elastic aerogels: Effects of the supercritical medium on structure-sensitive properties. Russ. J. Inorg. Chem. 2015, 60, 488–492. [Google Scholar] [CrossRef]
- Cervin, N.T.; Aulin, C.; Larsson, P.T.; Wågberg, L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2012, 19, 401–410. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. Ftir spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Lv, M.; Shi, Y.; Cao, D. Super hydrophilic poly(ethylene terephthalate) (pet)/poly(vinyl alcohol) (pva) composite fibrous mats with improved mechanical properties prepared via electrospinning process. Colloid. Surf. A 2013, 436, 417–424. [Google Scholar] [CrossRef]
- Gupta, D.; Chaudhary, H.; Gupta, C. Sericin based bioactive coating for polyester fabric. Indian J. Fibre Text. Res. 2015, 40, 70–80. [Google Scholar]
- Zheng, Q.; Javadi, A.; Sabo, R.; Cai, Z.; Gong, S. Polyvinyl alcohol (pva)–cellulose nanofibril (cnf)–multiwalled carbon nanotube (mwcnt) hybrid organic aerogels with superior mechanical properties. RSC Adv. 2013, 3, 20816–20823. [Google Scholar] [CrossRef]
- Rao, A.V.; Haranath, D. Effect of methyltrimethoxysilane as a synthesis component on the hydrophobicity and some physical properties of silica aerogels. Microporous Mesoporous Mater. 1999, 30, 267–273. [Google Scholar]
- Venkateswara Rao, A.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non Cryst. Solids 2003, 330, 187–195. [Google Scholar] [CrossRef]
- Sachithanadam, M.; Joshi, S.C. Novel fabrication methods. In Silica Aerogel Composites; Springer: Singapore, 2016; p. 139. [Google Scholar]
- Lee, Y.; Joo, C. Sound absorption properties of recycled polyester fibrous assembly absorbers. AUTEX Res. J. 2003, 3, 78–84. [Google Scholar]
- Sun, F.; Banks-Lee, P.; Peng, H. Sound absorption in an anisotropic periodically layered fluid-saturated porous medium. Appl. Acoust. 1993, 39, 65–76. [Google Scholar] [CrossRef]
- Amares, S.; Sujatmika, E.; Hong, T.; Durairaj, R.; Hamid, H. A Review: Characteristics of Noise Absorption Material; IOP Publishing: Bristol, UK, 2017; p. 908. [Google Scholar]
- Seddeq, H.S. Factors influencing acoustic performance of sound absorptive materials. Aust. J. Basic Appl. Sci. 2009, 3, 4610–4617. [Google Scholar]
- Hassan, N.N.M.; Rus, A.Z.M. Influences of thickness and fabric for sound absorption of biopolymer composite. Appl. Mech. Mater. 2013, 393, 102–107. [Google Scholar] [CrossRef]
- Shahani, F.; Soltani, P.; Zarrebini, M. The analysis of acoustic characteristics and sound absorption coefficient of needle punched nonwoven fabrics. J. Eng. Fabr. Fibers 2014, 9, 84–92. [Google Scholar]
- Koizumi, T.; Tsujiuchi, N.; Adachi, A. The development of sound absorbing materials using natural bamboo fibers. WIT Trans. Built Environ. 2002, 59. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Pekala, R.W. Thermal properties of organic and inorganic aerogels. J. Mater. Res. 2011, 9, 731–738. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chang, T.F.; Lu, S.Y.; Chang, Y.C. Preparation of monolithic silica aerogel of low thermal conductivity by ambient pressure drying. J. Am. Ceram. Soc. 2007, 90, 2003–2007. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 2005; Volume 1. [Google Scholar]
- Simmler, H.; Brunner, S.; Heinemann, U.; Schwab, H.; Kumaran, K.; Mukhopadhyaya, P.; Quénard, D.; Sallée, H.; Noller, K.; Kükükpinar-Niarchos, E. Vacuum Insulation Panels. Study on Vip-Components and Panels for Service Life Prediction of Vip in Building Applications (Subtask A); IEA/ECBCS Annex: Paris, France, 2005; p. 39. [Google Scholar]
