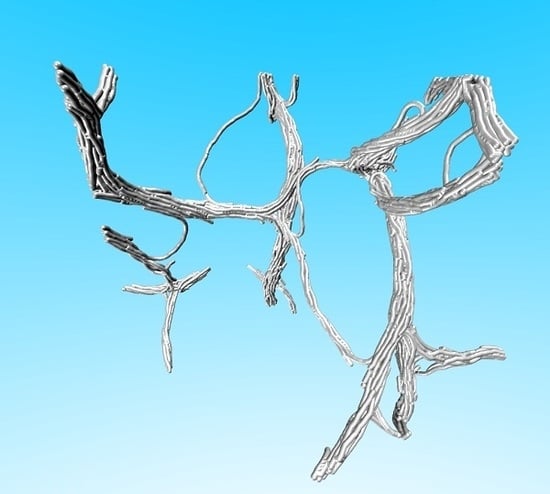
Fiber Network Formation in Semi-Flexible Polymer Solutions: An Exploratory Computational Study
Abstract
:1. Introduction
2. Results and Discussion
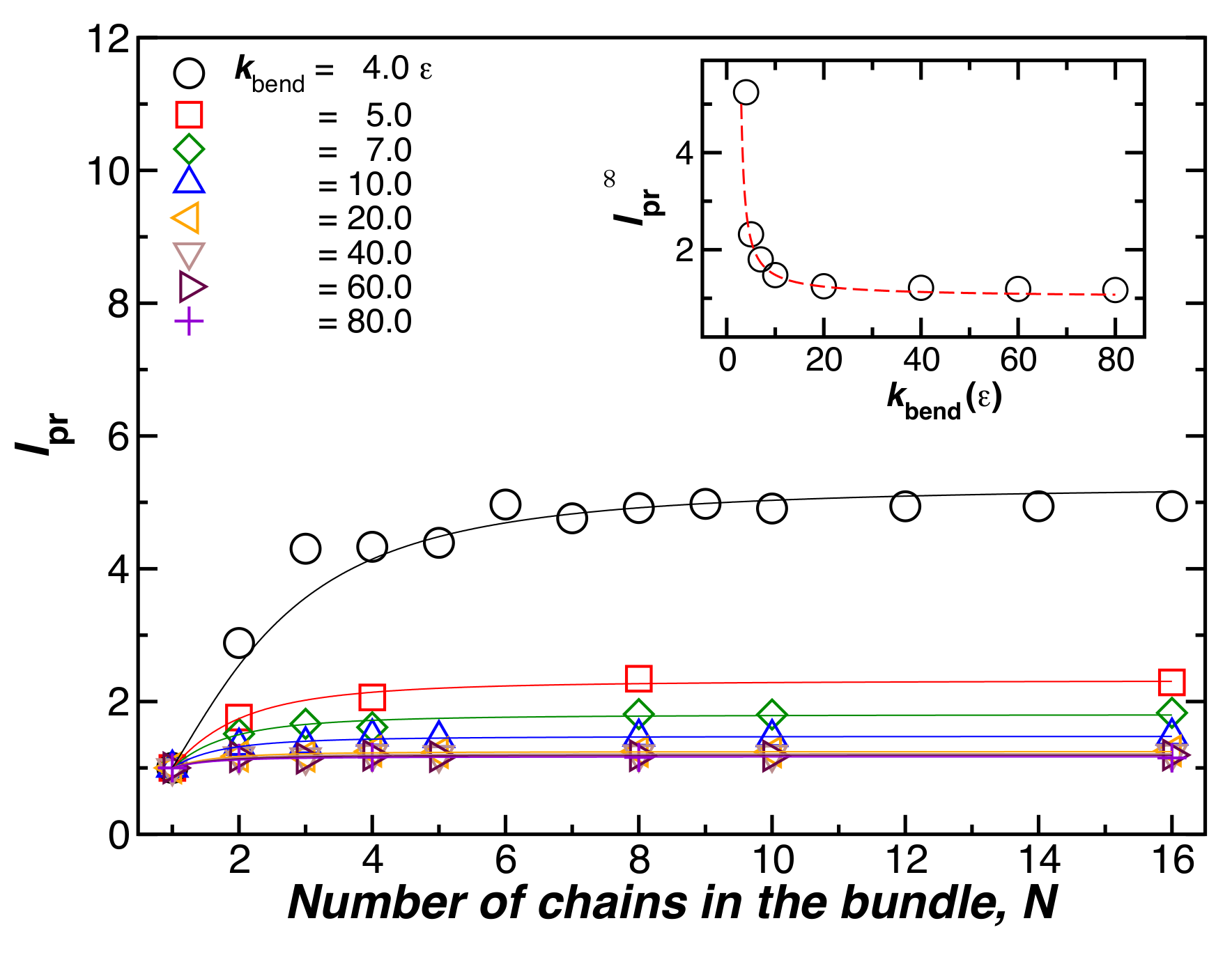
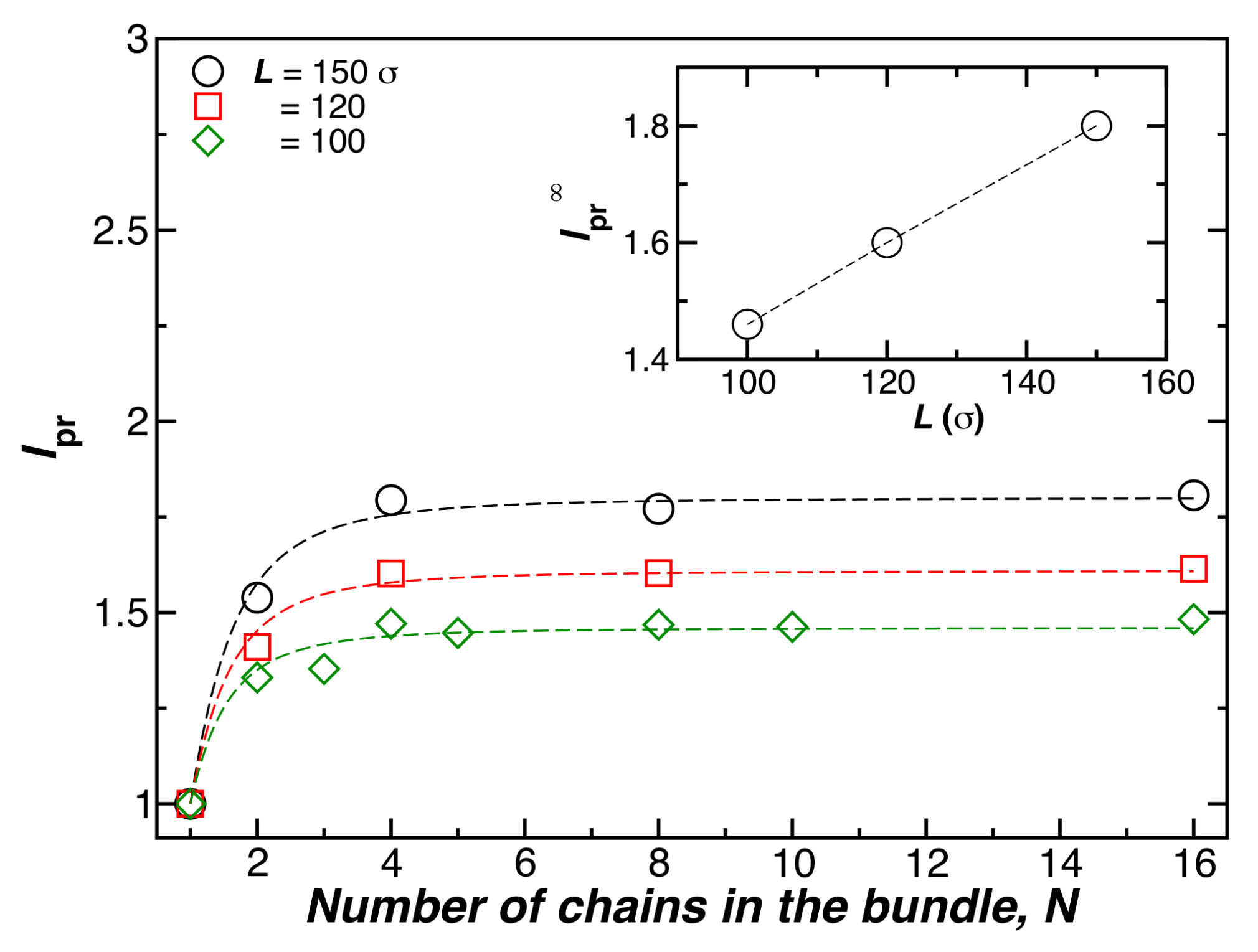
2.1. Calculation of Bundle Characteristic Rigidity
2.1.1. Effect of Individual Chain Stiffness on Fiber Bundle Stiffness
2.1.2. Effect of the Chain Stiffness on the Bundle Rigidity
2.1.3. Effect of the Chain Length L on Fiber Bundle Rigidity
2.1.4. Effect of the Chain Attractive Interaction on the Bundle Rigidity
2.2. Melting of Model Fiber Bundles
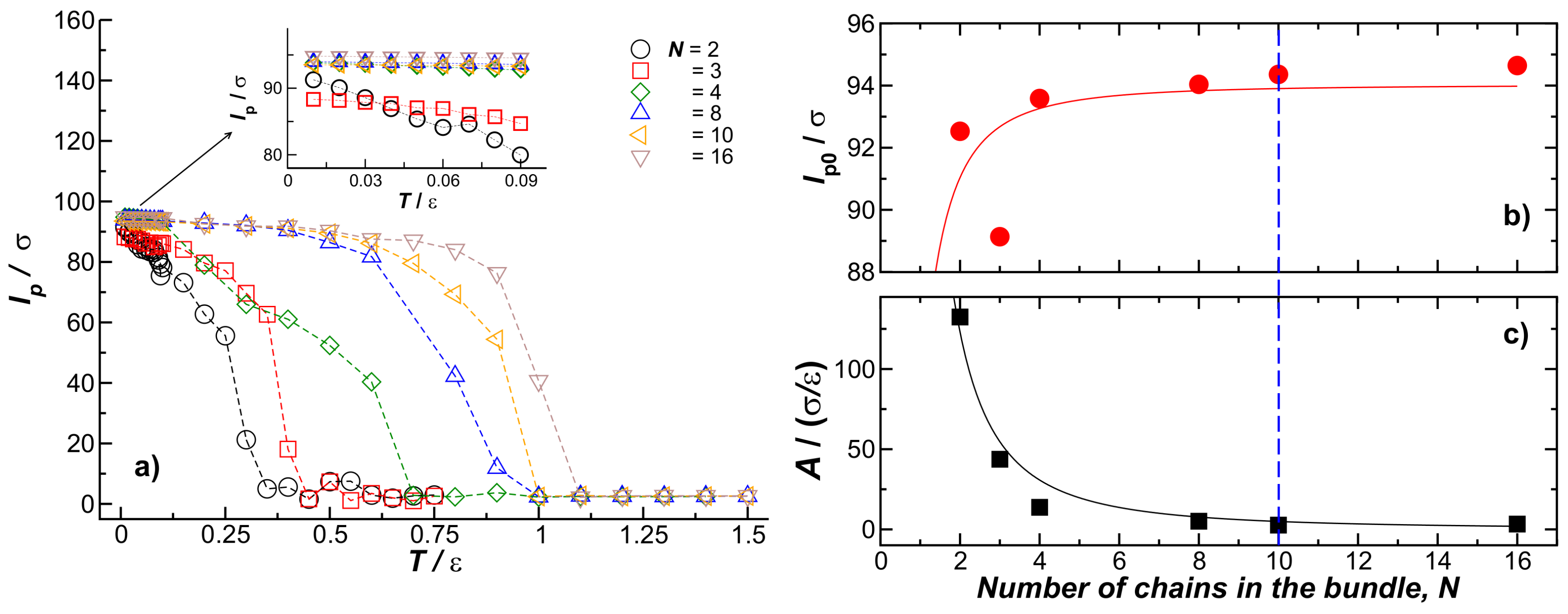
2.3. Temperature Dependence of Bundle Persistence Length
3. Conclusions
4. Materials and Methods
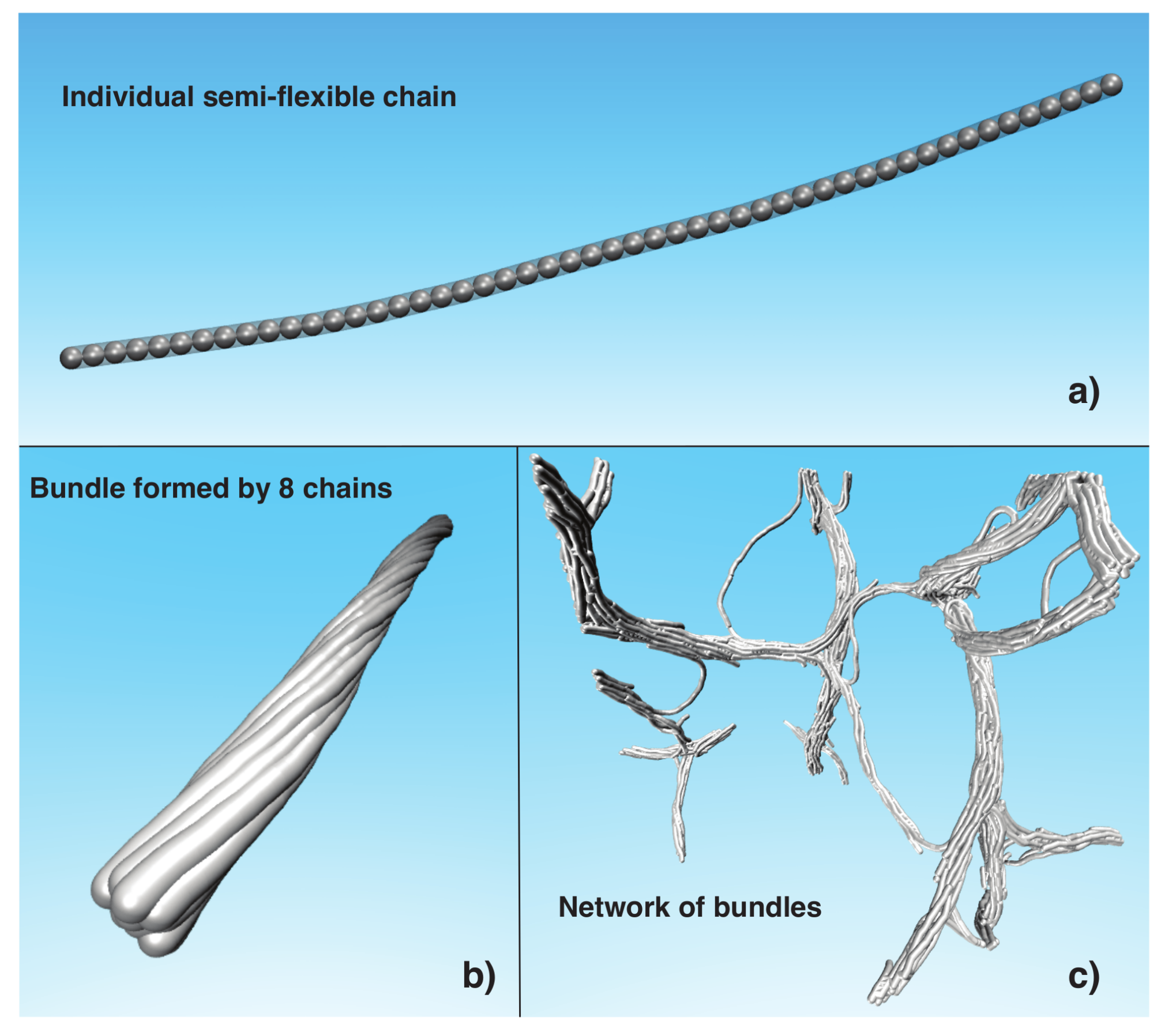
4.1. Computational Model
4.2. Exploratory Study of the Formation of Fiber Networks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cai, C.; Lin, J.; Chen, T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Raeburn, J.; Zamith Cardoso, A.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143–5156. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, K.; Miller, W.G. Network structure in gels of rod-like polypeptides. Nature 1981, 289, 813. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J. Controll. Release 1987, 6, 297–305. