Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications
Abstract
:1. Introduction
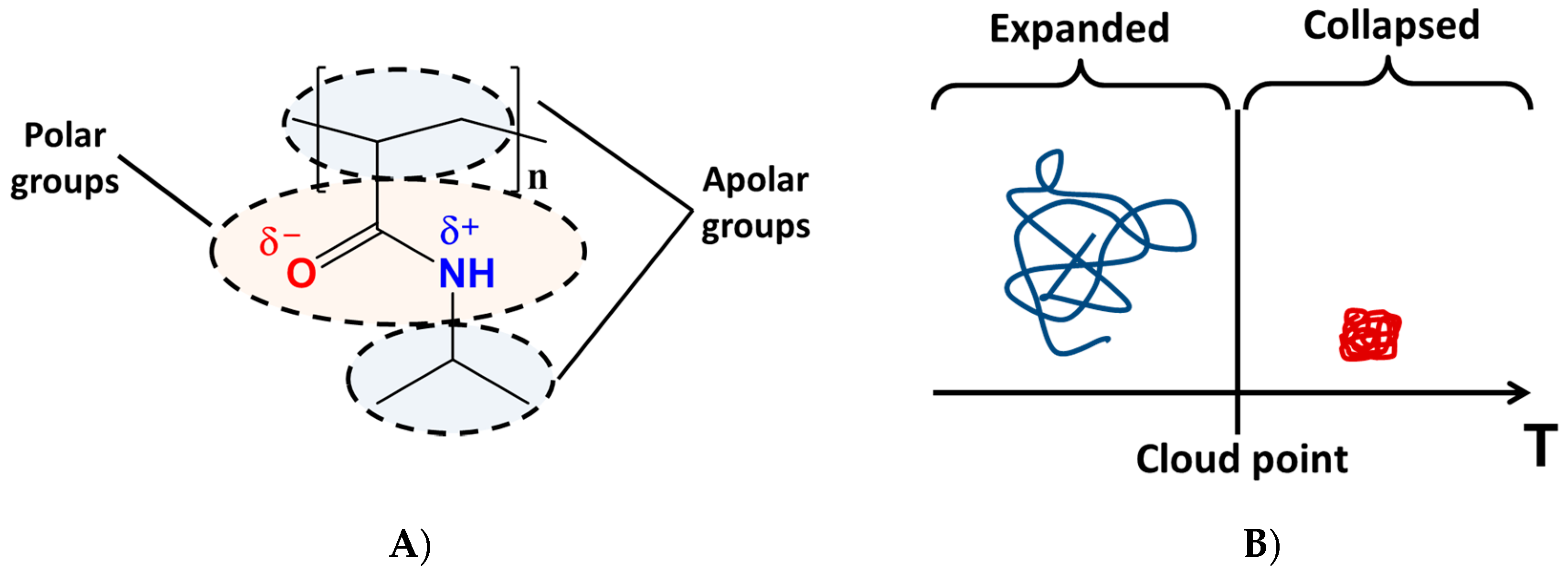
2. The Role of the Hydrogen-Bonding Interactions
3. Biocompatibility and Biodegradability of PNIPAAm-Based Hydrogels
4. Poly(N-isopropylacrylamide) Copolymers and Graft Polymers
4.1. Poly(N-isopropylacrylamide) Thermo-Responsive Hydrogels with Synthetic Polymers
4.2. Poly(N-isopropylacrylamide) Thermo-Responsive Hydrogels with Polyssacharides
4.3. Poly(N-isopropylacrylamide) Thermo-Responsive Hydrogels with Polypeptides and Proteins
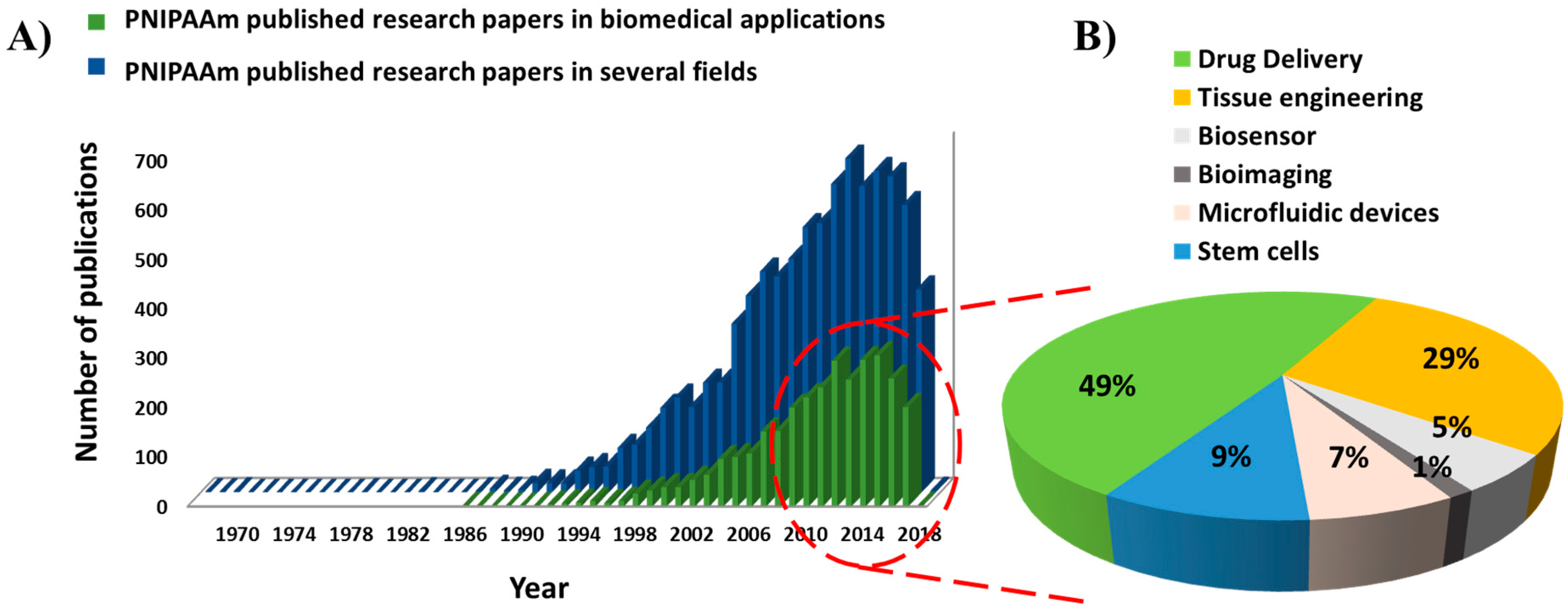
5. Novel Applications of PNIPAAm-Based Hydrogel Copolymers and Grafted Polymers
6. Brief Conclusions and Future Outlooks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Omidian, H.; Park, K. Introduction to Hydrogels. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer Science & Business Media: New York, NY, USA, 2010; pp. 1–16. ISBN 978-1-4419-5919-5. [Google Scholar]
- Wang, X.; Sun, Y.; Peng, C.; Luo, H.; Wang, R.; Zhang, D. Transitional Suspensions Containing Thermosensitive Dispersant for Three-Dimensional Printing. ACS Appl. Mater. Interface 2015, 7, 26131–26136. [Google Scholar] [CrossRef] [PubMed]
- Dalmont, H.; Pinprayoon, O.; Saunders, B.R. Study of pH responsive microgels containing methacrylic acid: Effects of particle composition and added calcium. Langmuir 2008, 24, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ahiabu, A.; Serpe, M.J. Controlled drug release from the aggregation-disaggregation behaviour of pH-responsive microgels. ACS Appl. Mater. Interfaces 2014, 6, 13749–13756. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Cohen Stuart, A.M.; Huck, T.S.W.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, B.G.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.M.; Xu, W.; Serpe, M.J. Optical devices constructed from multiresponsive microgels. Angew. Chem. Int. Ed. 2014, 53, 4827–4831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, G.; Hu, J.; Lu, X.; Li, J. Temperature, ionic strength and pH induced electrochemical switching of smart polymer interfaces. Chem. Commun. 2006, 4820–4822. [Google Scholar] [CrossRef]
- Shibayama, M.; Ikkai, F.; Inamoto, S.; Nomura, S.; Han, C.C. pH and salt concentration dependence of the microstructure of poly(N-isopropylacrylamide-co-acrylic acid) gels. J. Chem. Phys. 1996, 105, 4358–4366. [Google Scholar] [CrossRef]
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef] [PubMed]
- Häring, M.; Schiller, J.; Mayr, J.; Grijalvo, S.; Eritja, R.; Díaz, D.D. Magnetic Gel Composites for Hyperthermia Cancer Therapy. Gels 2015, 1, 135–161. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Y.; Serpe, M.J. Electrochemically color tunable poly (N-isopropylacrylamide) microgel-based etalons. J. Mater. Chem. C 2014, 2, 3873–3878. [Google Scholar] [CrossRef]
- Armelin, E.; Pérez-Madrigal, M.M.; Alemán, C.; Díaz, D.D. Current status and challenges of biohydrogels for applications as supercapacitors and secondary batteries. J. Mat. Chem. A 2016, 4, 8952–8968. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Mano, J. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Adv. Eng. Mat. 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Yoshida, R.; Okano, T. Stimuli-Responsive Hydrogels and Their Application to Functional Materials. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer Science & Business Media: New York, NY, USA, 2010; pp. 19–43. ISBN 978-1-4419-5919-5. [Google Scholar]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New Directions in Thermoresponsive Polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Geng, Y.; Discher, D.E.; Yang, S. Temperature-Controlled Assembly and Release from Polymer Vesicles of Poly(ethylene oxide)-blockpoly(N-isopropylacrylamide). Adv. Mater. 2006, 18, 2905–2909. [Google Scholar] [CrossRef]
- Zhou, C.; Hillmyer, M.A.; Lodge, T.P. Micellization and Micelliar Aggregation of Poly(ethylene-altpropylene)-b-poly(ethyleneoxide)-b-poly(N-isopropylacrylamide) Triblock Terpolymers in Water. Macromolecules 2011, 44, 1635–1641. [Google Scholar] [CrossRef]
- Zhou, C.; Hillmyer, M.A.; Lodge, T.P. Efficient Formation of Multicompartment Hydrogels by Stepwise Self-Assembly of Thermoresponsive ABC Triblock Terpolymers. J. Am. Chem. Soc. 2012, 134, 10365–10368. [Google Scholar] [CrossRef] [PubMed]
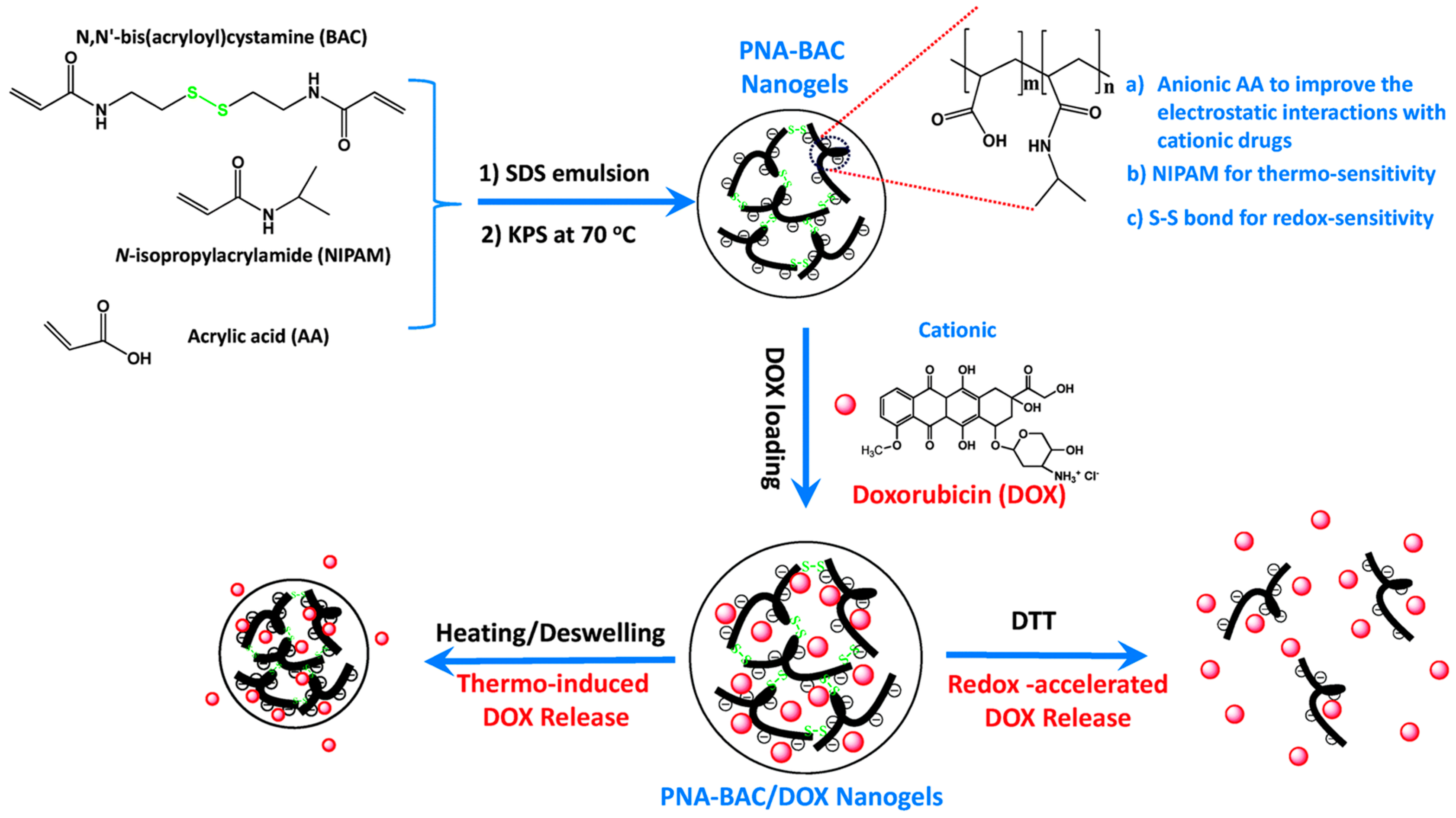
- Gupta, M.K.; Martin, J.R.; Werfel, T.A.; Shen, T.; Page, J.M.; Duvall, C.L. Cell Protective, ABC Triblock Polymer-Based Thermoresponsive Hydrogels with ROS-Triggered Degradation and Drug Release. J. Am. Chem. Soc. 2014, 136, 14896–14902. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Tachibana, M.; Umezu, M.; Matsunaga, Y.T. Bio-inspired smart hydrogel with temperature dependent properties and enhanced cell attachment. J. Mater. Chem. B 2016, 4, 1740–1746. [Google Scholar] [CrossRef]
