Synthesis of Helical Phenolic Resin Bundles through a Sol-Gel Transcription Method
Abstract
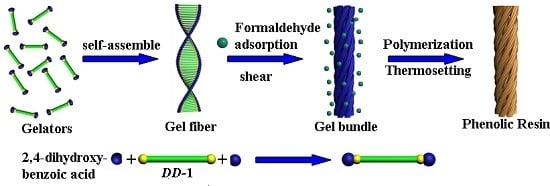
:1. Introduction
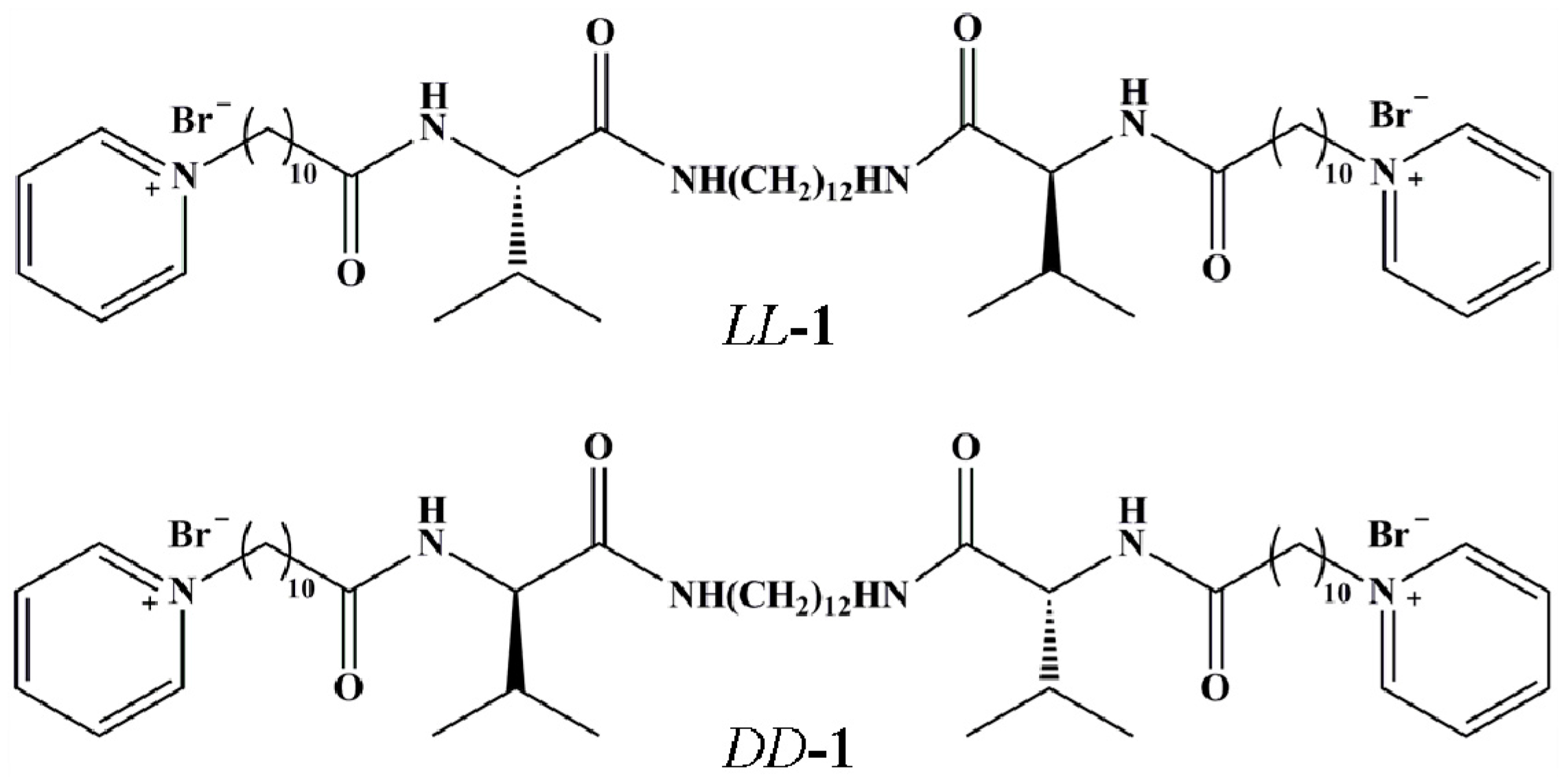
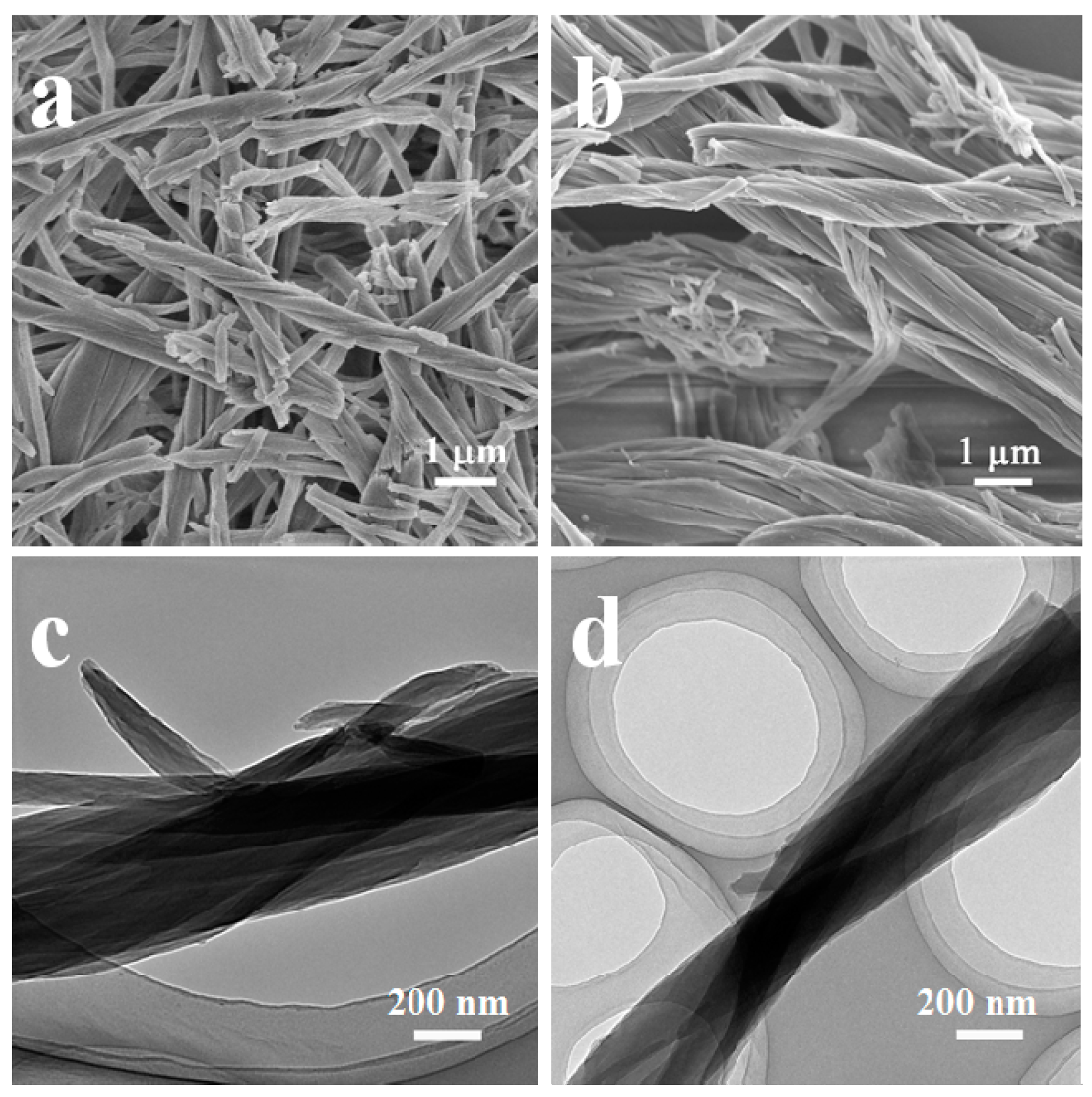
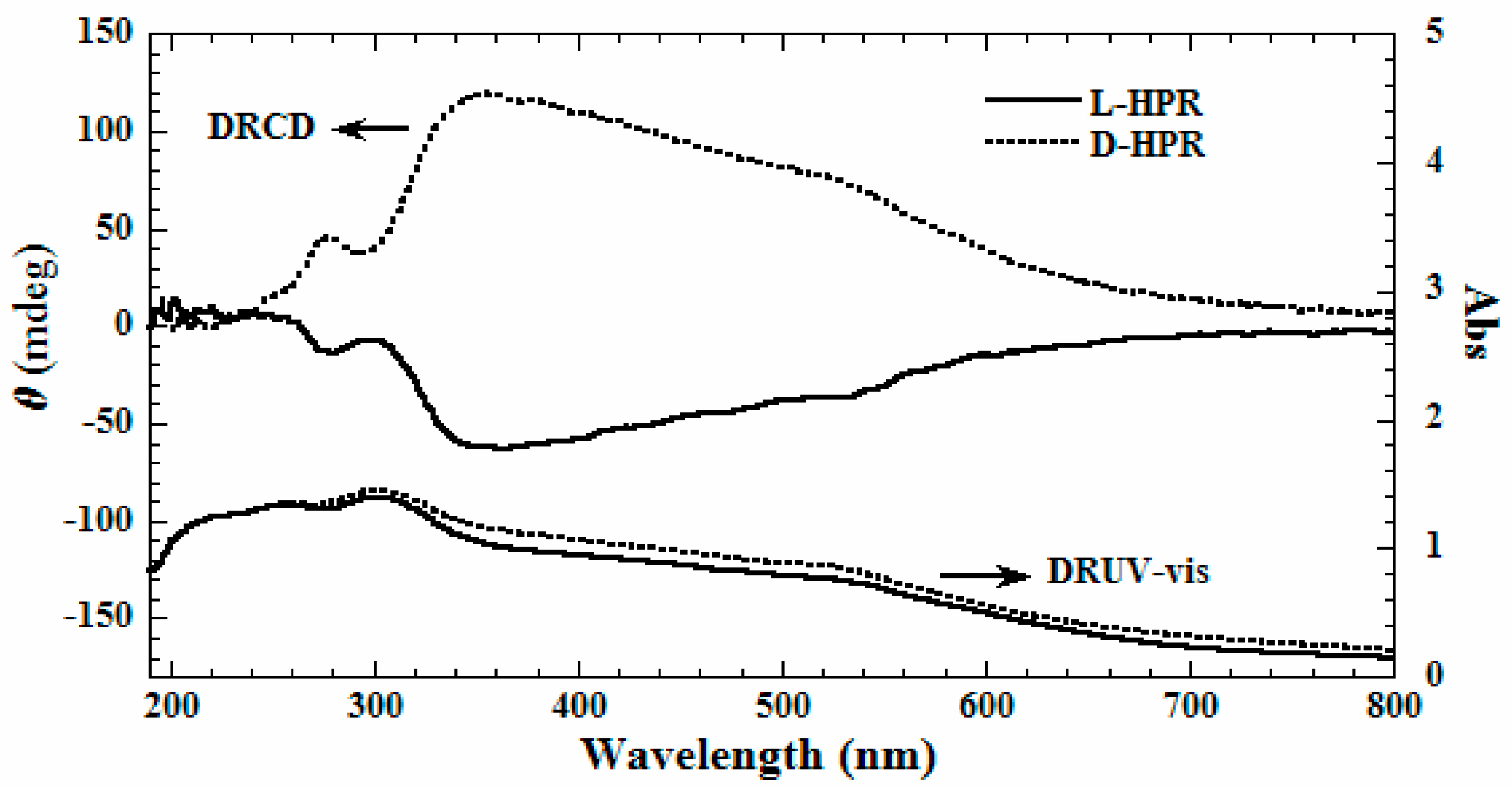
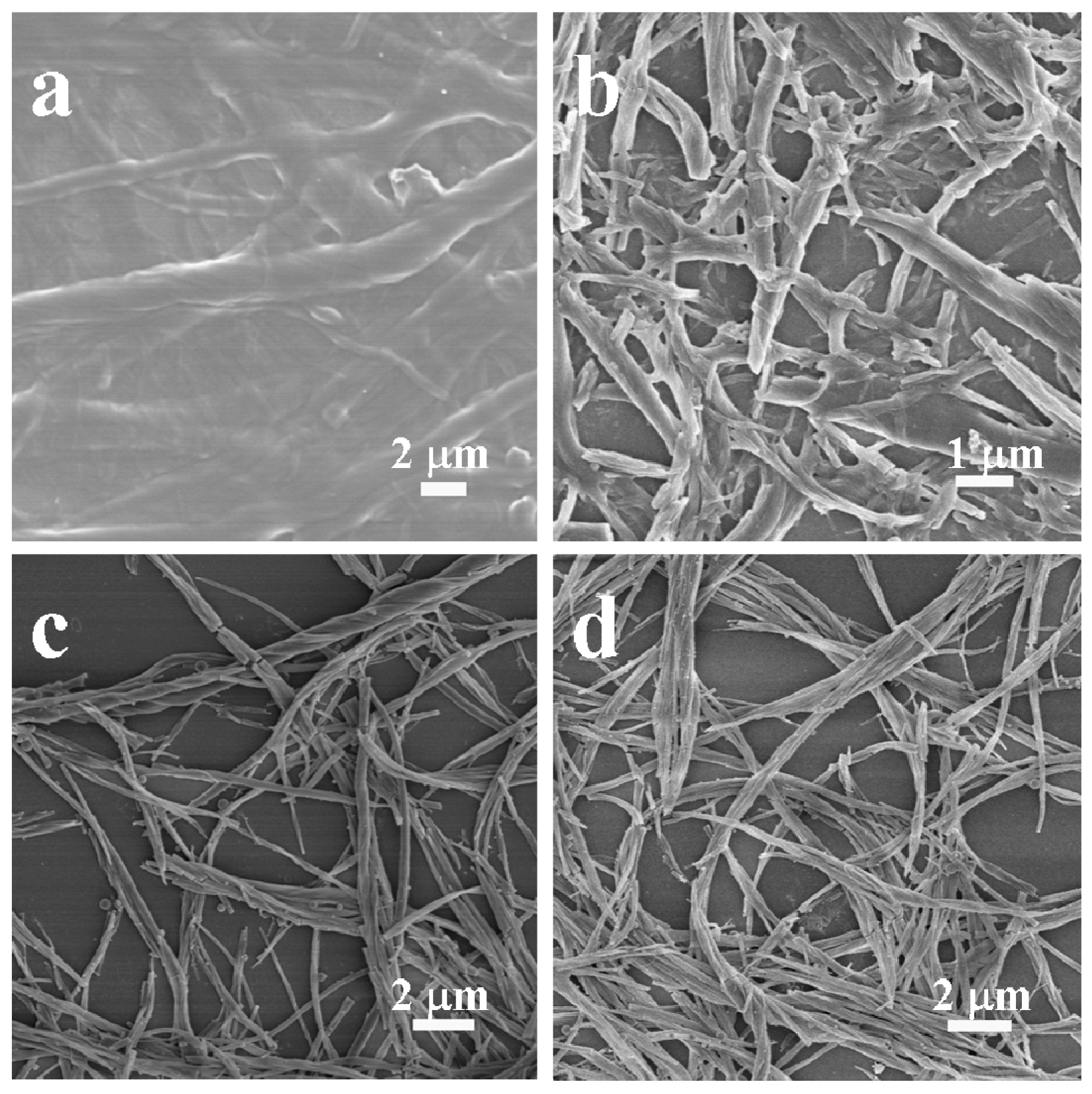
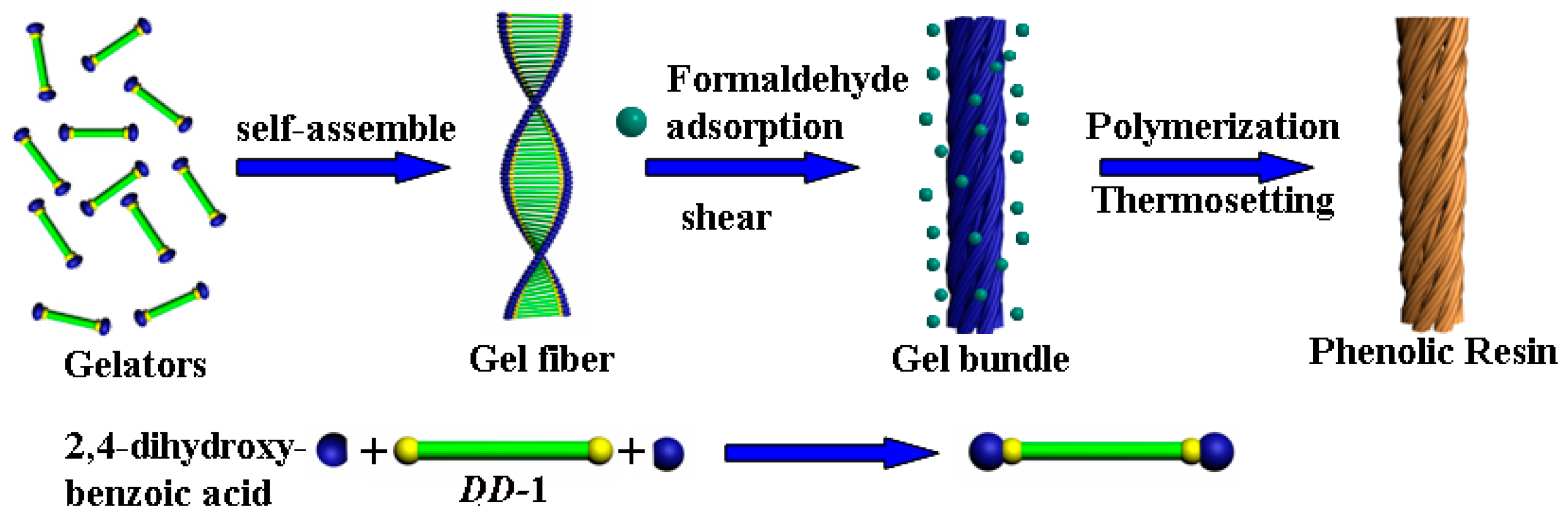
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Methods
4.2. Materials
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dong, J.; Liu, Y.; Cui, Y. Chiral porous organic frameworks for asymmetric heterogeneous catalysis and gas chromatographic separation. Chem. Commun. 2014, 50, 14949–14952. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, J.; Tao, Q.; Jiang, L.; Lu, T. Enantioselective recognition and separation of racemic 1-phenylethanol by a pair of 2D chiral coordination polymers. Inorg. Chem. 2013, 52, 11694–11696. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Basu, K.; Benoit, C.; Cirtiu, C.; Vali, H.; Moores, A. Cellulose nanocrystals as chiral inducers: Enantioselective catalysis and transmission electron microscopy 3D characterization. J. Am. Chem. Soc. 2015, 137, 6124–6127. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Li, Y.; Yuan, Y.; Lin, S.; Li, B.; Wang, M.; Yang, Y. A single-step approach for fabrication of vancomycin-bonded silica monolith as chiral stationary phase. Chem. Asian J. 2014, 9, 2866–2871. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Guo, Y.; Li, B.; Li, Y. Preparation of Single-Handed Helical Carbon/Silica and Carbonaceous Nanotubes by Using 4,4′-Biphenylene-Bridged Polybissilsesquioxane. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 1149–1154. [Google Scholar] [CrossRef]
- Moshe, H.; Levi, G.; Sharon, D.; Mastai, Y. Atomic layer deposition of enantioselective thin film of alumina on chiral self-assembled-monolayer. Surf. Sci. 2014, 629, 88–93. [Google Scholar] [CrossRef]
- Okazaki, Y.; Cheng, J.; Dedovets, D.; Kemper, G.; Delville, M.; Durrieu, M.; Ihara, H.; Takafuji, M.; Pouget, E.; Oda, R. Chiral Colloids: Homogeneous Suspension of Individualized SiO2 Helical and Twisted Nanoribbons. ACS Nano 2014, 8, 6863–6872. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, K.; Yamada, M.; Kimura, M.; Shirai, H. Prominent Gelation and Chiral Aggregation of Alkylamides Derived from trans-1,2-Diaminocyclohexane. Angew. Chem. Int. Ed. 1996, 35, 1949–1951. [Google Scholar] [CrossRef]
- Huang, Z.; Yao, Y.; Han, L.; Che, S. Control of Chiral Nanostructures by Self-Assembly of Designed Amphiphilic Peptides and Silica Biomineralization. Chem. Eur. J. 2014, 20, 17068–17076. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Che, S. Formation mechanism of achiral amphiphile-templated helical mesoporous silicas. J. Phys. Chem. B 2008, 112, 10466–10474. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Crudden, C. Chiral hybrid mesoporous silicas: Assembly of uniform hollow nanospheres and helical nanotubes with tunable diameters. Chem. Mater. 2012, 24, 3839–3846. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Q.; Tian, G.; Wei, F. Nanoscale Emerging double helical nanostructures. Nanoscale 2014, 6, 9339–9354. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gu, D.; Zou, Y.; Wu, Z.; Li, F.; Che, R.; Deng, Y.; Tu, B.; Zhao, D. A Low-Concentration Hydrothermal Synthesis of Biocompatible Ordered Mesoporous Carbon Nanospheres with Tunable and Uniform Size. Angew. Chem. Int. Ed. 2010, 49, 7987–7991. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, Y.; Guo, Q.; Shi, J.; Liu, L. Tuning the pore size and structure of mesoporous carbons synthesized using an evaporation-induced self-assembly method. Mater. Lett. 2011, 65, 2130–2132. [Google Scholar] [CrossRef]
- Li, J.; Qi, J.; Liu, C.; Zhou, L.; Song, H.; Yu, C.; Shen, J.; Sun, X.; Wang, L. A Fabrication of ordered mesoporous carbon hollow fiber membranes via a confined soft templating approach. J. Mater. Chem. A 2014, 2, 4144–4149. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Li, Y.; Li, B.; Zhang, C.; Yang, Y. Preparation of single-handed helical carbonaceous nanotubes using 3-aminophenol-formaldehyde resin. RSC Adv. 2015, 5, 39946–39951. [Google Scholar] [CrossRef]
- Valkama, S.; Nykänen, A.; Kosonen, H.; Ramani, R.; Tuomisto, F.; Engelhardt, P.; Brinke, G.T.; Ikkala, O.; Ruokolainen, J. Hierarchical Porosity in Self-Assembled Polymers: Post-Modification of Block Copolymer–Phenolic Resin Complexes by Pyrolysis Allows the Control of Micro- and Mesoporosity. Adv. Funct. Mater. 2007, 17, 183–190. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, C.; Li, J.; Chen, H.; Li, B.; Li, Y.; Yang, Y. Synthesis of Helical Phenolic Resin Bundles through a Sol-Gel Transcription Method. Gels 2017, 3, 9. https://doi.org/10.3390/gels3010009
Shao C, Li J, Chen H, Li B, Li Y, Yang Y. Synthesis of Helical Phenolic Resin Bundles through a Sol-Gel Transcription Method. Gels. 2017; 3(1):9. https://doi.org/10.3390/gels3010009
Chicago/Turabian StyleShao, Changzhen, Jiangang Li, Hao Chen, Baozong Li, Yi Li, and Yonggang Yang. 2017. "Synthesis of Helical Phenolic Resin Bundles through a Sol-Gel Transcription Method" Gels 3, no. 1: 9. https://doi.org/10.3390/gels3010009