Improved Concrete Materials with Hydrogel-Based Internal Curing Agents
Abstract
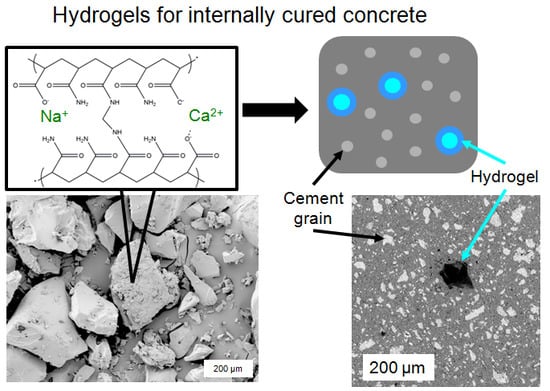
:1. Introduction
2. Results
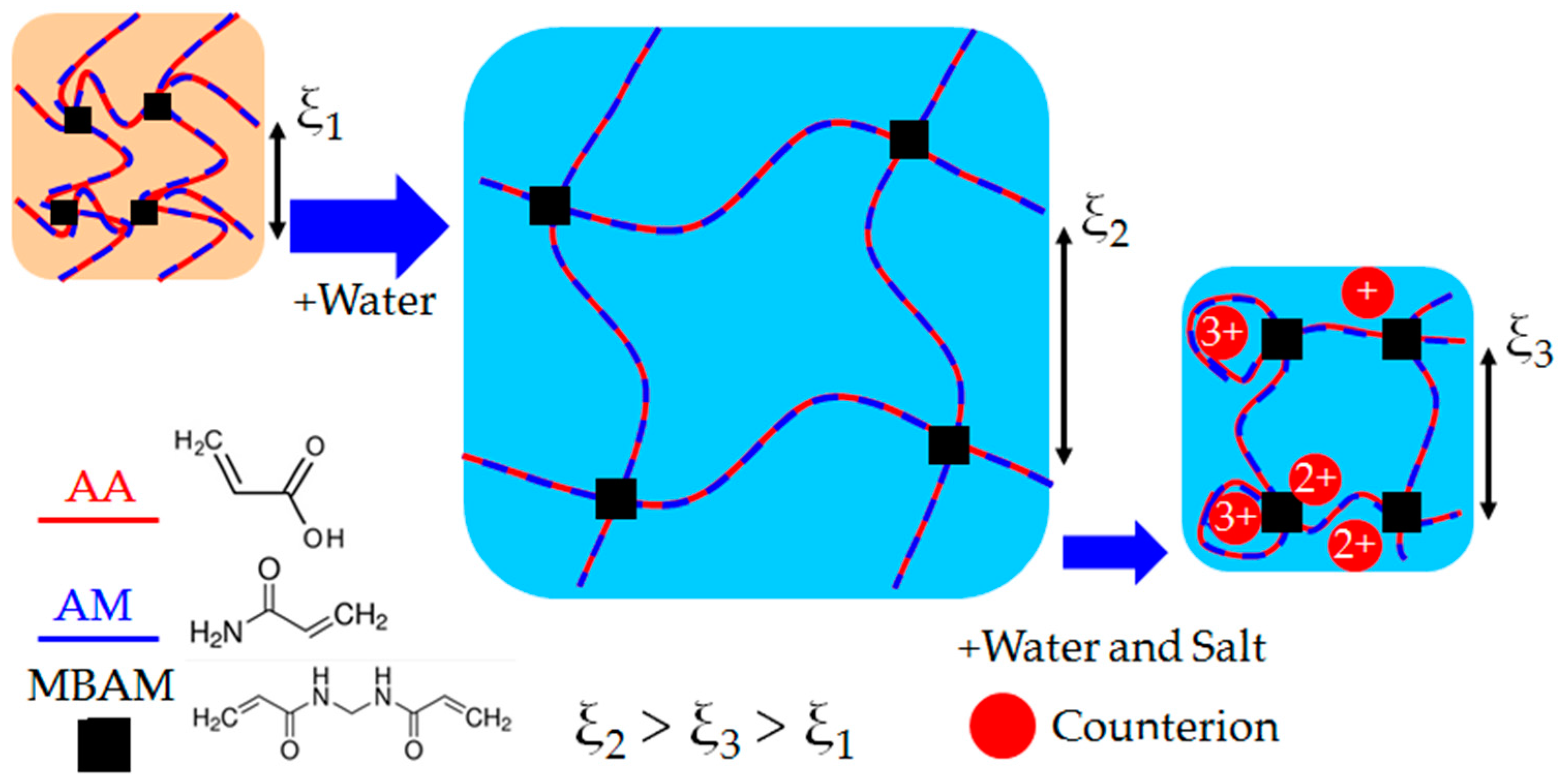
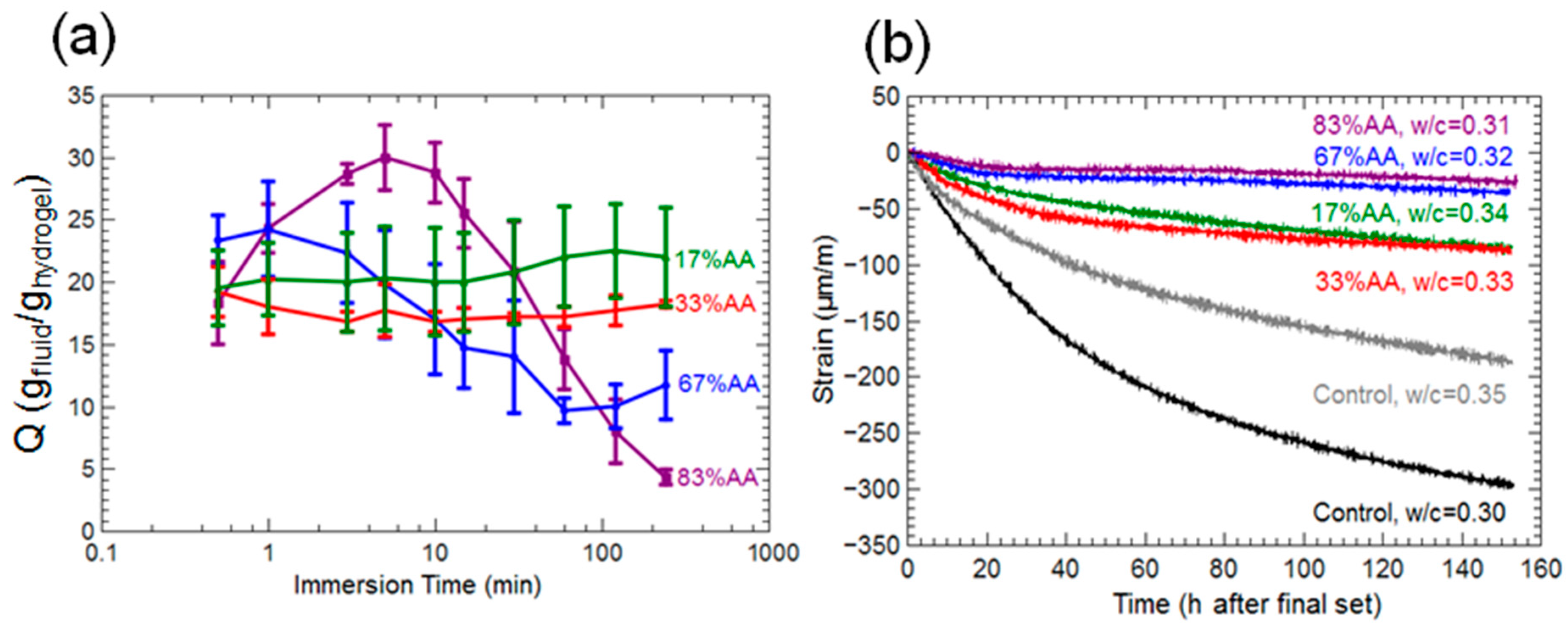
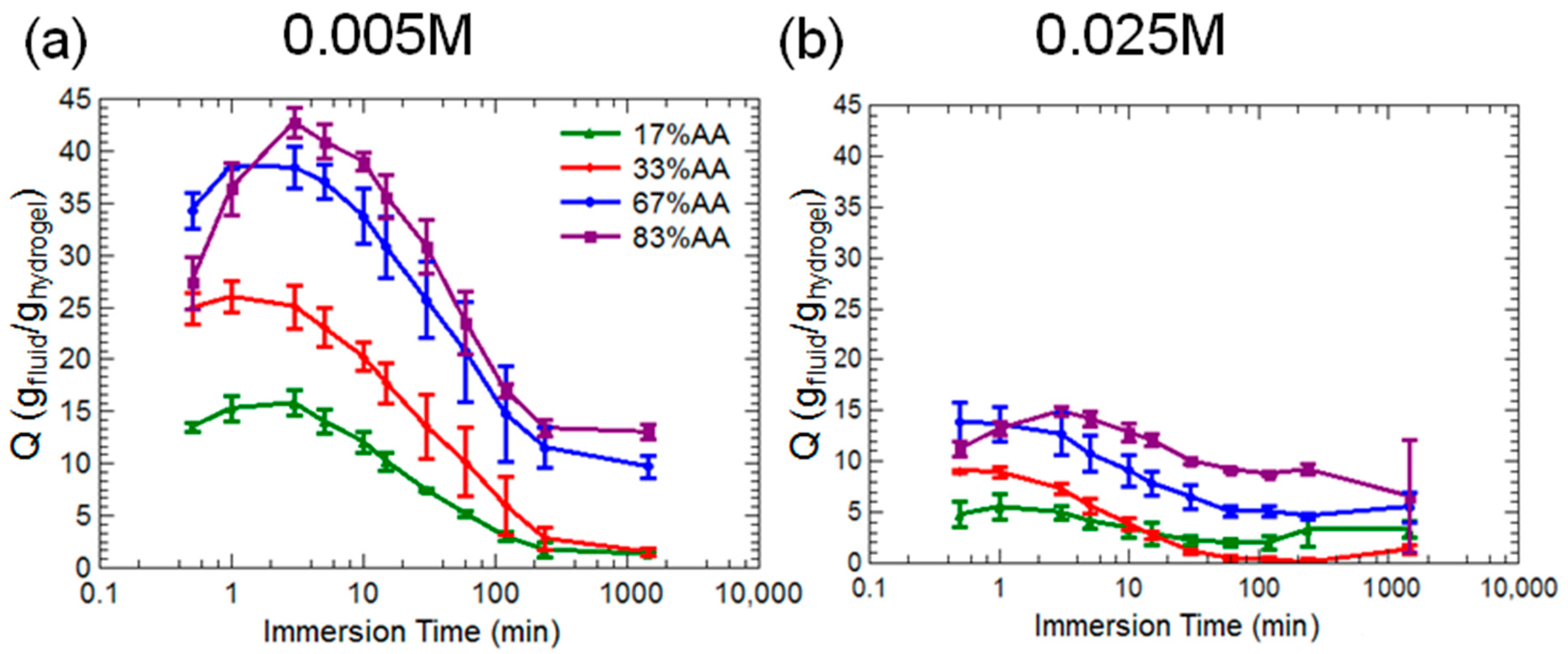
2.1. Hydrogel Swelling in Salt Solutions
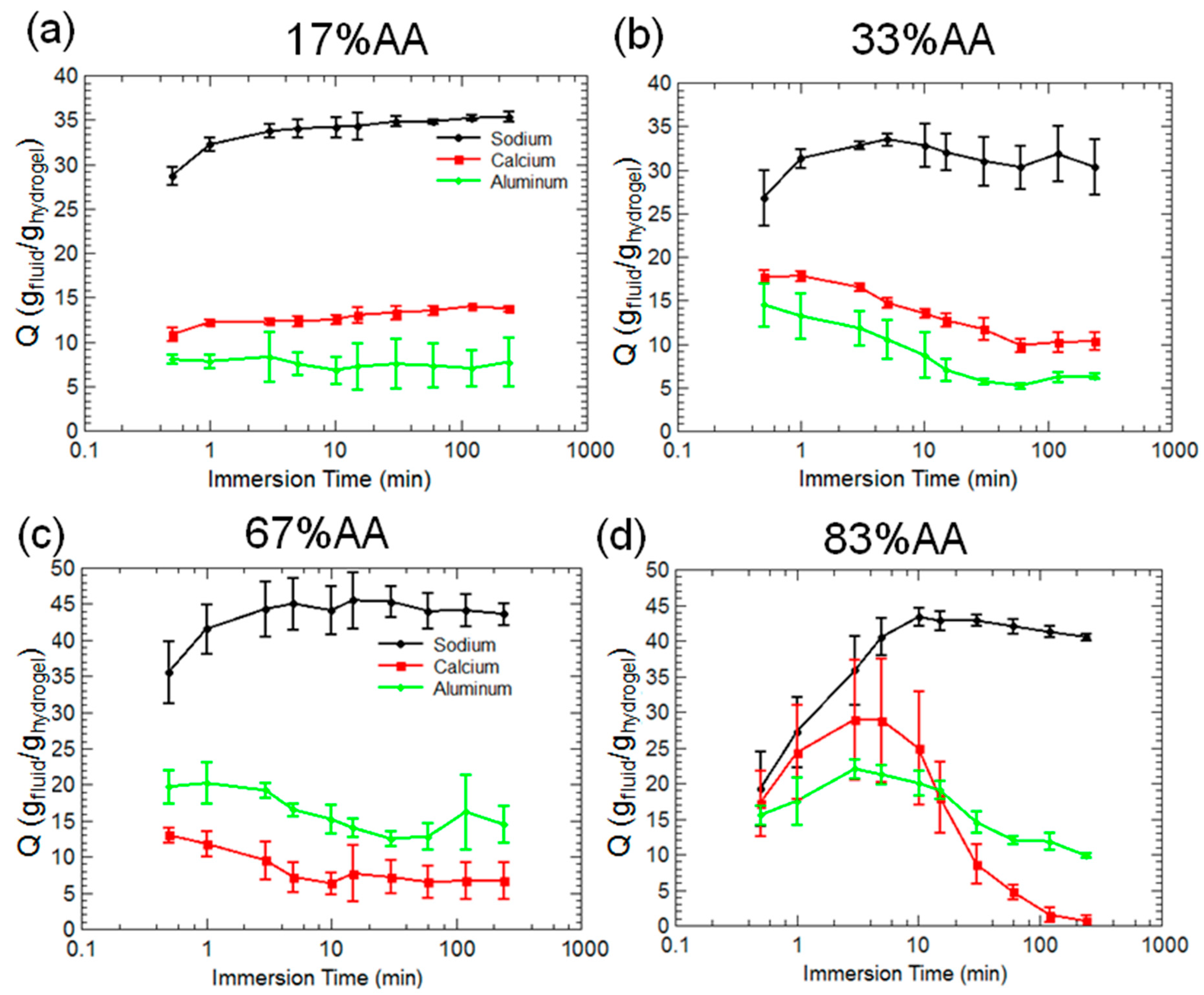
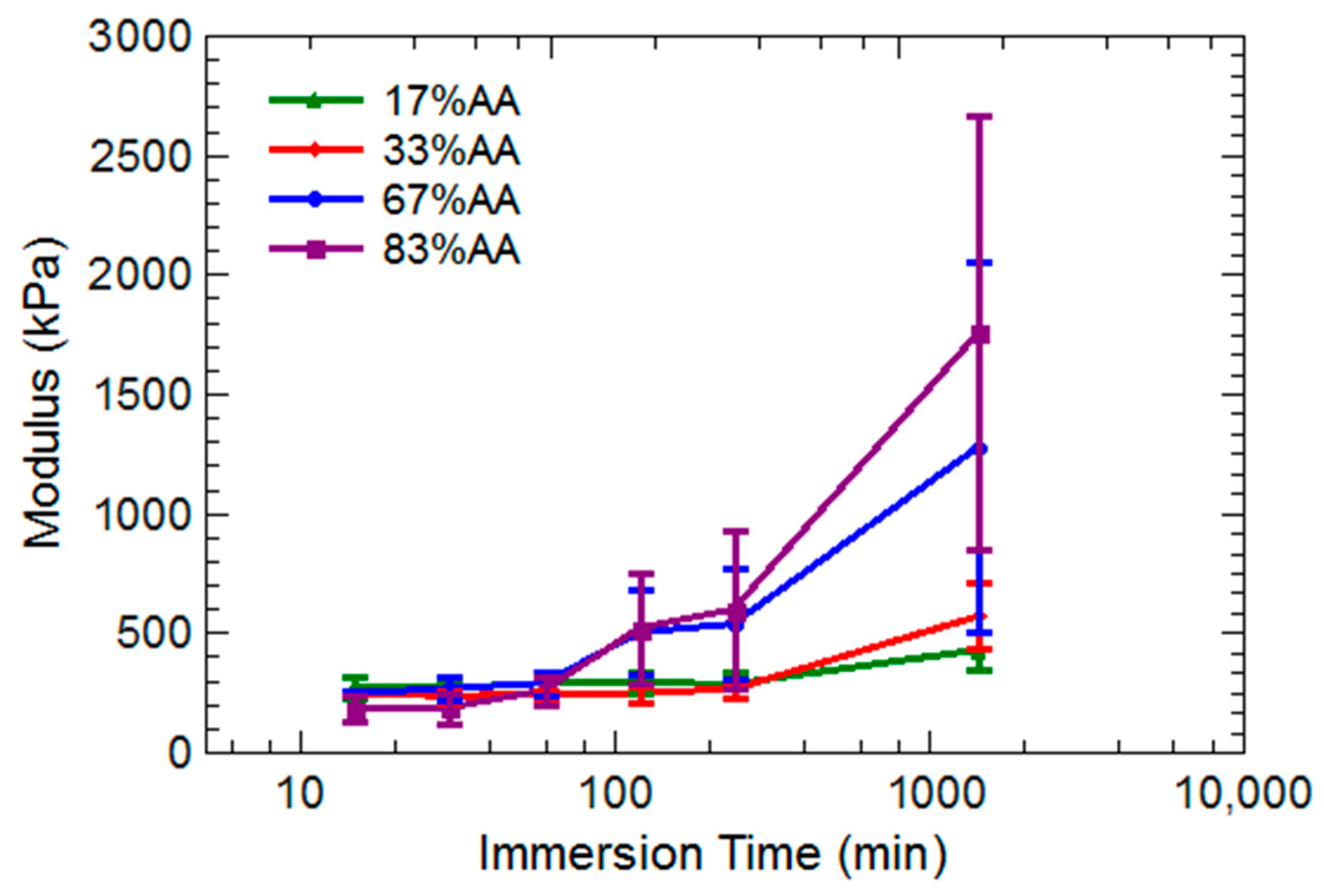
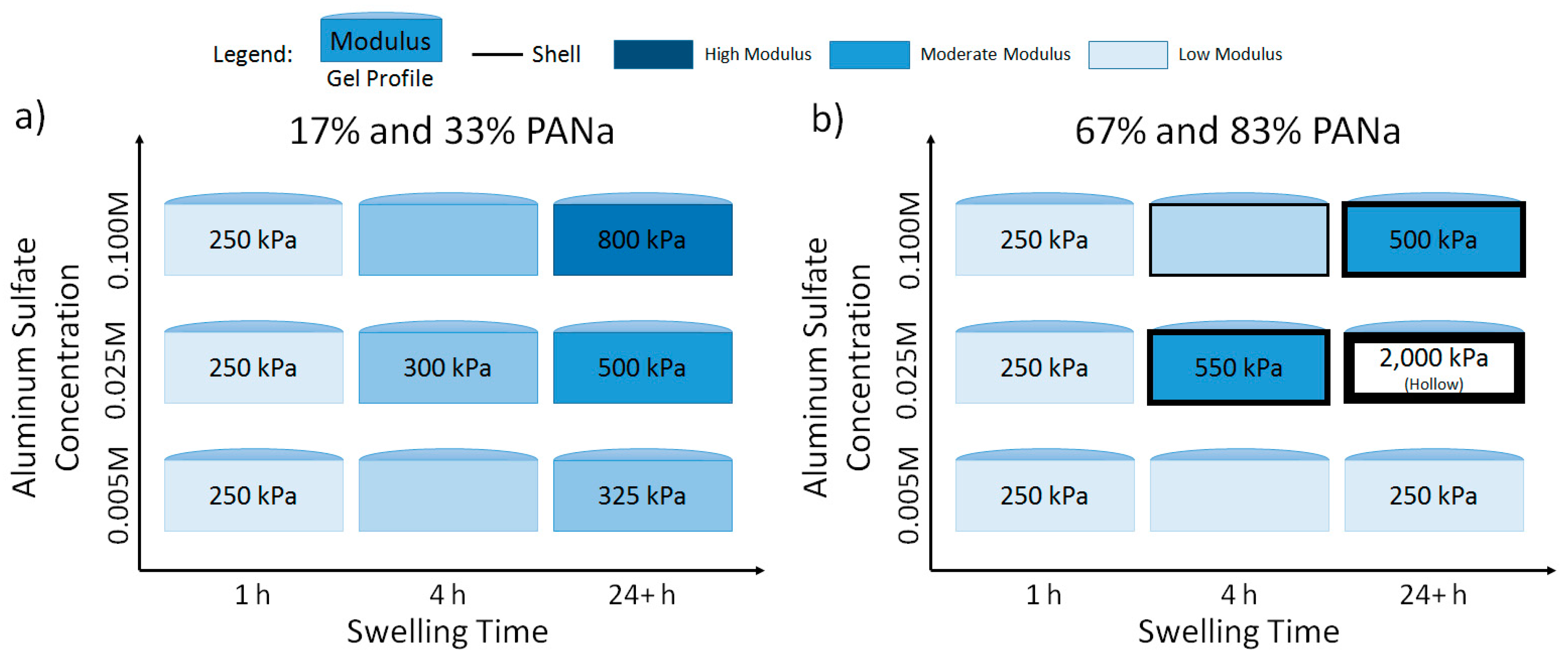
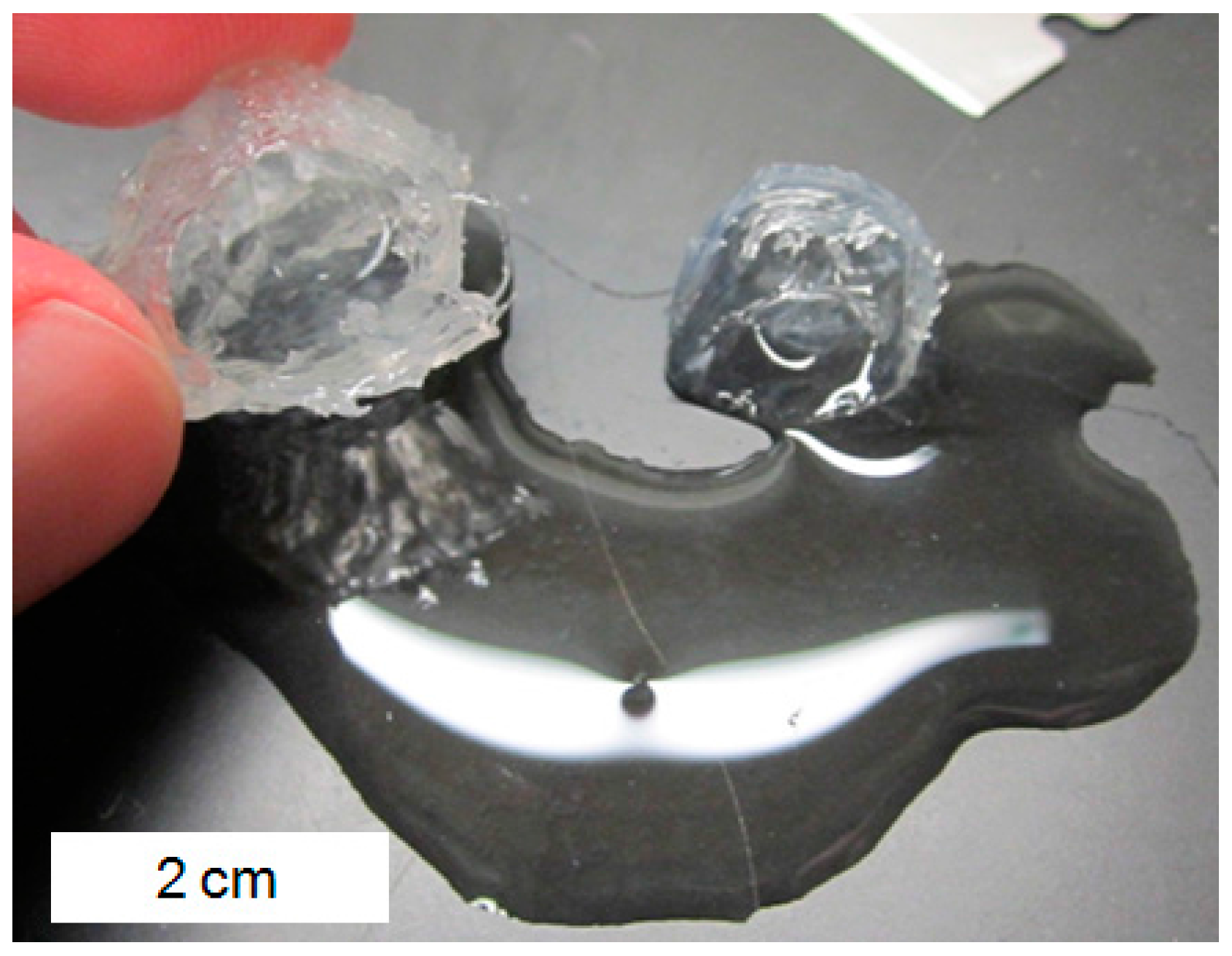
2.2. Hydrogel Behavior in Aluminum Solutions
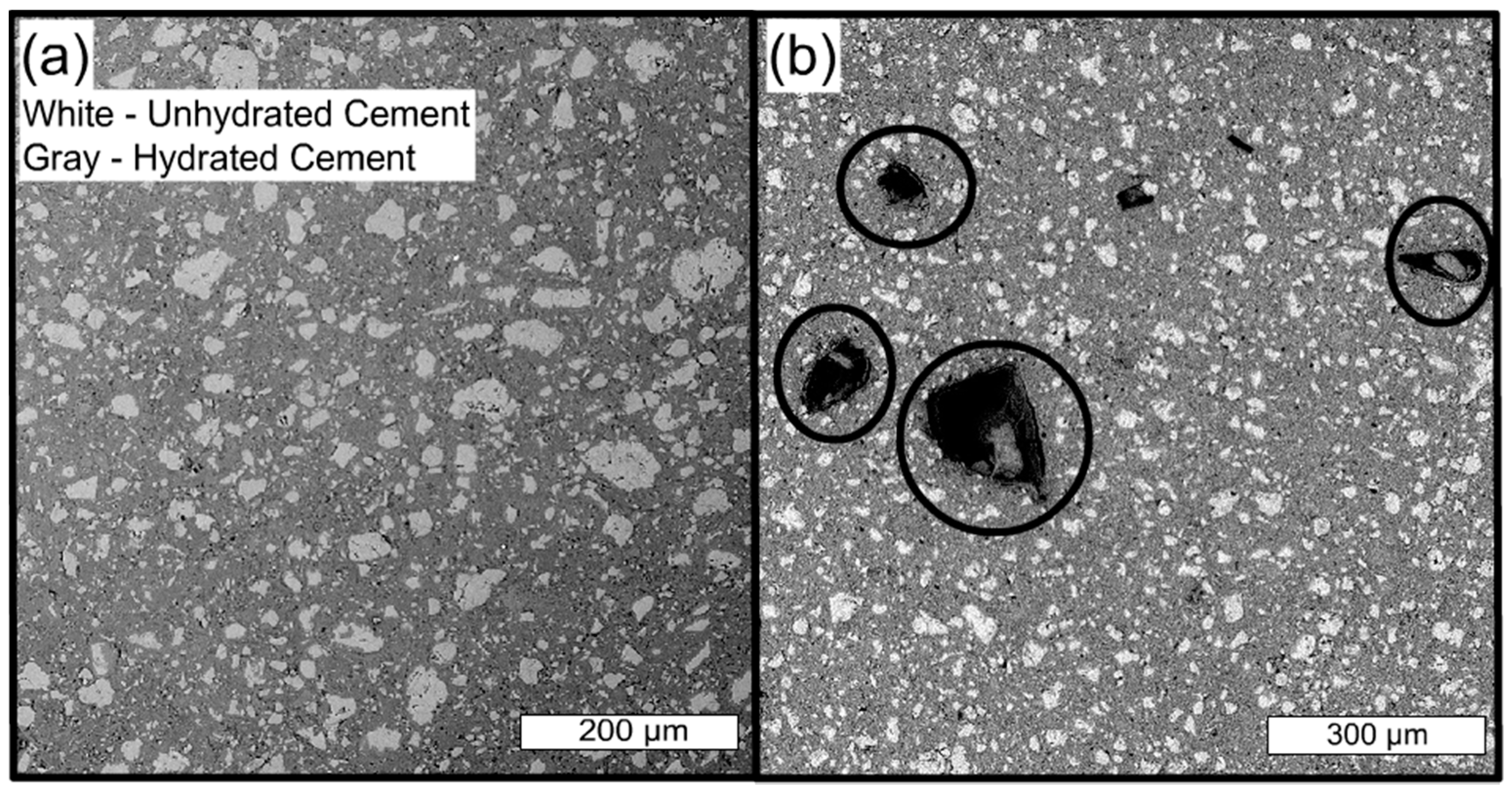
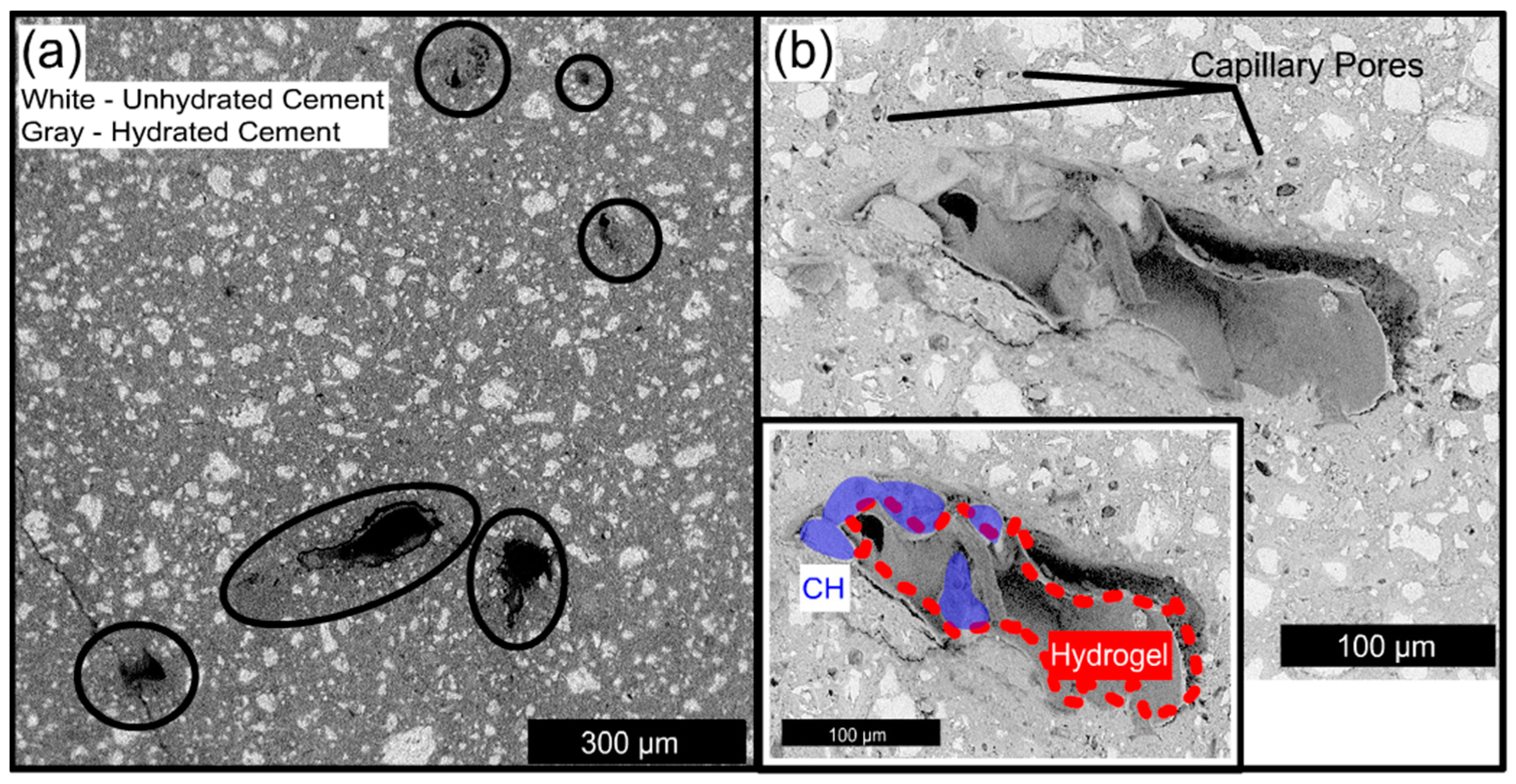
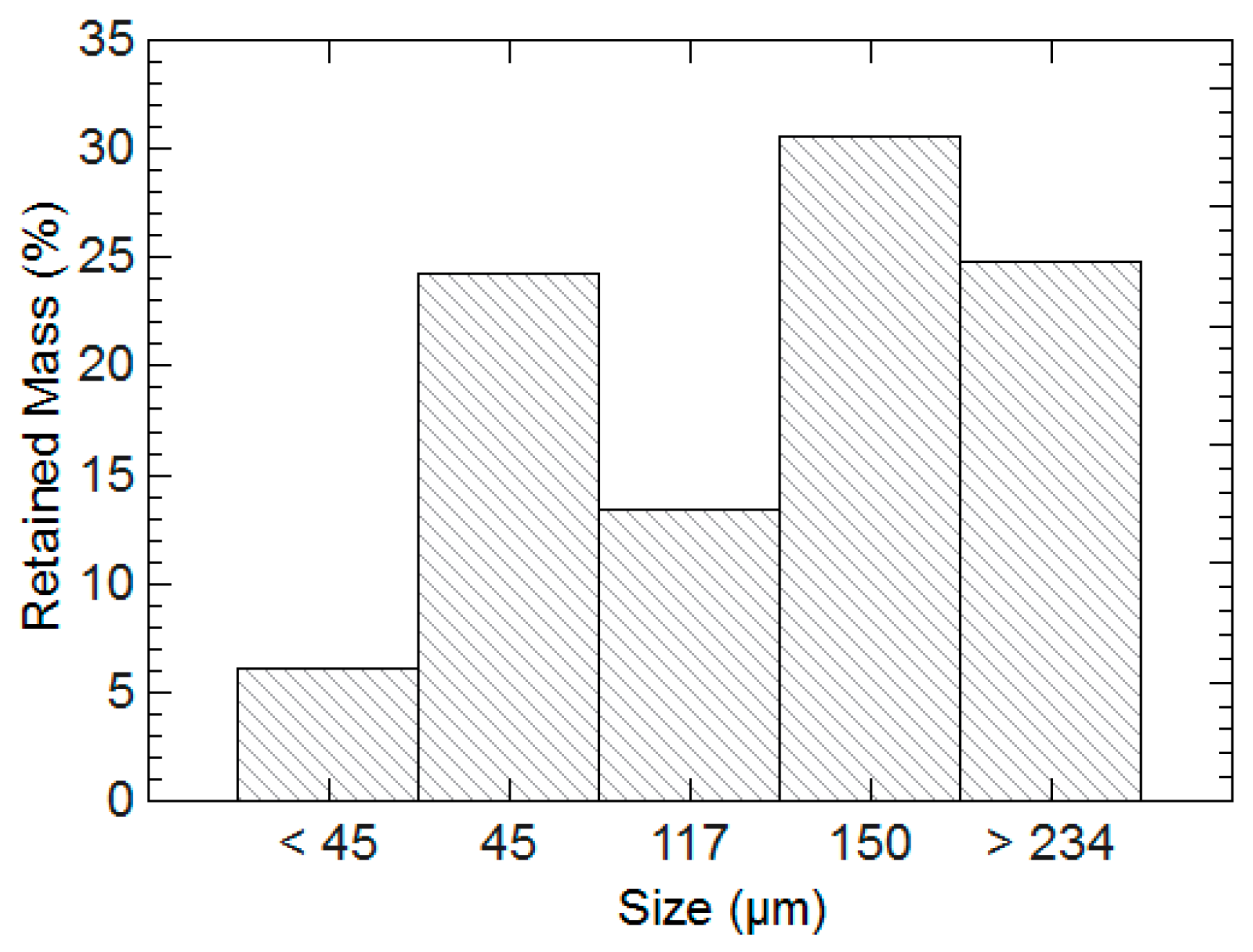
2.3. Influence of Hydrogel Chemistry on Cement Paste Microstructure
3. Discussion