- Mi, L.; Xue, H.; Li, Y.; Jiang, S.A. Thermoresponsive Antimicrobial Wound Dressing Hydrogel Based on a Cationic Betaine Ester. Adv. Funct. Mater. 2011, 21, 4028–4034. [Google Scholar] [CrossRef]
- Patenaude, M.; Hoare, T. Injectable, Mixed Natural-Synthetic Polymer Hydrogels with Modular Properties. Biomacromolecules 2012, 13, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.; Itayagoshi, M.; Hagiwara, T.; Yamaguchi, M.; Kanamori, T.; Shinbo, T.; Wang, P.C. Poly(N-isopropylacrylamide)-graft-polypropylene membranes containing adsorbed antibody for cell separation. Biomaterials 2005, 26, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Charan, H.; Kinzel, J.; Glebe, U.; Anand, D.; Garakani, T.M.; Zhu, L.; Bocola, M.; Schwaneberg, U.; Böker, A. Grafting PNIPAAm from β-barrel shaped transmembrane nanopores. Biomaterials 2016, 107, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Hilt, J.Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release 2008, 130, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, A.; Kikuchi, A.; Yamato, M.; Kanazawa, H.; Okano, T. Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials 2008, 29, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Klaikherd, A.; Nagamani, C.; Thayumanavan, S. Multi-stimuli sensitive amphiphilic block copolymer assemblies. J. Am. Chem. Soc. 2009, 131, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.L.; Ma, Z.; Nelson, D.M.; Hashizume, R.; Guan, J.; Tobita, K.; Wagner, W.R. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials 2009, 30, 4357–4368. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, M.; Bai, H.; Chen, P.; Xia, F.; Han, D.; Jiang, L. Antiplatelet and thermally responsive poly(N-isopropylacrylamide) surface with nanoscale topography. J. Am. Chem. Soc. 2009, 131, 10467–10472. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, S.; Chang, P.E.J.; Rumpel, H.; Kee, I.H.C.; Ng, R.T.H.; Chow, P.K.H.; Tan, C.K.; Ramanujan, R.V. Thermoresponsive core-shell magnetic nanoparticles for combined modalities of cancer therapy. Nanotechnology 2009, 20, 305101. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R. Self-oscillating gels driven by the belousov-zhabotinsky reaction as novel smart materials. Adv. Mater. 2010, 22, 3463–3483. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. Smart core-shell hybrid nanogels with ag nanoparticle core for cancer cell imaging and gel shell for pH-regulated drug delivery. Chem. Mater. 2010, 22, 1966–1976. [Google Scholar] [CrossRef]
- Stoychev, G.; Puretskiy, N.; Ionov, L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter 2011, 7, 3277–3279. [Google Scholar] [CrossRef]
- Lin, J.B.; Isenberg, B.C.; Shen, Y.; Schorsch, K.; Sazonova, O.V.; Wong, J.Y. Thermo-responsive poly(N-isopropylacrylamide) grafted onto microtextured poly(dimethylsiloxane) for aligned cell sheet engineering. Colloids Surf. B Biointerfaces 2012, 99, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ma, P.; Cheng, Z.; Kang, X.; Zhang, X.; Hou, Z.; Li, C.; Yang, D.; Zhai, X.; Lin, J. Up-conversion cell imaging and pH-induced thermally controlled drug release from NaYF 4:Yb 3+/Er 3+@hydrogel core-shell hybrid microspheres. ACS Nano 2012, 6, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.H.; Lu, Y.; Peng, J.; Chen, J.F.; Yu, S.H. Photothermally sensitive poly(N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels as remote light-controlled liquid microvalves. Adv. Mater. 2012, 22, 4017–4022. [Google Scholar] [CrossRef]
- Yang, J.; Shen, D.; Zhou, L.; Li, W.; Li, X.; Yao, C.; Wang, R.; El-Toni, A.M.; Zhang, F.; Zhao, D. Spatially confined fabrication of core-shell gold nanocages@Mesoporous silica for near-infrared controlled photothermal drug release. Chem. Mater. 2013, 25, 3030–3037. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lü, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; Xing, M.; Wang, C. A PNIPAAm-Based Thermosensitive Hydrogel Containing SWCNTs for Stem Cell Transplantation in Myocardial Repair. Biomaterials 2014, 35, 5679–5688. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Cheng, Y.; Zhang, Z.; Zhang, P.; Chen, X.; Wei, Y. In vitro study of electroactive tetraaniline-containing thermosensitive hydrogels for cardiac tissue engineering. Biomacromolecules 2014, 15, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Bakarich, S.E.; Gorkin, R., III; Panhuis, M.; Spinks, G.M. 4D Printing with Mechanically Robust, Thermally Actuating Hydrogels. Macromol. Rapid Commun. 2015, 36, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Müller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Psarra, E.; König, U.; Ueda, Y.; Bellmann, C.; Janke, A.; Bittrich, E.; Eichhorn, P.; Uhlmann, K.J. Nanostructured Biointerfaces: Nanoarchitectonics of Thermoresponsive Polymer Brushes Impact Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2015, 7, 12516–12529. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.H.; Morales, Y.; Cabral, T. Ocular Biocompatibility of Poly-N-Isopropylacrylamide (pNIPAM). J. Ophthalmol. 2016, 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, D.; Jin, G.; Tan, B.H.; He, C. Facile Layer-by-Layer Self-Assembly toward Enantiomeric Poly(lactide) Stereocomplex Coated Magnetite Nanocarrier for Highly Tunable Drug Deliveries. ACS Appl. Mater. Interfaces 2016, 8, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hou, J.; Chen, R.; Xin, Z.; Shi, H.; Wong, S.C.; Yin, J.; Shi, Q. Cell-inspired biointerfaces constructed from patterned smart hydrogels for immunoassays in whole blood. J. Mater. Chem. B 2017, 5, 2315–2321. [Google Scholar] [CrossRef]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-Responsive Poly(N-Isopropylacrylamide)-Cellulose Nanocrystals Hybrid Hydrogels for Wound Dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Gao, B.; Li, Y.; Duan, Q.; Kakuchi, T. Comb-shaped, temperature-tunable and water-soluble porphyrin-based thermoresponsive copolymer for enhanced photodynamic therapy. Mater. Sci. Eng. C 2018, 82, 155–162. [Google Scholar] [CrossRef]
- Ito, D.; Kubota, K. Solution properties and thermal behaviour of poly(N-propylacrylamide) in water. Macromolecules 1997, 30, 7828–7834. [Google Scholar] [CrossRef]
- Ono, Y.; Shikata, T. Hydration and Dynamic Behaviour of Poly(N-isopropylacrylamide)s in Aqueous Solution: A Sharp Phase Transition at the Lower Critical Solution Temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on Thermo Responsive Polymers: Phase Behaviour, Drug Delivery and Biomedical Applications. Asian J. Pharm.Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chen, K.S.; Run-Chu, L. Drying Methods Affecting the Particle Sizes, Phase Transition, Deswelling/Reswelling Processes and Morphology of Poly(N-isopropylacrylamide) Microgel Beads. Polymer 1999, 40, 6307–6312. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Tanaka, T.; Sato, H.; Ozaki, Y. Conformational Change of Poly(N-isopropylacrylamide) during the Coil-Globule Transition Investigated by Attenuated Total Reflection/Infrared Spectroscopy and Density Functional Theory Calculation. J. Phys. Chem. A 2002, 106, 3429–3435. [Google Scholar] [CrossRef]
- Dybal, J.; Trchova, M.; Schmidt, P. The Role of Water in Structural Changes of Poly(N-isopropylacrylamide) and Poly(N-isopropylmethacrylamide) Studied by FTIR, Raman Spectroscopy and Quantum Chemical Calculations. Vib. Spectrosc. 2009, 51, 44–51. [Google Scholar] [CrossRef]
- Boutris, C.; Chatzi, E.G.; Kiparissides, C. Characterization of the LCST Behaviour of Aqueous Poly(N-isopropylacrylamide) Solutions by Thermal and Cloud Point Techniques. Polymer 1997, 38, 2567–2570. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Wu, J.; Chen, T. Thermo-responsive Textiles. In Handbook of Smart Textiles; Tao, X., Ed.; Springer Science & Business Media: Singapore, 2015; Chapter 34; pp. 919–952. ISBN 978-981-4451-46-8. [Google Scholar]
- Pérez-Madrigal, M.M.; Torras, J.; Casanovas, J.; Häring, M.; Alemán, C.; Diaz Diaz, D. Paradigm Shift for Preparing Versatile M2+-Free Gels from Unmodified Sodium Alginate. Biomacromolecules 2017, 18, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
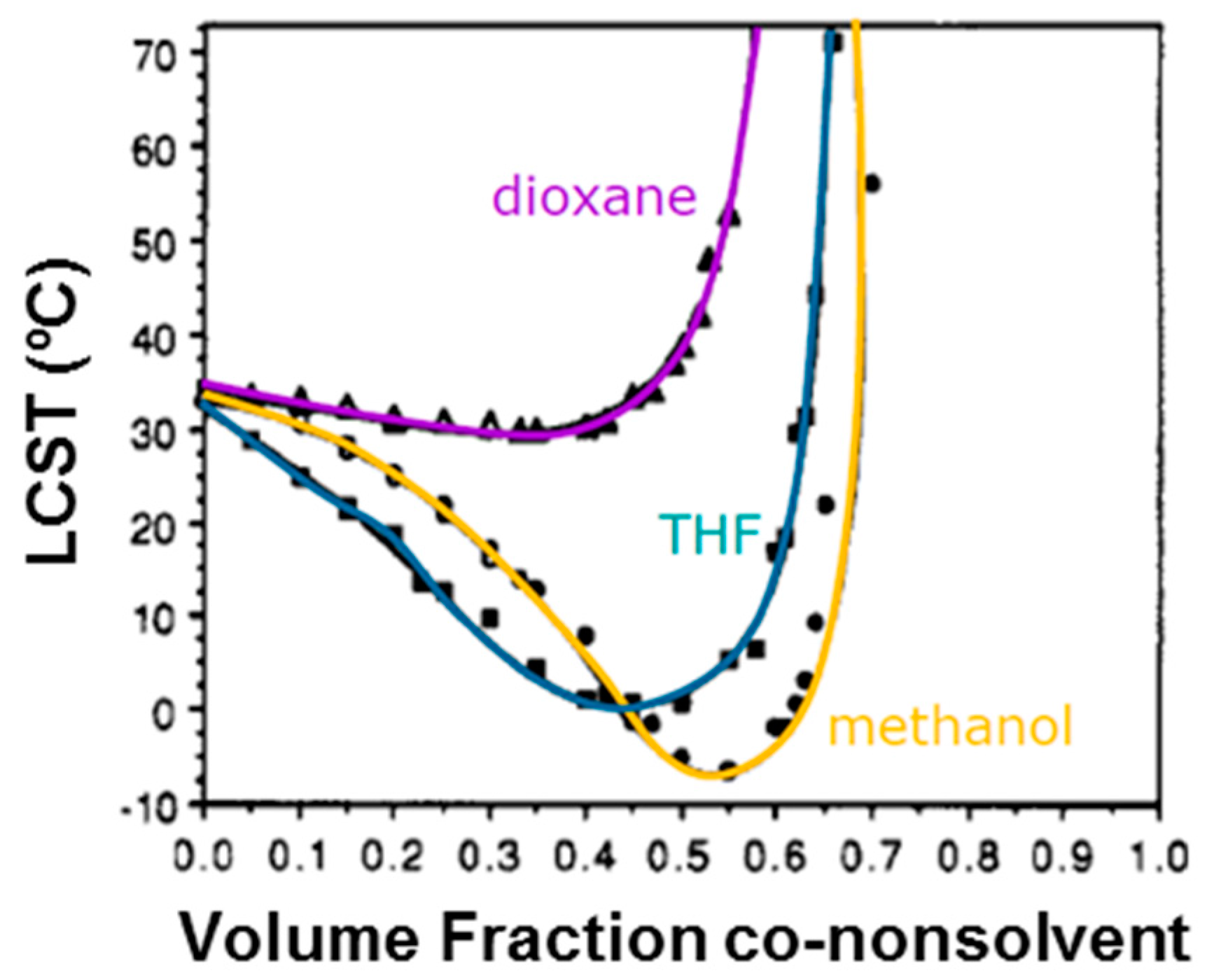
- Zhang, G.; Wu, C. The Water/Methanol Complexation Induced Reentrant Coil-to-Globule-to-Coil Transition of Individual Homopolymer Chains in Extremely Dilute Solution. J. Am. Chem. Soc. 2001, 123, 1376–1380. [Google Scholar] [CrossRef]
- Tanaka, F.; Koga, T.; Kojima, H.; Winnik, F.M. Temperature- and Tension-Induced Coil-Globule Transition of Poly(N-isopropylacrylamide) Chains in Water and Mixed Solvent of Water/Methanol. Macromolecules 2009, 42, 1321–1330. [Google Scholar] [CrossRef]
- Tanaka, F.; Koga, T.; Kojima, H.; Xue, N.; Winnik, F.M. Preferential Adsorption and co-nonsolvency of Thermoresponsive Polymers in Mixed Solvents of Water/Methanol. Macromolecules 2011, 44, 2978–2989. [Google Scholar] [CrossRef]
- Kojima, H.; Tanaka, F. Reentrant Volume Phase Transition of Cross-linked Poly(N-isopropylacrylamide) Gels in Mixed Solvents of Water/Methanol. Soft Matter 2012, 8, 3010–3020. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ringsdorf, H.; Venzmer, J. Methanol-Water as a Co-nonsolvent System for Poly(N-isopropylacrylamide). Macromolecules 1990, 23, 2415–2416. [Google Scholar] [CrossRef]
- Schild, H.G.; Muthukumar, M.; Tirrell, D.A. Cononsolvency in Mixed Aqueous Solutions of Poly(N-isopropylacrylamide). Macromolecules 1991, 24, 948–952. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Dubovik, A.S.; Kuznetsov, D.V.; Grinberg, N.V.; Grosberg, A.Y.; Tanaka, T. Studies of the Thermal Volume Transition of Poly(N-isopropylacrylamide) Hydrogels by High-Sensitivity Differential Scanning Microcalorimetry. 2. Thermodynamic Functions. Macromolecules 2000, 33, 8685–8692. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 722–3742. [Google Scholar] [CrossRef] [PubMed]