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Douglas, J.F. The conundrum of gel formation by molecular nanofibers, wormlike micelles, and filamentous proteins: Gelation without cross-links? Soft Matter 2012, 8, 8539–8546. [Google Scholar] [CrossRef]
- Wilder, E.A.; Hall, C.K.; Khan, S.A.; Spontak, R.J. Effects of Composition and Matrix Polarity on Network Development in Organogels of Poly(ethylene glycol) and Dibenzylidene Sorbitol. Langmuir 2003, 19, 6004–6013. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Kundu, S. Probing Gelation and Rheological Behavior of a Self-Assembled Molecular Gel. Langmuir 2017, 33, 7769–7779. [Google Scholar] [CrossRef] [PubMed]
- Lott, J.R.; McAllister, J.W.; Wasbrough, M.; Sammler, R.L.; Bates, F.S.; Lodge, T.P. Fibrillar Structure in Aqueous Methylcellulose Solutions and Gels. Macromolecules 2013, 46, 9760–9771. [Google Scholar] [CrossRef]
- Lott, J.R.; McAllister, J.W.; Arvidson, S.A.; Bates, F.S.; Lodge, T.P. Fibrillar Structure of Methylcellulose Hydrogels. Biomacromolecules 2013, 14, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nieuwendaal, R.; Dimitriadis, E.K.; Hammouda, B.; Douglas, J.F.; Xu, B.; Horkay, F. Supramolecular Self-Assembly of a Model Hydrogelator: Characterization of Fiber Formation and Morphology. Gels 2016, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Cifra, P. Differences and limits in estimates of persistence length for semi-flexible macromolecules. Polymer 2004, 45, 5995–6002. [Google Scholar] [CrossRef]