3.1. Hydrogel Swelling Kinetics in Salt Solutions
3.2. Hydrogel Swelling Behavior in Aluminum Sulfate Solutions
3.3. Influence of Hydrogel Chemistry on Cement Paste Microstructure
4. Conclusions
5. Materials and Methods
5.1. Hydrogel Synthesis
5.2. Swelling and Compression Tests
5.3. Paste Batching
5.4. Backscattered Electron Microscopy
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Habert, G.; Arribe, D.; Dehove, T.; Espinasse, L.; le Roy, R. Reducing environmental impact by increasing the strength of concrete: Quantification of the improvement to concrete bridges. J. Clean. Prod. 2012, 35, 250–262. [Google Scholar] [CrossRef]
- Portland Cement Association. United States Cement Industry; Portland Cement Association: Skokie, IL, USA, 2016. [Google Scholar]
- Walraven, J. High Performance Concrete: A Material with a Large Potential. J. Adv. Concr. Technol. 2009, 7, 145–156. [Google Scholar] [CrossRef]
- Aïtcin, P.-C.; Neville, A. High-Performance Concrete Demystified. Concr. Int. 1993, 15, 21–26. [Google Scholar]
- Aïtcin, P.C. The durability characteristics of high performance concrete: A review. Cem. Concr. Compos. 2003, 25, 409–420. [Google Scholar] [CrossRef]
- Kodur, V.K.R.; Phan, L. Critical factors governing the fire performance of high strength concrete systems. Fire Saf. J. 2007, 42, 482–488. [Google Scholar] [CrossRef]
- Hernández-Olivares, F.; Barluenga, G. Fire performance of recycled rubber-filled high-strength concrete. Cem. Concr. Res. 2004, 34, 109–117. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Lura, P.; Jensen, O.M.; van Breugel, K. Autogenous shrinkage in high-performance cement paste: An evaluation of basic mechanisms Modeling of autogenous relative humidity change and autogenous. Cem. Concr. Res. 2003, 33, 223–232. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials—I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Beushausen, H.; Gillmer, M.; Alexander, M. The influence of superabsorbent polymers on strength and durability properties of blended cement mortars. Cem. Concr. Compos. 2014, 52, 73–80. [Google Scholar] [CrossRef]
- Farzanian, K.; Teixeira, K.P.; Rocha, I.P.; de Sa Carneiro, L.; Ghahremaninezhad, A. The mechanical strength, degree of hydration, and electrical resistivity of cement pastes modified with superabsorbent polymers. Constr. Build. Mater. 2016, 109, 156–165. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jensen, O.M. Chloride migration in concrete with superabsorbent polymers. Cem. Concr. Compos. 2015, 55, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Schröfl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- De Sensale, G.R.; Goncalves, A.F. Effects of Fine LWA and SAP as Internal Water Curing Agents. Int. J. Concr. Struct. Mater. 2014, 8, 229–238. [Google Scholar] [CrossRef]
- Friedrich, S. Application of Super Absorbent Polymers (SAP) in Concrete Construction; Springer Science & Business Media: Dordrecht, Netherlands, 2012; pp. 13–19. [Google Scholar]