- Boere, K.W.M.; Soliman, B.G.; Rijkers, D.T.S.; Hennink, W.E.; Vermonden, T. Thermoresponsive Injectable Hydrogels Cross-Linked by Native Chemical Ligation. Macromolecules 2014, 47, 2430–2438. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Zhao, C.; Kong, W.; Dai, Q.; Chen, Q.; Liu, C.; Sun, R. Xylan-Based Temperature/pH Sensitive Hydrogels for Drug Controlled Release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, R.P.; Oprea, A.M.; Cheaburu, C.N.; Nistor, M.T.; Novac, O.; Ghiciuc, C.M.; Profire, L.; Vasile, C. Biocompatible and Biodegradable Alginate/Poly(N-isopropylacrylamide) Hydrogels for Sustained Theophylline Release. J. Appl. Polym. Sci. 2014, 131, 40733. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Matsushita, S.; Guan, J. Differentiation of Cardiosphere-Derived Cells into a Mature Cardiac Lineage Using Biodegradable Poly(N-isopropylacrylamide) Hydrogels. Biomaterials 2011, 32, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, M.; Hoare, T. Injectable, Degradable Thermoresponsive Poly(N-isopropylacrylamide) Hydrogels. ACS Macro Lett. 2012, 1, 409–413. [Google Scholar] [CrossRef]
- Gan, J.; Guan, X.X.; Zheng, J.; Guo, H.; Wu, K.; Liang, L.; Lu, M. Biodegradable, Thermoresponsive PNIPAM-Based Hydrogel Scaffolds for the Sustained Release of Levofloxacin. RSC Adv. 2016, 6, 32967–32978. [Google Scholar] [CrossRef]
- Galperin, A.; Long, T.J.; Garty, S.; Ratner, B.D. Synthesis and Fabrication of a Degradable Poly(N-isopropyl acrylamide) Scaffold for Tissue Engineering Applications. J. Biomed. Mater. Res. A 2013, 101A, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; van Lith, R.; Baler, K.; Hoshi, R.A.; Ameer, G.A. A Thermoresponsive Biodegradable Polymer with Intrinsic Antioxidant Properties. Biomacromolecules 2014, 15, 3942–3952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained Release of Stromal Cell Derived Factor-1 from an Antioxidant Thermoresponsive Hydrogel Enhances Dermal Wound Healing in Diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable Block Copolymers as Injectable Drug-Delivery Systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Nakayama, Y.; Matsuda, T. In Vivo Evaluation of Poly(N-isopropylacrylamide) (PNIPAM)-grafted Gelatin as an In Situ-Formable Scaffold. J. Artif. Organs 2004, 7, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, S.H.; Park, K.D.; Jung, M.C.; Yang, W.I.; Han, S.W.; Noh, J.Y.; Lee, J.W. Chondrogenic Differentiation of Human Mesenchymal Stem Cells Using a Thermosensitive Poly(N-isopropylacrylamide) and Water-Soluble Chitosan Copolymer. Biomaterials 2004, 2, 5743–5751. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.; Ping, Q. Poly(N-isopropylacrylamide)–Chitosan as Thermosensitive In Situ Gel-Forming System for Ocular Drug Delivery. J. Control. Release 2007, 120, 186–194. [Google Scholar] [CrossRef] [PubMed]
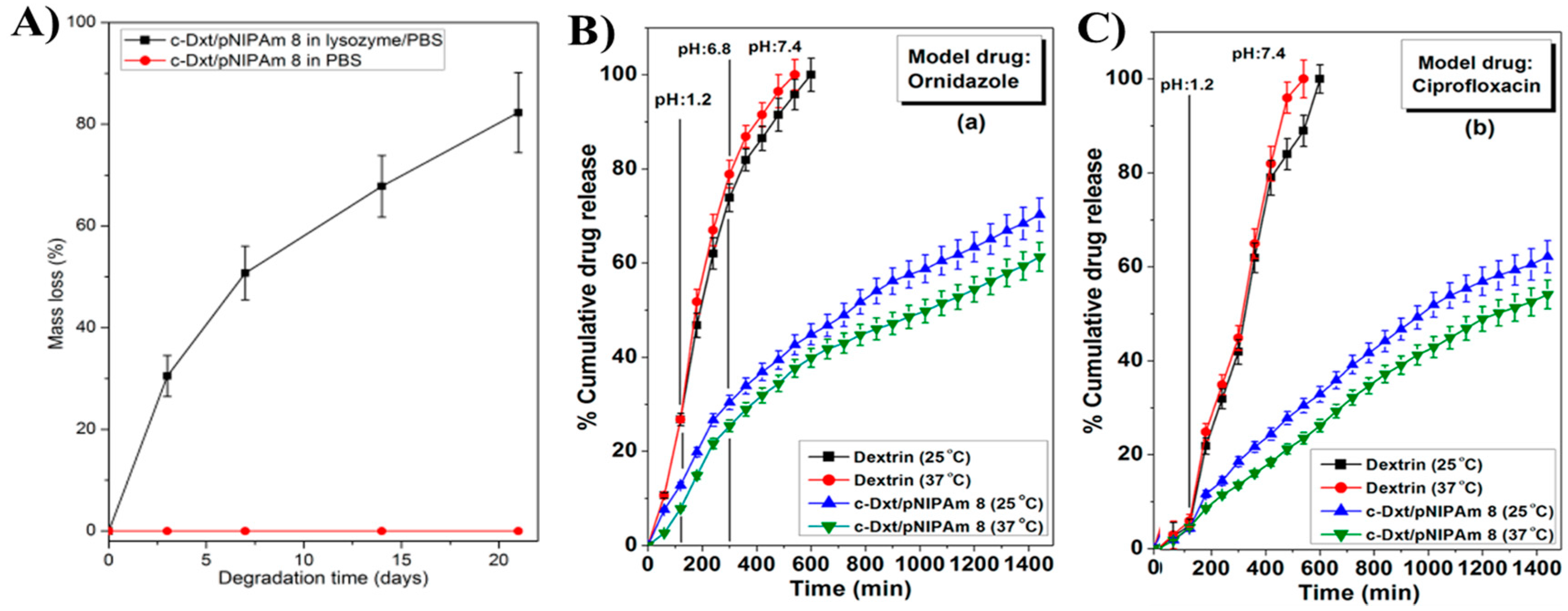
- Das, D.; Ghosh, P.; Ghosh, A.; Haldar, C.; Dhara, S.; Panda, A.B.; Pal, S. Stimulus-Responsive, Biodegradable, Biocompatible, Covalently Cross-Linked Hydrogel Based on Dextrin and Poly(N-isopropylacrylamide) for in Vitro/in Vivo Controlled Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 14338–14351. [Google Scholar] [CrossRef] [PubMed]
- Rzaev, Z.M.O.; Dinçer, S.; Pişkin, E. Functional Copolymers of N-isopropylacrylamide for Bioengineering Applications. Prog. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/“living” Radical Polymerization. Atom Transfer Radical Polymerization in the Presence of Transition-Metal Complexes. J. Am. Chem. Soc. 1995, 117, 5614. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris- (triphenylphosphine) ruthenium (II)/Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization. Macromolecules 1995, 28, 1721. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/Living Radical Polymerization: Features, Developments, and Perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the Design of Functional Materials for Biomedical Applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Kali, G.; Silva, T.B.; Sigg, S.J.; Seidi, F.; Renggli, K.; Bruns, N. ATRPases: Using nature’s catalysts in atom transfer radical polymerizations. ACS Symp. Ser. 2012, 1100, 171–181. [Google Scholar] [CrossRef]
- Sigg, S.J.; Seidi, F.; Renggli, K.; Silva, T.B.; Kali, G.; Bruns, N. Horseradish peroxidase as a catalyst for atom transfer radical polymerization. Macromol. Rapid Commun. 2011, 32, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- The Matyjaszewski Polymer Group. Available online: https://www.cmu.edu/maty/materials/Synthesis_of_well_defined_macromolecules/graft-copolymers.html#graft (accessed on 11 August 2017).
