Mechanical, Swelling, and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
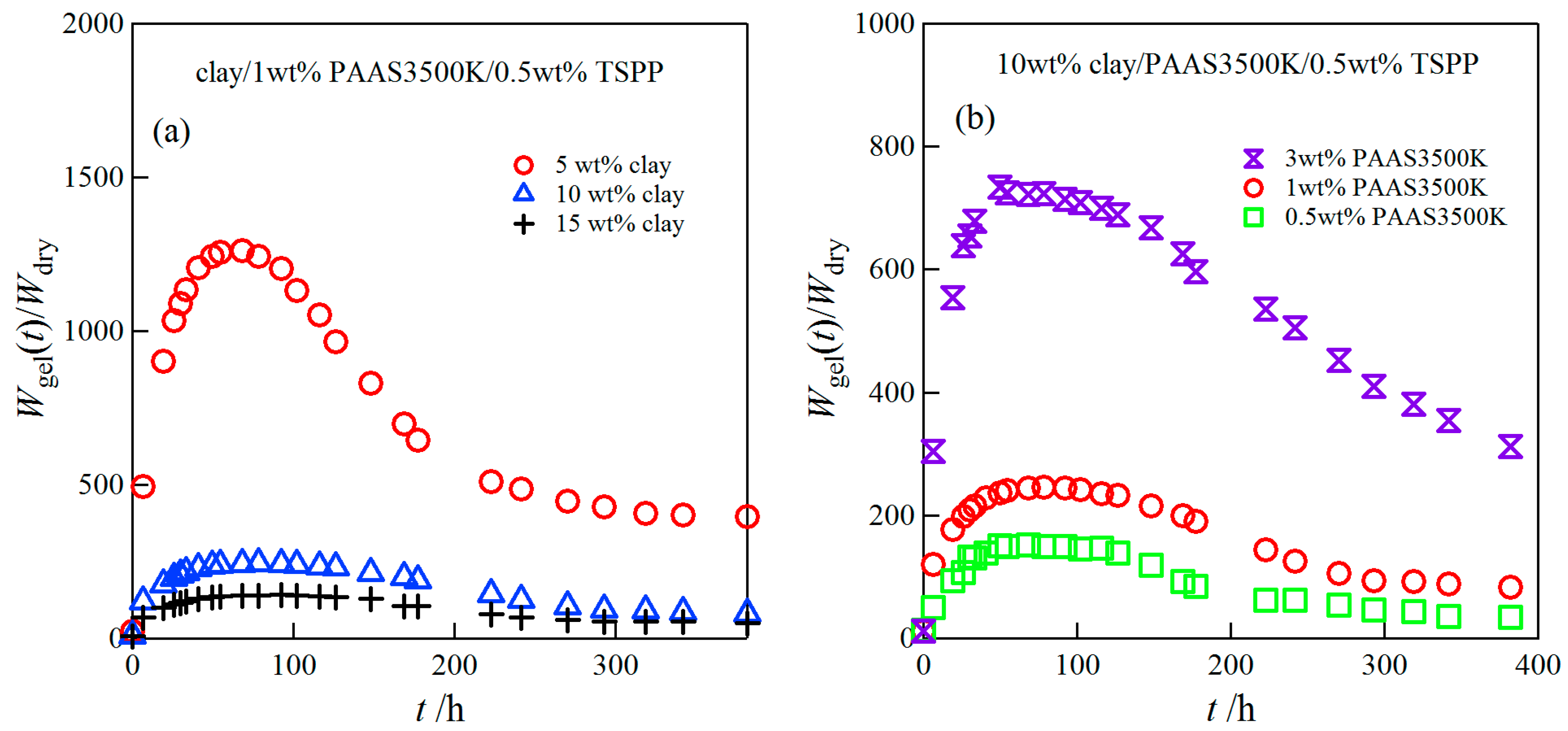
2.1. Time Course of Swelling Ratios for Clay/PAAS Hydrogels
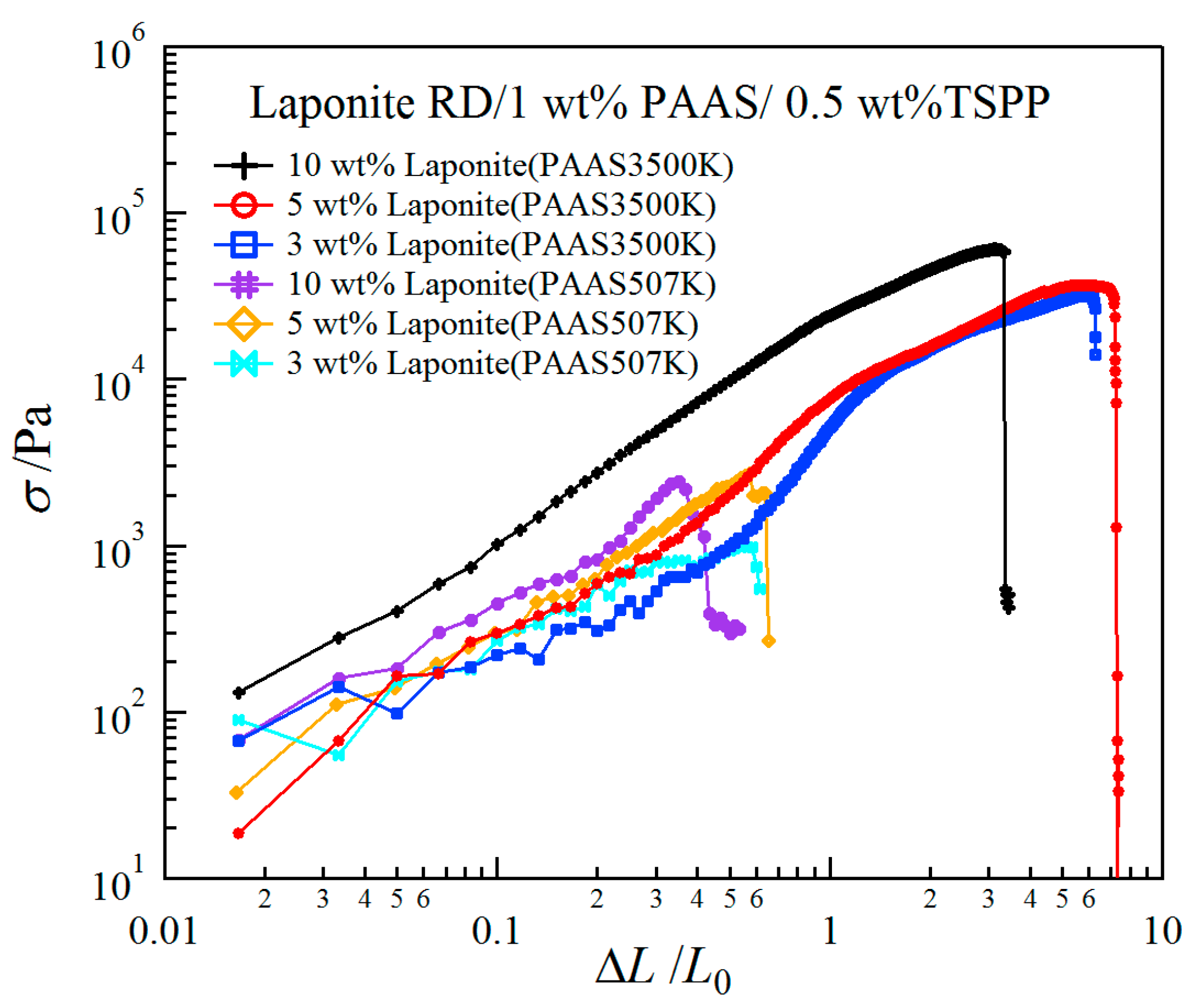
2.2. Mechanical Properties of Clay/PAAS Hydrogels
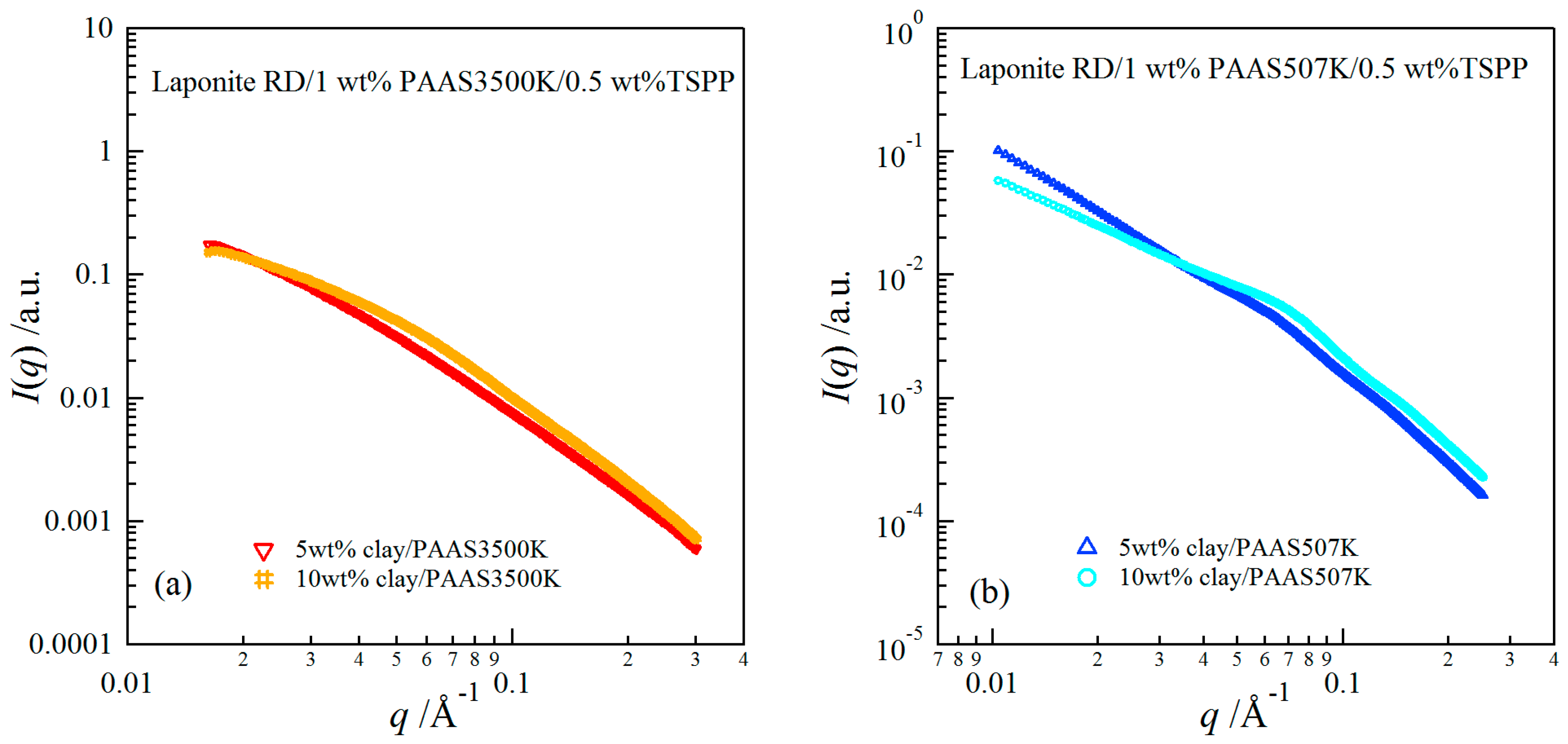
2.3. Structures of Clay/PAAS Hydrogels
3. Summary
4. Experiments
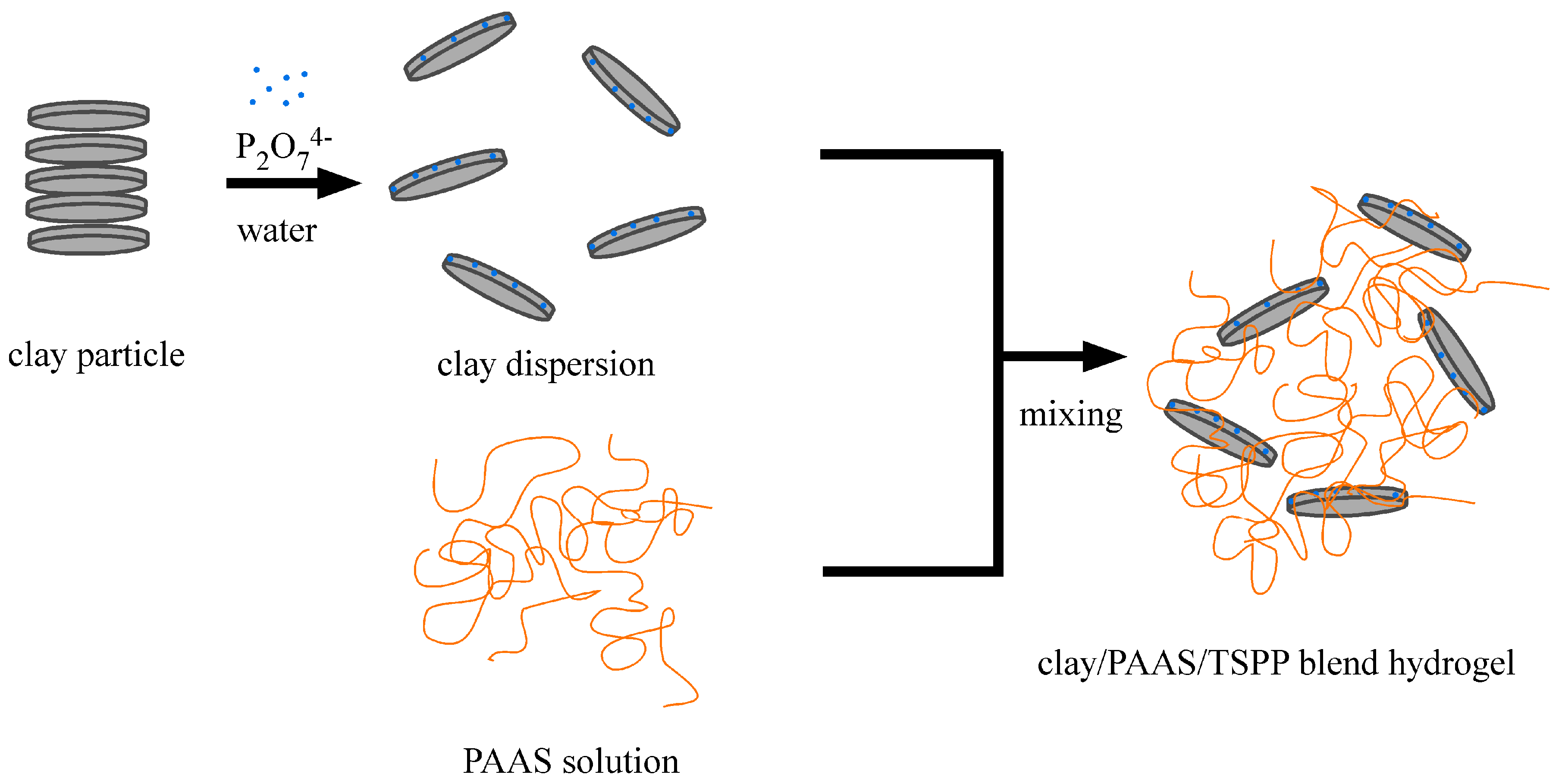
4.1. Samples and Sample Preparation
4.2. Mechanical Measurements
4.3. Swelling Measurements
4.4. Synchrotron Small-Angle X-ray Scattering
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haraguchi, K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym. J. 2011, 43, 223–241. [Google Scholar] [CrossRef]