- Jensen, O.M.; Lura, P. Techniques and materials for internal water curing of concrete. Mater. Struct. 2006, 39, 817–825. [Google Scholar] [CrossRef]
- Kelly, S.L.; Krafcik, M.J.; Erk, K.A. Synthesis and Characterization of Superabsorbent Polymer Hydrogels Used as Internal Curing Agents in High-Performance Concrete: Impact of Particle Shape on Mortar Compressive Strength. Int. Congr. Polym. Concr. 2017. submitted. [Google Scholar]
- Browning, J.; Darwin, D.; Reynolds, D.; Pendergrass, B. Lightweight Aggregate as Internal Curing Agent to Limit Concrete Shrinkage. ACI Mater. J. 2011, 108, 638–644. [Google Scholar]
- Cusson, D.; Hoogeveen, T. Internal curing of high-performance concrete with pre-soaked fine lightweight aggregate for prevention of autogenous shrinkage cracking. Cem. Concr. Res. 2008, 38, 757–765. [Google Scholar] [CrossRef]
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The pore solution of blended cements: A review. Mater. Struct. 2016, 49, 3341–3367. [Google Scholar] [CrossRef]
- Schröfl, C.; Snoeck, D.; Mechtcherine, V. A review of characterisation methods for superabsorbent polymer (SAP) samples to be used in cement-based construction materials: Report of the RILEM TC 260-RSC. Mater. Struct. 2017, 50, 197. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry, 15th ed.; Cornell University Press: New York, NY, USA, 1992. [Google Scholar]
- Knovel Critical Tables, 2nd ed.; Knovel: New York, NY, USA, 2008.