- Saldivar-Guerra, E.; Vivaldo-Lima, E. Handbook of Polymer Synthesis, Characterization, and Processing, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-0-470-63032-7. [Google Scholar]
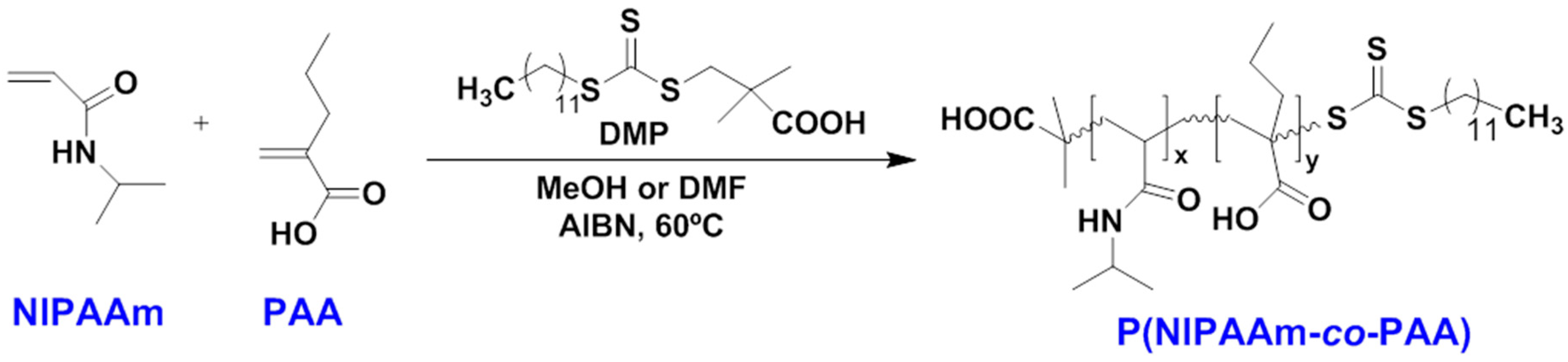
- Garbern, J.C.; Hoffman, A.S.; Stayton, P.S. Injectable pH- and Temperature-Responsive poly(N-isopropylacrylamide-co-propylacrylic acid) Copolymers for Delivery of Angiogenic Growth Factors. Biomacromolecules 2010, 11, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Gan, T.; Guan, Y.; Zhang, Y. Thermogelable PNIPAM Microgel Dispersion as 3D Cell Scaffold: Effect of Syneresis. J. Mater. Chem. 2010, 20, 5937–5944. [Google Scholar] [CrossRef]
- Lue, S.J.; Chen, B.W.; Shih, C.M.; Chou, F.Y.; Lai, J.Y.; Chiu, W.Y. Micron- and Nano-Sized Poly(N-isopropylacrylamide-co-acrylic acid) Latex Syntheses and their Applications for Controlled Drug Release. J. Nanosci. Nanotechnol. 2013, 13, 5305–5315. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Gonçalves, M.; Yi, P.; Capelo, D.; Zhang, Y.; Rodrigues, J.; Liu, C.; Tomás, H.; Li, Y.; He, P. Thermo/Redox/pH-Triple Sensitive Poly(N-isopropylacrylamide-co-acrylic acid) Nanogels for Anticancer Drug Deliver. J. Mater. Chem. B 2015, 3, 4221–4230. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogels: From Soft Contact Lenses and Implants to Self-Assembled Nanomaterials. J. Polym. Sci. A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.; Hobzova, R.; Pradny, M.; Duskova, M. Hydrogels Contact Lenses. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer Science & Business Media: New York, NY, USA, 2010; pp. 303–318. ISBN 978-1-4419-5919-5. [Google Scholar]
- Li, Z.; Fan, Z.; Xu, Y.; Lo, W.; Wang, X.; Niu, H.; Li, X.; Xie, X.; Khan, M.; Guan, J. pH-Sensitive and Thermosensitive Hydrogels as Stem-Cell Carriers for Cardiac Therapy. ACS Appl. Mater. Interfaces 2016, 8, 10752–10760. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Z.; Xu, Y.; Niu, H.; Xie, X.; Liu, Z.; Guan, J. Thermosensitive and Highly Flexible Hydrogels Capable of Stimulating Cardiac Differentiation of Cardiosphere-Derived Cells under Static and Dynamic Mechanical Training Condition. ACS Appl. Mater. Interfaces 2016, 8, 15948–15957. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsieh, Y.L. Preparation and Properties of Cellulose Nanocrystals: Rods, Spheres, and Network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cao, M.; Li, H.; Li, L.; Xu, W. Synthesis and Characterization of Thermo-Sensitive Semi-IPN Hydrogels Based on Poly(ethylene glycol)-co-poly(ε-caprolactone) Macromer, N-isopropylacrylamide, and Sodium Alginate. Carbohydr. Res. 2010, 345, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, R.P.; Mitchell, G.R.; Vasile, C. Multi-Responsive Hydrogels Based on N-isopropylacrylamide and Sodium Alginate. Polym. Int. 2011, 60, 222–233. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Sun, H.; Xing, D.; Yao, F. Nano-Hydroxyapatite Formation Via Co-Precipitation with Chitosan-g-poly(N-isopropylacrylamide) in Coil and Globule States for Tissue Engineering Application. Front. Chem. Sci. Eng. 2013, 7, 388–400. [Google Scholar] [CrossRef]
- Kajjari, P.B.; Manjeshwar, L.S.; Aminabhavi, T.M. Novel pH- and Temperature-Responsive Blend Hydrogel Microspheres of Sodium Alginate and PNIPAAm-g-GG for Controlled Release of Isoniazid. AAPS PharmSciTech 2012, 13, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Song, W.; Blanco-Fernandez, B.; Alvarez-Lorenzo, C.; Mano, J.F. Synthesis of Temperature-Responsive Dextran-MA/PNIPAAm Particles for Controlled Drug Delivery Using Superhydrophobic Surfaces. Pharm. Res. 2011, 28, 1294–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Madrigal, M.M.; del Valle, L.J.; Armelin, E.; Michaux, C.; Roussel, G.; Perpète, E.A.; Alemán, C. Polypyrrole-Supported Membrane Proteins for Bio-Inspired Ion Channels. ACS Appl. Mater. Interfaces 2015, 7, 1632–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puiggalí-Jou, A.; Pérez-Madrigal, M.M.; del Valle, L.J.; Armelin, E.; Casas, M.T.; Michaux, C.; Perpète, E.A.; Estrany, F.; Alemán, C. Confinement of a β-Barrel Protein in Nanoperforated Free-Standing Nanomembranes for Ion Transport. Nanoscale 2016, 8, 16922–16935. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Dorbatt, V.; Tolstyka, Z.P.; Maynard, H.D. Synthesis of Aminooxy End-Functionalized pNIPAAm by RAFT Polymerization for Protein and Polysaccharide Conjugation. Macromolecules 2009, 42, 7650–7656. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Nguyen, T.H.; Maynard, H.D. Thermoprecipitation of Glutathione S-Transferase by Glutathione-Poly-(N-Isopropylacrylamide) Prepared by RAFT Polymerization. Macromol. Rapid Commun. 2010, 31, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.M.; Prabhakaran, P.; Rome, L.H.; Maynard, H.D. Smart Vaults: Thermally-Responsive Protein Nanocapsules. ACS Nano 2013, 7, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Wang, S.T. Poly(N-isopropylacrylamide)-Based Thermo-Responsive Surfaces with Controllable Cell Adhesion. Sci. China Chem. 2014, 57, 552–557. [Google Scholar] [CrossRef]
- Cross, M.C.; Toomey, R.G.; Gallant, N.D. Protein-Surface Interactions on Stimuli-Responsive Polymeric Biomaterials. Biomed. Mater. 2016, 11, 022002. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Khan, J.; Saraf, S.; Saraf, S. Polyethylene Glycol (PEG)–Poly(N-isopropylacrylamide) (PNIPAAm) Based Thermosensitive Injectable Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Teotia, A.K.; Sami, H.; Kumar, A. Thermo-Responsive Polymers: Structure and Design of Smart Materials. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–43. ISBN 9780857097170. [Google Scholar]
- Zhu, Y.; Jiang, H.; Ye, S.; Yoshizumi, T.; Wagner, W.R. Tailoring the degradation rates of thermally responsive hydrogels designed for soft tissue injection by varying the autocatalytic potential. Biomaterials 2015, 53, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Okano, T. Temperature-responsive polymers for cell culture and tissue engineering applications. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 203–233. ISBN 9780857097170. [Google Scholar]
- Smink, A.M.; de Haan, B.J.; Paredes-Juarez, G.A.; Wolters, A.H.; Kuipers, J.; Giepmans, B.N.; Schwab, L.; Engelse, M.A.; van Apeldoorn, A.A.; de Koning, E.; et al. Selection of polymers for application in scaffolds applicable for human pancreatic islet transplantation. Biomed. Mater. 2016, 11, 035006. [Google Scholar] [CrossRef] [PubMed]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Thermoresponsive Surfaces for Cell Culture and Enzyme-Free Cell Detachment. Prog. Polym. Sci. 2010, 35, 1311–1324. [Google Scholar] [CrossRef]
- Hernández, R.; Sacristán, J.; Asín, L.; Torres, T.E.; Ibarra, M.R.; Goya, G.F.; Mijangos, C. Magnetic Hydrogels Derived from Polysaccharides with Improved Specific Power Absorption: Potential Devices for Remotely Triggered Drug Delivery. J. Phys. Chem. B 2010, 114, 12002–12007. [Google Scholar] [CrossRef] [PubMed]
- Sanz, B.; von Bilderling, C.; Tuninetti, J.S.; Pietrasanta, L.; Mijangos, C.; Longo, G.S.; Azzaroni, O.; Giussi, J.M. Thermally-Induced Softening of PNIPAm-Based Nanopillar Arrays. Soft Matter 2017, 13, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.M.; Yeong, W.Y. Smart Hydro-Gels for 3D Bioprinting. Int. J. Bioprint. 2015, 1, 3–14. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Chua, C.K.; Yeong, W.Y. Bioprinting: Principles and Applications; World Scientific Publishing Company Incorporated: Singapore, 2015; ISBN 978-9814612104. [Google Scholar]
- Yu, Q.; Ista, L.K.; López, G.P. Nanopatterned Antimicrobial Enzymatic Surfaces Combining Biocidal and Fouling Release Properties. Nanoscale 2014, 6, 4750–4757. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, X.; Fangteng, J.; Wang, S.; Fang, L.; Shen, H.; Xiang, S.; Sun, H.; Yang, B. Bioinspired Polyethylene Terephthalate Nanocone Arrays with Underwater Superoleophobicity and Anti-Bioadhesion Properties. Nanoscale 2014, 6, 13845–13853. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chua, K.C.; Mironov, V. A perspective on 4D bioprinting. Int. J. Bioprint. 2016, 2, 3–5. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Lequieu, W.; Du Prez, F.E. Segmented polymer networks based on poly(N-isopropyl acrylamide) and poly(tetrahydrofuran) as polymer membranes with thermo-responsive permeability. Polymer 2004, 45, 749–757. [Google Scholar] [CrossRef]
- Kali, G.; Vavra, S.; László, K.; Iván, B. Thermally Responsive Amphiphilic Conetworks and Gels Based on Poly(N-isopropylacrylamide) and Polyisobutylene. Macromolecules 2013, 46, 5337–5344. [Google Scholar] [CrossRef]





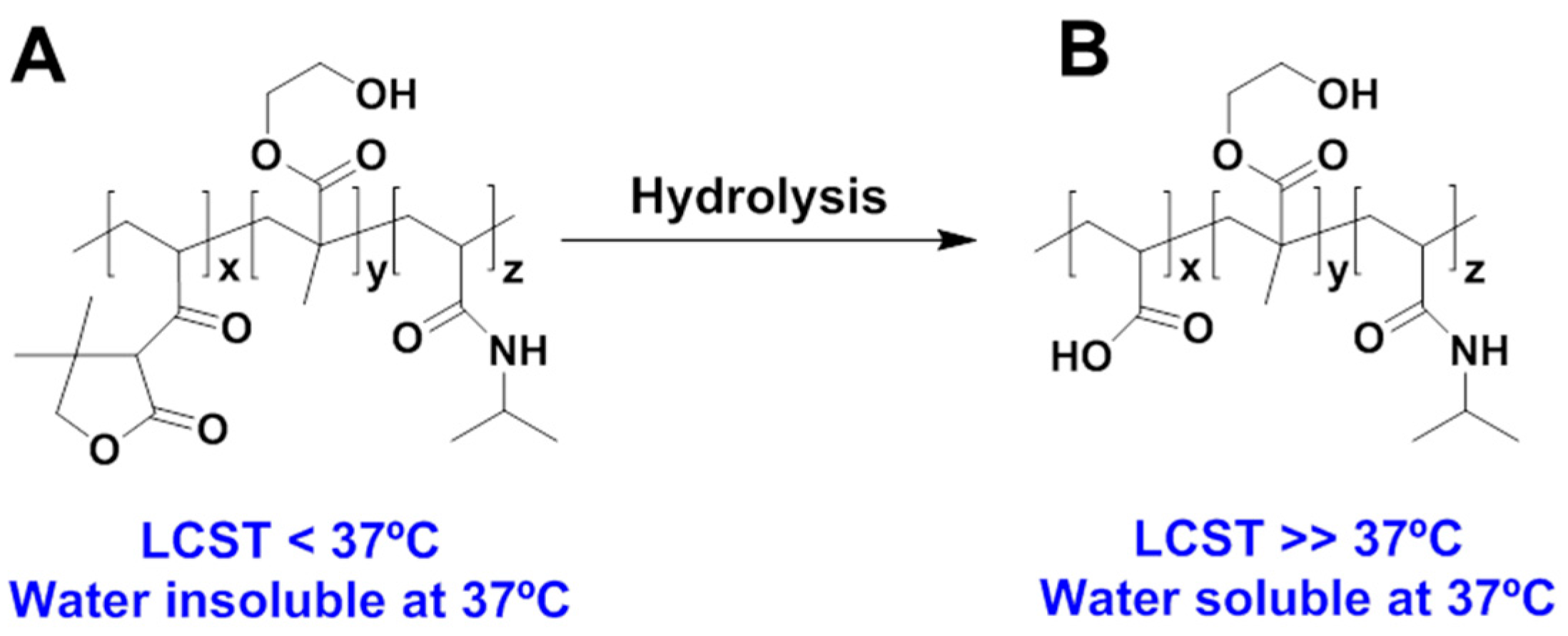
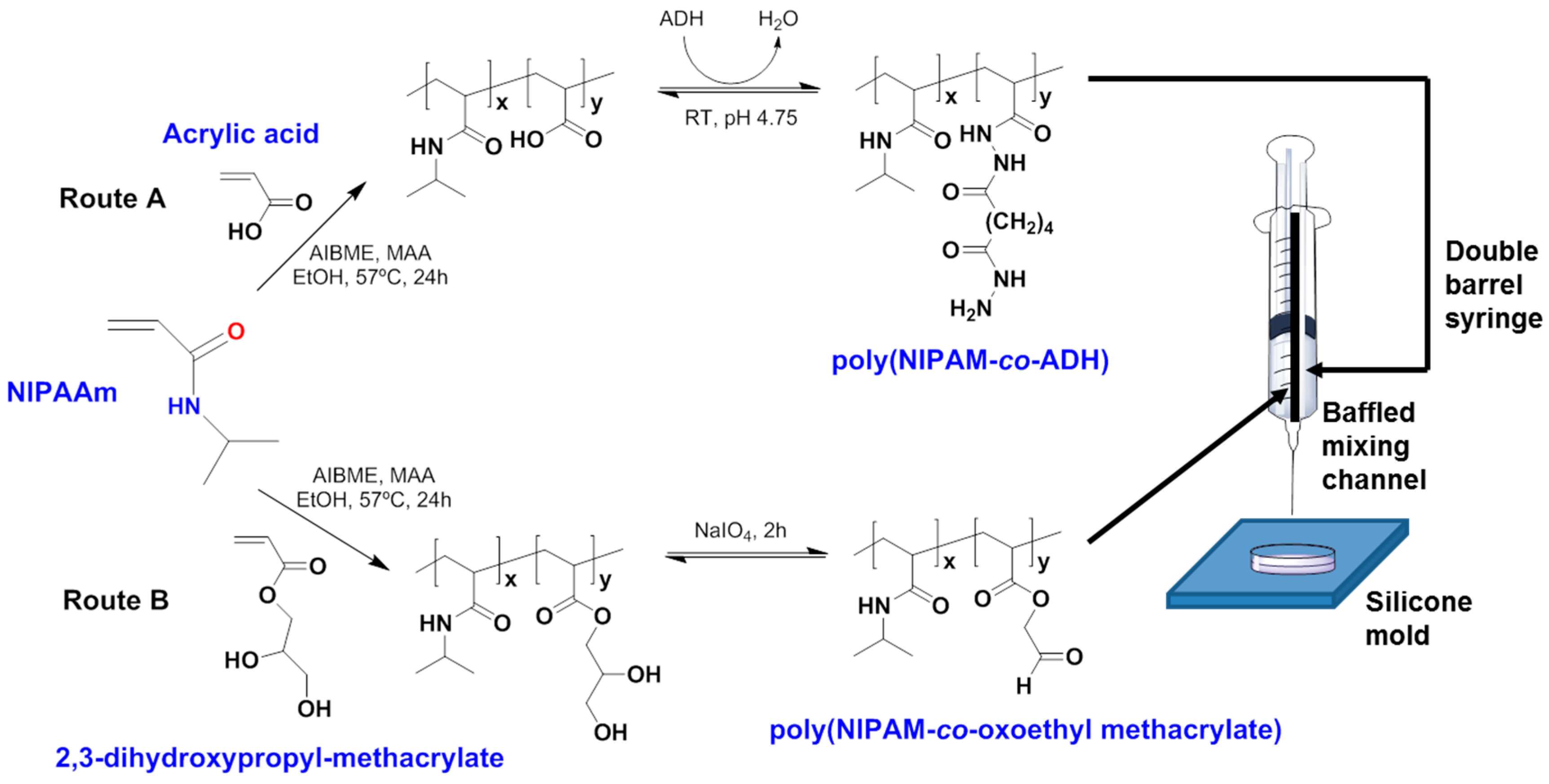

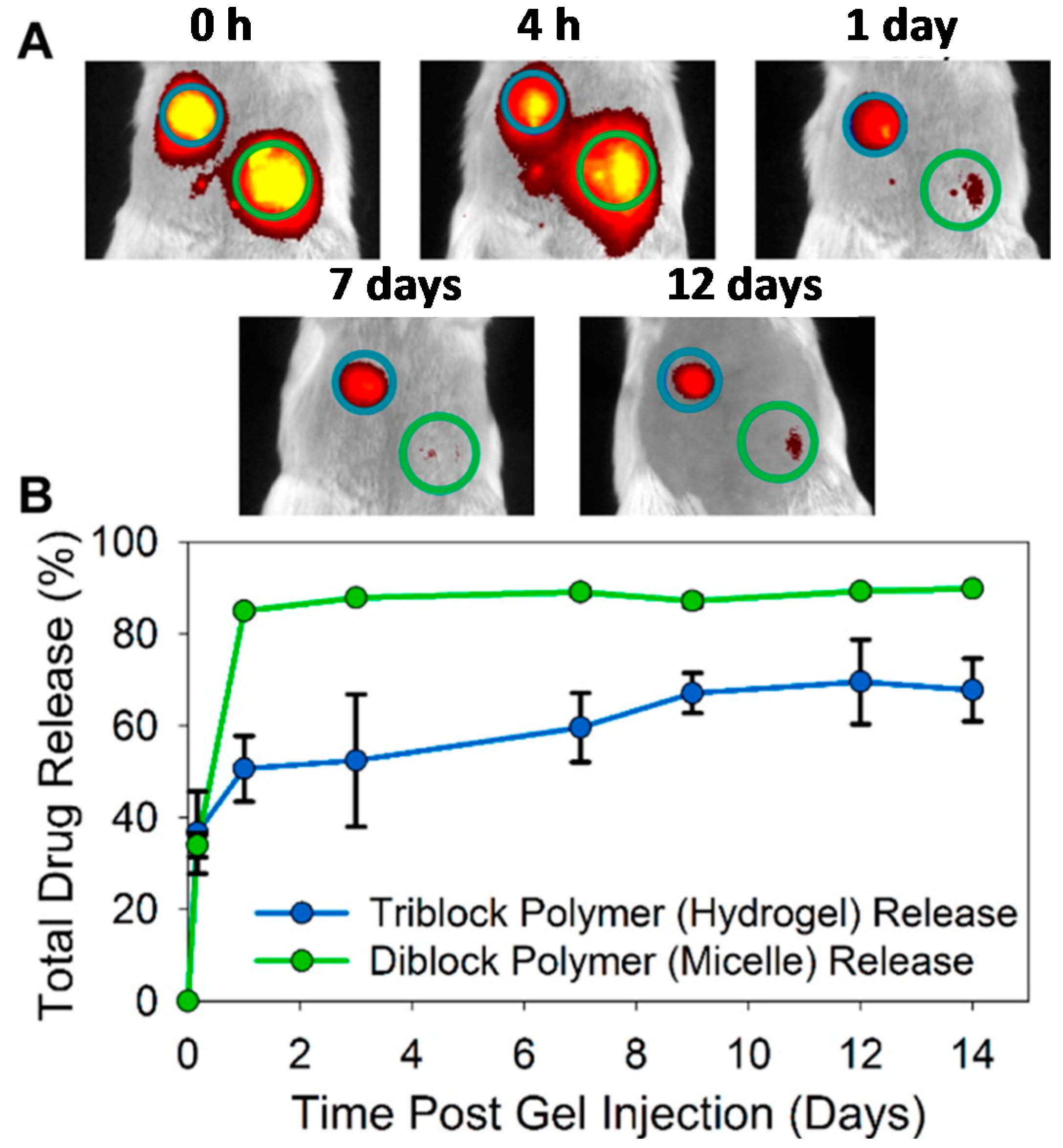


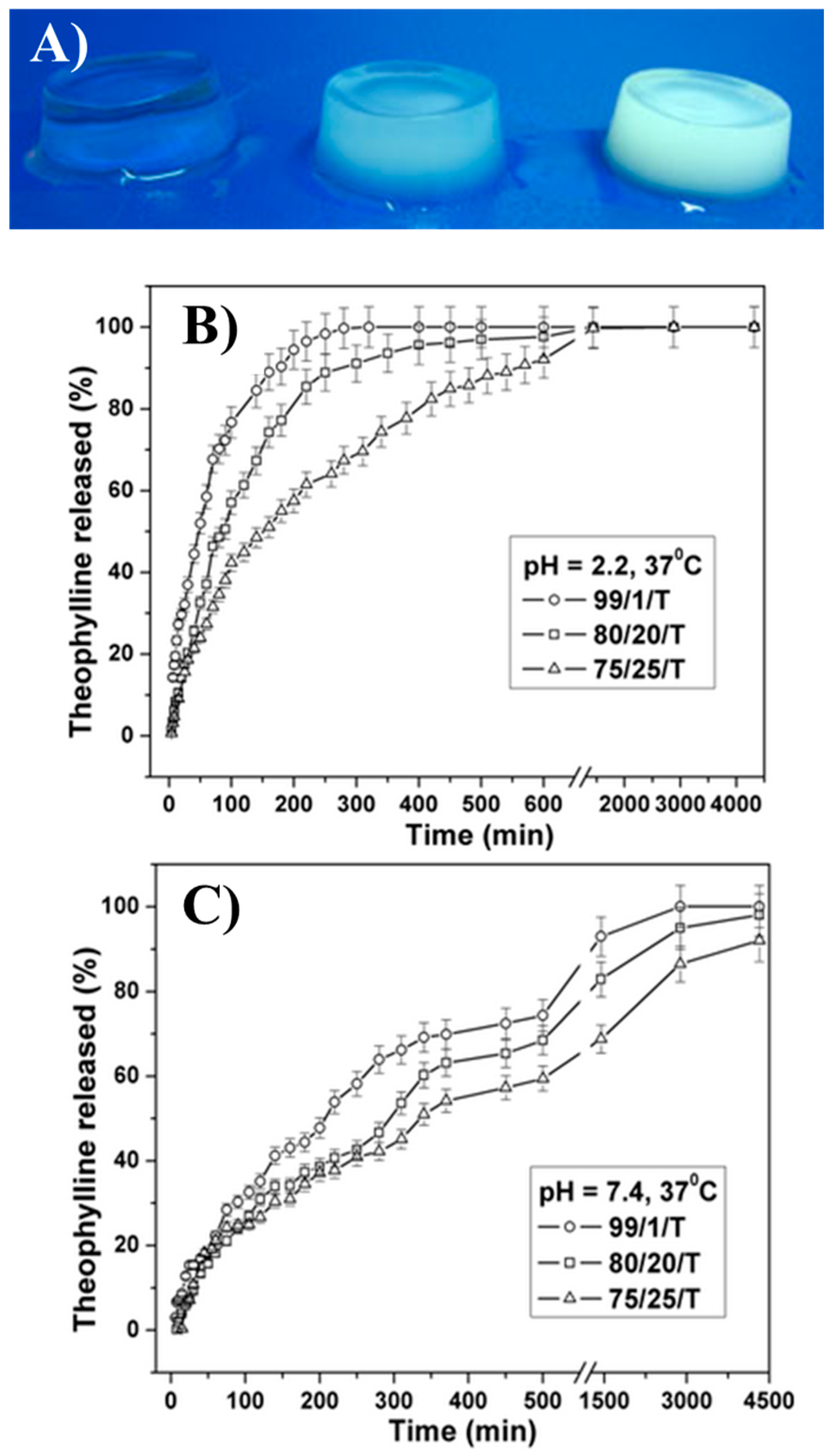
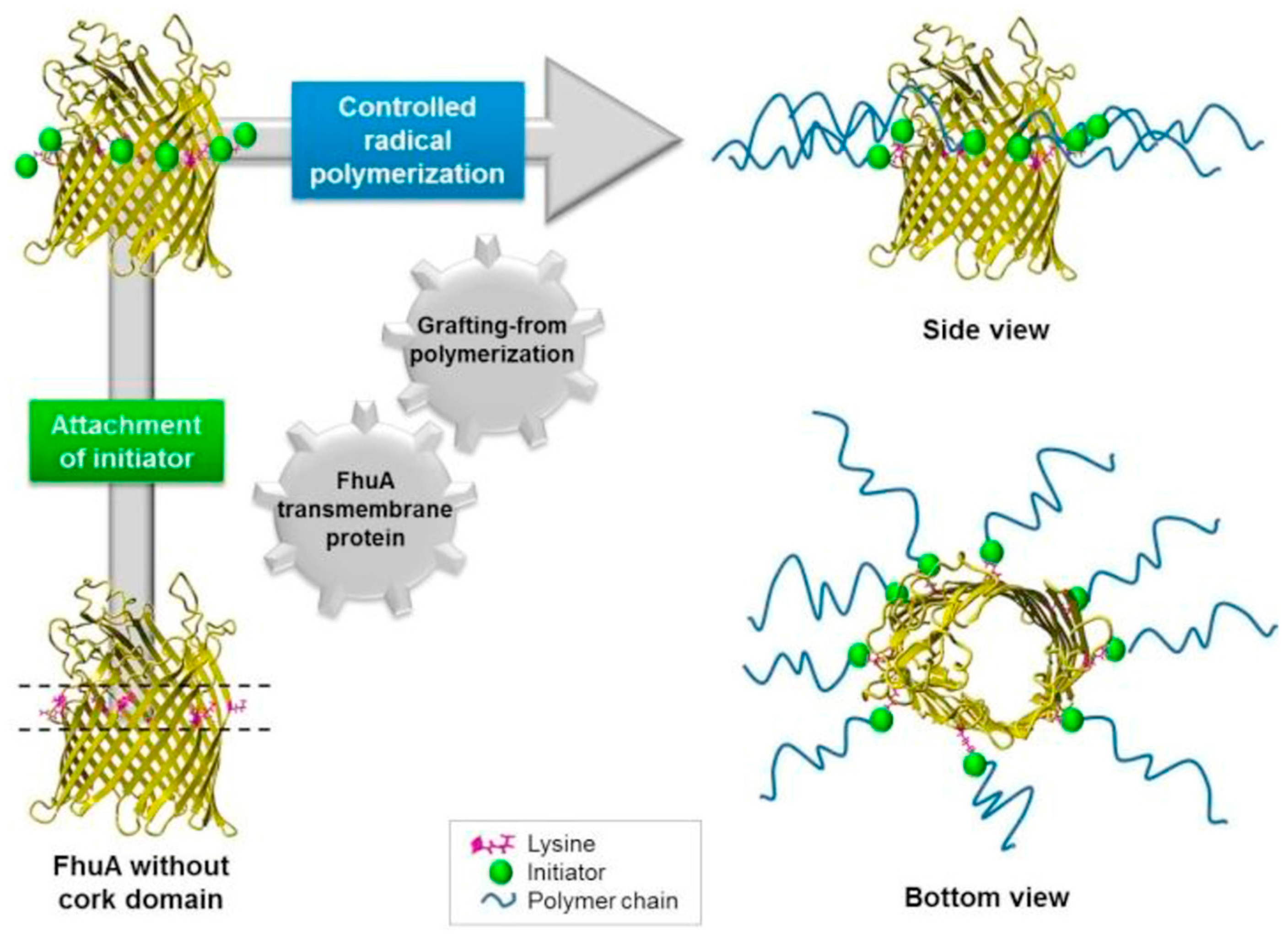