- Kiran, M.; Nandanwar, A.; Naidu, M.V.; Rajulu, K.C.V. Effect of density on thermal conductivity of bamboo mat board. Int. J. Agric. For. 2012, 2, 257–261. [Google Scholar]
- Cabeza, L.F.; Castell, A.; Medrano, M.; Martorell, I.; Pérez, G.; Fernández, I. Experimental study on the performance of insulation materials in mediterranean construction. Energy Build. 2010, 42, 630–636. [Google Scholar] [CrossRef]
- Yang, H.; Xu, S.; Jiang, L.; Dan, Y. Thermal decomposition behavior of poly (vinyl alcohol) with different hydroxyl content. J. Macromol. Sci. B 2012, 51, 464–480. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Oh, C.-S.; Kim, Y.-H.; Ahn, Y.-S.; Yeo, J.-G. Methyltrimethoxysilane based monolithic silica aerogels via ambient pressure drying. Microporous Mesoporous Mater. 2007, 100, 350–355. [Google Scholar] [CrossRef]
- Vegt, A.K.; Govaert, L.E. Vraagstukken Polymeren: Behorende Bij Polymeren, Van Keten Tot Kunststof; VSSD: Delft, The Netherlands, 2005. [Google Scholar]
- Mathis, N. Transient thermal conductivity measurements: Comparison of destructive and nondestructive techniques. High Temp. High Press. 2000, 32, 321–327. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Impedance and Absorption of Acoustical Materials Using a Tube, Two Microphones and a Digital Frequency Analysis System; ASTM E1050-12; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
| Samples | Fiber Length (mm) | Fiber Denier | Fiber Concentration (wt.%) | Thickness (mm) | Density, ρa (g/cm3) | Porosity, φ (%) |
|---|---|---|---|---|---|---|
| PPA 1 | 64 | 7D | 0.5 | 30 | 0.007 ± 0.001 | 99.47 ± 0.05 |
| PPA 2 | 64 | 7D | 1.0 | 30 | 0.014 ± 0.001 | 99.00 ± 0.05 |
| PPA 3 | 64 | 7D | 2.0 | 30 | 0.026 ± 0.001 | 98.14 ± 0.05 |
| PPA 4 | 64 | 3D | 2.0 | 30 | 0.024 ± 0.001 | 98.26 ± 0.05 |
| PPA 5 | 64 | 15D | 2.0 | 30 | 0.024 ± 0.001 | 98.26 ± 0.05 |
| PPA 6 | 32 | 15D | 2.0 | 30 | 0.024 ± 0.001 | 98.26 ± 0.05 |
| PPA 7 | 64 | 7D | 2.0 | 10 | 0.024 ± 0.001 | 98.23 ± 0.05 |
| PPA 8 | 64 | 7D | 2.0 | 20 | 0.024 ± 0.001 | 98.25 ± 0.05 |
| Samples | Fiber Concentration (wt.%) | Density (g/cm3) | Thermal Conductivity, Kavg (W/m.K) | Compressive Young’s Modulus, E (kPa) |
|---|---|---|---|---|
| PPA 1 | 0.5 | 0.007 ± 0.001 | 0.035 ± 0.001 | 1.16 ± 0.05 |
| PPA 2 | 1.0 | 0.014 ± 0.001 | 0.036 ± 0.001 | 1.76 ± 0.08 |
| PPA 3 | 2.0 | 0.026 ± 0.001 | 0.037 ± 0.001 | 2.76 ± 0.16 |
| PPA 4 | 2.0 | 0.024 ± 0.001 | 0.037 ± 0.001 | 2.87 ± 0.17 |
| PPA 5 | 2.0 | 0.024 ± 0.001 | 0.038 ± 0.001 | 2.61 ± 0.17 |
| PPA 6 | 2.0 | 0.024 ± 0.001 | 0.037 ± 0.001 | 2.45 ± 0.21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, H.W.; Le, D.K.; Ng, G.N.; Zhang, X.; Phan-Thien, N.; Kureemun, U.; Duong, H.M. Advanced Recycled Polyethylene Terephthalate Aerogels from Plastic Waste for Acoustic and Thermal Insulation Applications. Gels 2018, 4, 43. https://doi.org/10.3390/gels4020043
Koh HW, Le DK, Ng GN, Zhang X, Phan-Thien N, Kureemun U, Duong HM. Advanced Recycled Polyethylene Terephthalate Aerogels from Plastic Waste for Acoustic and Thermal Insulation Applications. Gels. 2018; 4(2):43. https://doi.org/10.3390/gels4020043
Chicago/Turabian StyleKoh, Hong Wei, Duyen K. Le, Gek Nian Ng, Xiwen Zhang, Nhan Phan-Thien, Umeyr Kureemun, and Hai M. Duong. 2018. "Advanced Recycled Polyethylene Terephthalate Aerogels from Plastic Waste for Acoustic and Thermal Insulation Applications" Gels 4, no. 2: 43. https://doi.org/10.3390/gels4020043