- Vargas-Lara, F.; Starr, F.W.; Douglas, J.F. Molecular rigidity and enthalpy-entropy compensation in DNA melting. Soft Matter 2017, 13, 8309–8330. [Google Scholar] [CrossRef] [PubMed]
- Schiffels, D.; Liedl, T.; Fygenson, D.K. Nanoscale Structure and Microscale Stiffness of DNA Nanotubes. ACS Nano 2013, 7, 6700–6710. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.; Li, J.; Nelson, P.C.; Perkins, T.T.; Betterton, M. Elasticity of Short DNA Molecules: Theory and Experiment for Contour Lengths of 0.6–7 µm. Biophys. J. 2007, 93, 4360–4373. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Lattanzi, G.; Jonáš, A.; Frey, T.S.E.; Florin, E.L. Thermal fluctuations of grafted microtubules provide evidence of a length-dependent persistence length. Proc. Natl. Acad. Sci. USA 2006, 103, 10248–10253. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Fitzpatrick, A.W.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of Intermolecular Forces in Defining Material Properties of Protein Nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Corradini, M.G.; Weiss, R.G.; Raghavan, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.S.; Briehl, R.W.; Ferrone, F.A.; Josephs, R. Twisted Protein Aggregates and Disease: The Stability of Sickle Hemoglobin Fibers. Phys. Rev. Lett. 2003, 90, 128103. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.M.A.E.; Semmrich, C.; Ramos, L.; Bausch, A.R. Helical twist controls the thickness of F-actin bundles. Proc. Natl. Acad. Sci. USA 2008, 105, 8819–8822. [Google Scholar] [CrossRef] [PubMed]
- Bruss, I.R.; Grason, G.M. Topological defects, surface geometry and cohesive energy of twisted filament bundles. Soft Matter 2013, 9, 8327–8345. [Google Scholar] [CrossRef]
- Ramos Sasselli, I.; Halling, P.J.; Ulijn, R.V.; Tuttle, T. Supramolecular Fibers in Gels Can Be at Thermodynamic Equilibrium: A Simple Packing Model Reveals Preferential Fibril Formation versus Crystallization. ACS Nano 2016, 10, 2661–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, M.; Witten, T.A. Geometrical frustration yields fibre formation in self-assembly. Nat. Phys. 2017, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Gittes, F.; Mickey, B.; Nettleton, J.; Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 1993, 120, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Wachtman, J.B.; Tefft, W.E.; Lam, D.G.; Apstein, C.S. Exponential Temperature Dependence of Young’s Modulus for Several Oxides. Phys. Rev. 1961, 122, 1754–1759. [Google Scholar] [CrossRef]
- Anderson, O.L. Derivation of Wachtman’s Equation for the Temperature Dependence of Elastic Moduli of Oxide Compounds. Phys. Rev. 1966, 144, 553–557. [Google Scholar] [CrossRef]
- Claessens, M.M.A.E.; Bathe, M.; Frey, E.; Bausch, A.R. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat. Mater. 2006, 5, 748. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, L.; Agarwal, G. The influence of discoidin domain receptor 2 on the persistence length of collagen type I fibers. Biomaterials 2010, 31, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.; Grest, G.S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation. J. Chem. Phys. 1990, 92, 5057–5086. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Lara, F.; Douglas, J.F. Fiber Network Formation in Semi-Flexible Polymer Solutions: An Exploratory Computational Study. Gels 2018, 4, 27. https://doi.org/10.3390/gels4020027
Vargas-Lara F, Douglas JF. Fiber Network Formation in Semi-Flexible Polymer Solutions: An Exploratory Computational Study. Gels. 2018; 4(2):27. https://doi.org/10.3390/gels4020027
Chicago/Turabian StyleVargas-Lara, Fernando, and Jack F. Douglas. 2018. "Fiber Network Formation in Semi-Flexible Polymer Solutions: An Exploratory Computational Study" Gels 4, no. 2: 27. https://doi.org/10.3390/gels4020027