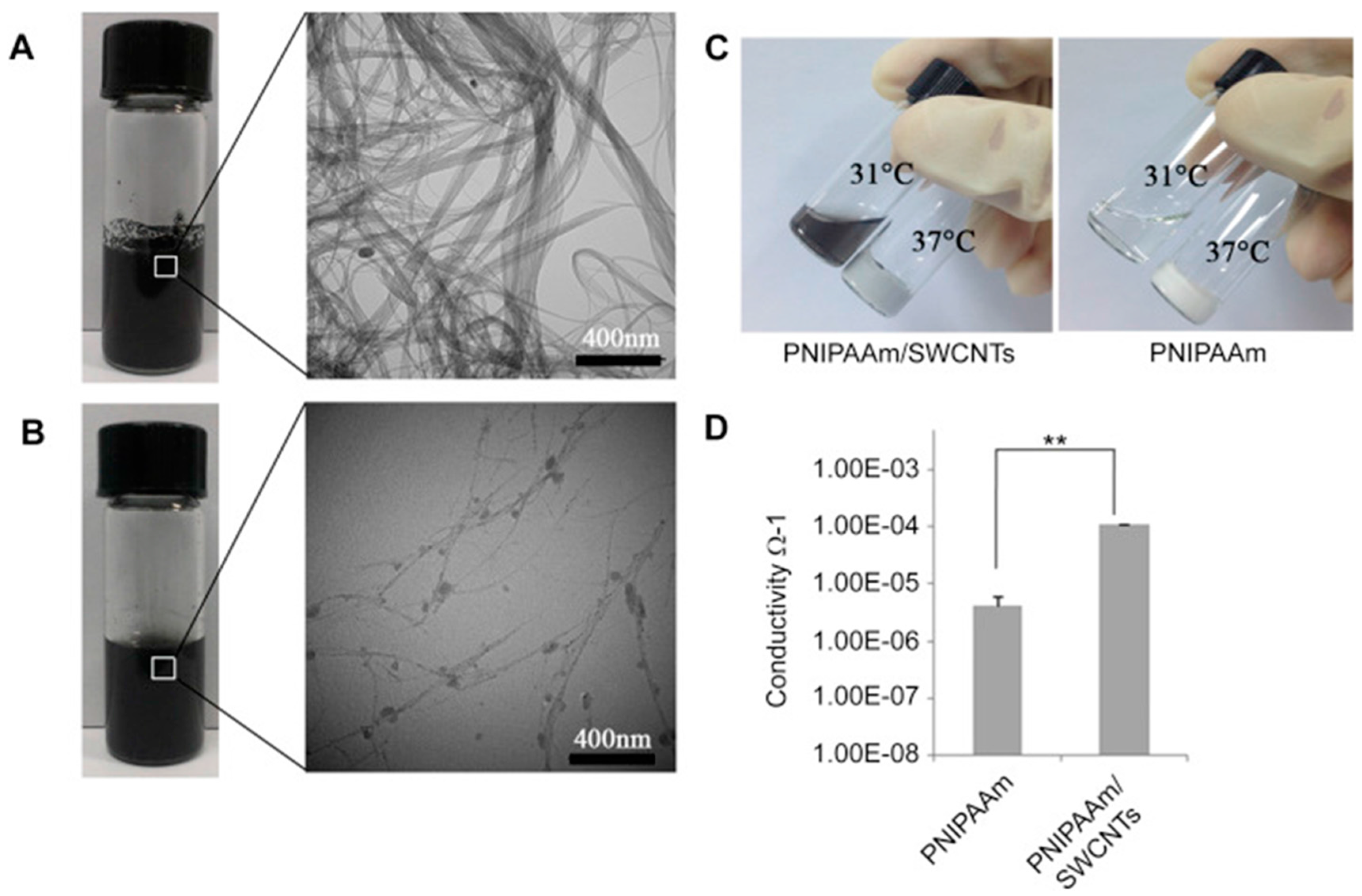
| Authors (Year) | Bioapplications (Bio-Area) * | Scientific Innovation | Improvements in the Biomedical Field |
|---|---|---|---|
| Satarkar et al. (2008) [29] | Remote controlled (RC) drug delivery (D.D.) | High-frequency alternating magnetic field (AMF) to trigger the on-demand pulsatile drug release from nanocomposites synthesized by incorporation of superparamagnetic Fe3O4 particles in PNIPAAm gels | Application of AMF resulted in uniform heating within the nanocomposites, leading to accelerated collapse and squeezing out large amounts of imbibed drug (release at a faster rate) |
| Mizutani et al. (2008) [30] | Tissue engineering for endothelial cells (T.E.) | ATRP of PNIPAAm brushes and their influence on the adhesion and the detachment of bovine carotid artery endothelial cells (ECs) | Improvement of surface hydrophilicity, presence of more extended chain conformations with relatively high chain mobility and chain hydration |
| Klaikherd et al. (2009) [31] | Tuning and control of drug delivery (D.D.) | Novel triple stimuli sensitive block assembly that responds to changes in temperature, pH and redox potential | Fine-tuning of the guest molecule release kinetics and possibility of achieving location-specific delivery |
| Tan et al. (2009) [32] | Injectable hydrogel for adipose tissue engineering (T.E./S.C.) | Synthesis of copolymer composed by hyaluronic acid and PNIPAAm (AHA-g-PNIPAAm) | Encapsulation of human adipose-derived stem cells (ASCs) within hydrogels showed the AHA-g-PNIPAAm copolymers were non-cytotoxic and preserved the viability of the entrapped cells |
| Fujimoto et al. (2009) [33] | Injectable hydrogel for ischemic cardiomyopathy (T.E.) | Biodegradable, thermo-responsive hydrogel based on copolymerization of NIPAAm, acrylic acid (AA) and hydroxyethyl methacrylate-poly(trimethylene carbonate) (HEMAPTMC) | Injection of the material prevented ventricular dilation and improved contractile function in a chronic rat infarction model |
| Chen et al. (2009) [34] | Blood-compatible materials (T.E.) | Surface-initiated ATRP for PNIPAAm grafting from silicon nanowire arrays | Largely reduced platelet adhesion in vitro, providing a new strategy for fabricating blood-compatible materials |
| Purushotham et al. (2009) [35] | Anticancer therapy (D.D.) | γ-Fe2O3 iron oxide magnetic nanoparticles (MNP) coated with PNIPAAm and loaded with anti-cancer drug (doxorubicin-(dox)) | Magnetic drug targeting followed by simultaneous hyperthermia and drug release |
| Yoshida (2010) [36] | Biomimetic actuators (B.S.) | Self-oscillating gels driven by the Belousov-Zhabotinsky reaction | Cyclic soluble–insoluble changes or swelling–deswelling changes without any on–off switching of external stimuli |
| Wu et al. (2010) [37] | Cancer cell imaging (D.D./B.I.) | Core-shell structured hybrid nanogels composed of Ag nanoparticle (NP) as core and PNIPAAm-co-acrylic acid gel as shell | Long circulation and specific accumulation on cells (for use as smart dosing of the pathological zones) |
| Stoychev et al. (2011) [38] | Yeast cells release (D.D.) | Star-like patterned polycaprolactone-PNIPAAm bilayers like proof of principle for thermo-responsive self-folding capsules | Reversibly encapsulate/release yeast cells in response to temperature signal |
| Lin et al. (2012) [39] | Cell sheets (S.C.) | Microtextured PNIPAAm-poly(dimethylsiloxane) (PDMS) synthesized by a method suitable for generating aligned vascular smooth muscle cell (VSMC) sheets | Inexpensive, biocompatible, oxygen permeable, and easily microtextured thermo-responsive substrate for producing cell sheets |
| Dai et al. (2012) [40] | In vivo bioimaging and cancer therapy (D.D./B.I.) | Microspheres of NaYF4:Yb3+/Er3+ coated with PNIPAAm-co-(methacrylic acid)] polymer used as carrier for the anticancer drug | Luminescent bioprobes that rapidly release the anticancer drug (doxorubicin hydrochloride, DOX) |
| Zhu et al. (2012) [41] | Nanogels as microfluidic devices (M.F.D.) | Photothermally sensitive PNIPAAm/graphene oxide (PNIPAAm/GO) nanocomposite synthesized by γ-irradiation | Nanocomposite phase transition is completely reversible via laser exposure or non-exposure |
| Yang et al. (2013) [42] | Nanocarriers for RC drug release (D.D.) | Near-infrared (NIR)-stimulus controlled drug release system based on Au-nanocage@mSiO2@PNIPAAm core–shell nanocarrier | Synergistic chemo-photothermal therapy effect that significantly enhances the cancer cell killing efficacy |