- Brannon-Peppas, L.; Peppas, N.A. Structural analysis of charged polymeric networks. Polym. Bull. 1988, 20, 285–289. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium Swelling Behavior of pH-Sensitive Hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Horkay, F.; Horkay, F.; Tasaki, I.; Tasaki, I.; Basser, P.J.; Basser, P.J. Osmotic swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules 2000, 1, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mussel, M.; Wilczynski, E.; Eliav, U.; Gottesman, J.; Wilk, M.; Nevo, U. Dynamics of water and sodium in gels under salt-induced phase transition. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1620–1628. [Google Scholar] [CrossRef]
- Chremos, A.; Douglas, J.F. Counter-ion distribution around flexible polyelectrolytes having different molecular architecture. Soft Matter 2016, 12, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Krafcik, M.J.; Erk, K.A. Characterization of superabsorbent poly(sodium-acrylate acrylamide) hydrogels and influence of chemical structure on internally cured mortar. Mater. Struct. 2016, 49, 4765–4778. [Google Scholar] [CrossRef]
- Horkay, F.; Tasaki, I.; Basser, P.J. Effect of monovalent-divalent cation exchange on the swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules 2001, 2, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Budtova, T.; Navard, P. Swelling-Induced Birefringence of a Polyelectrolyte Gel Strongly Interacting with Metal Ions. Macromolecules 1997, 30, 6556–6558. [Google Scholar] [CrossRef]
- Lapitsky, Y.; Kaler, E.W. Formation of surfactant and polyelectrolyte gel particles in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 250, 179–187. [Google Scholar] [CrossRef]
- Lawrence, P.G.; Lapitsky, Y. Ionically cross-linked poly(allylamine) as a stimulus-responsive underwater adhesive: Ionic strength and pH effects. Langmuir 2015, 31, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Chremos, A.; Douglas, J.F. Influence of higher valent ions on flexible polyelectrolyte stiffness and counter-ion distribution. J. Chem. Phys. 2016, 144, 164904. [Google Scholar] [CrossRef] [PubMed]
- Farzanian, K.; Ghahremaninezhad, A. The effect of the capillary forces on the desorption of hydrogels in contact with a porous cementitious material. Mater. Struct. 2017, 50, 216. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Lura, P.; Pesavento, F.; Gawin, D. Modeling of Water Migration during Internal Curing with Superabsorbent Polymers. J. Mater. Civ. Eng. 2012, 24, 1006–1016. [Google Scholar] [CrossRef]
- Wehbe, Y.; Ghahremaninezhad, A. Combined effect of shrinkage reducing admixtures (SRA) and superabsorbent polymers (SAP) on the autogenous shrinkage, hydration and properties of cementitious materials. Constr. Build. Mater. 2017, 138, 151–162. [Google Scholar] [CrossRef]
- Budtova, T. Absorption/release of polyvalent metal ions by a polyelectrolyte gel. J. Control. Release 1998, 54, 305–312. [Google Scholar] [CrossRef]
- Watanabe, T.; Lopez, C.G.; Douglas, J.F.; Ono, T.; Cabral, J.T. Microfluidic approach to the formation of internally porous polymer particles by solvent extraction. Langmuir 2014, 30, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Goransson, A.; Hansson, P. Shrinking Kinetics of Polyacrylate Gels in Surfactant Solution. J. Phys. Chem. B 2003, 107, 9203–9213. [Google Scholar] [CrossRef]
- Huang, Y.; Lawrence, P.G.; Lapitsky, Y. Self-assembly of stiff, adhesive and self-healing gels from common polyelectrolytes. Langmuir 2014, 30, 7771–7777. [Google Scholar] [CrossRef] [PubMed]
- Esteves, L.P.; Lukošiute, I.; Česniene, J. Hydration of cement with Superabsorbent polymers. J. Therm. Anal. Calorim. 2014, 118, 1385–1393. [Google Scholar] [CrossRef]
- ASTM International. ASTM C150–17 Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Zhu, Q.; Barney, C.W.; Erk, K.A. Effect of ionic crosslinking on the swelling and mechanical response of model superabsorbent polymer hydrogels for internally cured concrete. Mater. Struct. 2015, 48, 2261–2276. [Google Scholar] [CrossRef]


| Hydrogel Type | AA | AM | Water | NaOH Soln. | Crosslinker Soln. | Init. Soln. |
|---|---|---|---|---|---|---|
| 17 wt % AA | 0.5 | 2.5 | 6.2 | 0.8 | 4.0 | 0.5 each |
| 33 wt % AA | 1 | 2 | 5.4 | 1.6 | 4.0 | 0.5 each |
| 67 wt % AA | 2 | 1 | 3.8 | 3.2 | 4.0 | 0.5 each |
| 83 wt % AA | 2.5 | 0.5 | 3 | 4.0 | 4.0 | 0.5 each |
| Type | Cement (kg) | Water (kg) | w/c | Hydrogels (kg) | Q | WRA |
|---|---|---|---|---|---|---|
| Control | 200 | 70 | 0.35 | 0.4 | -- | 0.7 |
| 17 wt % AA | 200 | 70 | 0.35 | 0.4 | 22 | 0.7 |
| 33 wt % AA | 200 | 70 | 0.35 | 0.4 | 18.2 | 0.7 |
| 67 wt % AA | 200 | 70 | 0.35 | 0.4 | 11.7 | 0.7 |
| 83 wt % AA | 200 | 70 | 0.35 | 0.4 | 4.3 | 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krafcik, M.J.; Macke, N.D.; Erk, K.A. Improved Concrete Materials with Hydrogel-Based Internal Curing Agents. Gels 2017, 3, 46. https://doi.org/10.3390/gels3040046
Krafcik MJ, Macke ND, Erk KA. Improved Concrete Materials with Hydrogel-Based Internal Curing Agents. Gels. 2017; 3(4):46. https://doi.org/10.3390/gels3040046
Chicago/Turabian StyleKrafcik, Matthew J., Nicholas D. Macke, and Kendra A. Erk. 2017. "Improved Concrete Materials with Hydrogel-Based Internal Curing Agents" Gels 3, no. 4: 46. https://doi.org/10.3390/gels3040046