| Li et al. (2014) [43] | Stem cell transplantation in myocardial repair (S.C.) | A thermo-sensitive single-wall carbon nanotubes (SWCNTs)-modified PNIPAAm hydrogel (PNIPAAm/SWCNTs) | Enhancement of the engraftment of seeding cells in infarct myocardium |
| Gupta et al. (2014) [22] | Cyto-protective hydrogel for cell encapsulation (D.D.) | ABC triblock polymer poly-[(propylenesulfide)-block-(N,N-dimethylacrylamide)-block-(PNIPAAm)](PPS-b-PDMA-b-PNIPAAm) | Good syneresis, lack of degradability, and lack of inherent drug loading and environmentally responsive release mechanisms |
| Cui et al. (2014) [44] | Injectable hydrogels for cardiac therapy (T.E./S.C.) | Hydrogel composed by PNIPAAm and electroactive tetraaniline (TA) followed by the addition of 2-methylene-1,3-dioxepane (MDO) | 2-Methylene-1,3-dioxepane (MDO) and tetraaniline improves biodegradability, electrical properties, and antioxidant activities |
| Li et al. (2015) [45] | Self-healing hydrogel (T.E.) | Mussel-inspired tri-block copolymer PNIPAAm-co-(N-3,4-dihydroxyphenethyl acrylamide)]-b-poly(ethylene oxide) | Automatic healing from repeated structural damage and effective prevention of non-specific cell attachment and biofilm formation |
| Bakarich et al. (2015) [46] | Thermally actuating hydrogel for smart valves (T.E./B.S.) | 4D Printing of hydrogels made by interpenetrating network of alginate and PNIPAAm | Mechanically robust and thermally actuating 4D printed smart valve |
| Kesti et al. (2015) [47] | Bioink for articular cartilage (T.E.) | Blending of PNIPAAm grafted hyaluronan (HA-PNIPAAm) with methacrylated hyaluronan (HAMA) | High-resolution scaffolds with good viability printed layer-by-layer |
| Psarra et al. (2015) [48] | Protein adsorption and cell adhesion (T.E.) | Nanostructures of PNIPAAm (homo) and PNIPAAm-co-acrylic acid (binary) by atom transfer radical polymerization (ATRP) and investigation of the fibrinogen (FGN) adsorption responsiveness | Terminal hydrophobic moieties improved wettability, lower critical solution temperature (LCST), and morphology of both brush systems with consequent alteration of FGN adsorption |
| Lima et al. (2016) [49] | Ocular biocompatibility (T.E.) | Study of the safety of intravitreal injections of poly-N-isopropylacrylamide (PNIPAAm) tissue adhesive in rabbit eyes | Intravitreal injections of PNIPAAm were nontoxic in this animal study |
| Li et al. (2016) [50] | Stem-cell carriers for cardiac therapy (S.C.) | Free-radical polymerization of NIPAAm, propylacrylic acid, hydroxyethyl methacrylate-co-oligo(trimethylene carbonate), and methacrylate poly(ethylene oxide) methoxy ester | Innovative hydrogels that quickly solidify at the pH of an infarcted heart but cannot solidify at the pH of blood injectable through catheters, commonly used for minimally invasive surgeries |
| Zhao et al. (2017) [51] | Cell-inspired biointerface for use in immunoassays in blood (T.E./B.S.) | Biointerfaces constructed by patterning smart hydrogels poly(N-isopropylacrylamide-co-sodium acrylate) (PNIPAAm-co-PNaAc) on hydrophilic layers (poly(ethylene glycol), PEG), followed by immobilization of antibodies on the patterned hydrogels | Versatile and effective biointerfaces for antibody–antigen recognition, which offers a potential new approach for developing highly sensitive immunoassays in blood |
| Zubik et al. (2017) [52] | Wound dressing (T.E.) | PNIPAAm reinforced with cellulose nanocrystals (CNCs); for wound dressing purposes, metronidazole was used as a target drug | Injectable hydrogels as promising materials for wound dressing |
| Liu et al. (2018) [53] | Photosensitizer for cancer treatment (D.D.) | A novel comb-shaped porphyrin end-functionalized poly(NIPAAm)-b-poly[oligo (ethylene glycol methyl ether methacrylate)] | Photo-toxicity toward HeLa cancer cells and local accumulation on tumor tissues: photosensitizer in photodynamic anticancer therapy |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. https://doi.org/10.3390/gels3040036
Lanzalaco S, Armelin E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels. 2017; 3(4):36. https://doi.org/10.3390/gels3040036
Chicago/Turabian StyleLanzalaco, Sonia, and Elaine Armelin. 2017. "Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications" Gels 3, no. 4: 36. https://doi.org/10.3390/gels3040036