- Inaoka, K.; Kobayashi, M.; Okada, M.; Sato, K. Stability, occurrence and step morphology of polymorphs and polytypes of stearic-acid: II. Mono-lamella step morphology and composite polymorphic polytypic transformation. J. Cryst. Growth 1988, 87, 243–250. [Google Scholar] [CrossRef]
- Haraguchi, K.; Li, H.J.; Matsuda, K.; Takehisa, T.; Elliott, E. Mechanism of forming organic/inorganic network structures during in-situ free-radical polymerization in PNIPA-clay nanocomposite hydrogels. Macromolecules 2005, 38, 3482–3490. [Google Scholar] [CrossRef]
- Vand, V. Method for determining the signs of the structure factors of long-chain compounds. Acta Crystallogr. 1951, 4, 104–105. [Google Scholar] [CrossRef]
- Takeno, H.; Nakamura, W. Structural and mechanical properties of composite hydrogels composed of clay and a polyelectrolyte prepared by mixing. Colloid Polym. Sci. 2013, 291, 1393–1399. [Google Scholar] [CrossRef]
- Takeno, H.; Sato, C. Effects of molecular mass of polymer and composition on the compressive properties of hydrogels composed of laponite and sodium polyacrylate. Appl. Clay Sci. 2016, 123, 141–147. [Google Scholar] [CrossRef]
- Dijkstra, M.; Hansen, J.P.; Madden, P.A. Gelation of a clay colloid suspension. Phys. Rev. Lett. 1995, 75, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Takeno, H.; Kimura, Y. Molecularweight effects on tensile properties of blend hydrogels composed of clay and polymers. Polymer 2016, 85, 47–54. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Anema, S.G.; Zhu, C.J.; Havea, P.; Coker, C. Water holding capacity and swelling of casein hydrogels. Food Hydrocoll. 2015, 44, 372–379. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Ozmen, M.M.; Okay, O. Swelling behavior of strong polyelectrolyte poly(Nt-butylacrylamide-co-acrylamide) hydrogels. Eur. Polym. J. 2003, 39, 877–886. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Can, V.; Abdurrahmanoglu, S.; Okay, O. Unusual swelling behavior of polymer-clay nanocomposite hydrogels. Polymer 2007, 48, 5016–5023. [Google Scholar] [CrossRef]
- Ren, H.Y.; Zhu, M.; Haraguchi, K. Characteristic swelling-deswelling of polymer/clay nanocomposite gels. Macromolecules 2011, 44, 8516–8526. [Google Scholar] [CrossRef]
- Barnes, G.E. An apparatus for the determination of the workability and plastic limit of clays. Appl. Clay Sci. 2013, 80–81, 281–290. [Google Scholar] [CrossRef]
- Brostow, W.; Hagg Lobland, H.E.; Khoja, S. Brittleness and toughness of polymers and other materials. Mater. Lett. 2015, 159, 478–480. [Google Scholar] [CrossRef]
- De, B.; Voit, B.; Karak, N. Transparent luminescent hyperbranched epoxy/carbon oxide dot nanocomposites with outstanding toughness and ductility. ACS Appl. Mater. Interfaces 2013, 5, 10027–10034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Sun, H.; Wu, S. Transient dynamic behavior of polypropylene fiber reinforced mortar under compressive impact loading. Constr. Build. Mater. 2016, 111, 30–42. [Google Scholar] [CrossRef]
- Higgins, J.S.; Benoît, H. Polymers and Neutron Scattering; Clarendon Press, Oxford University Press: Oxford, UK, 1994; p. 436. [Google Scholar]
- Kroon, M.; Vos, W.L.; Wegdam, G.H. Structure and formation of a gel of colloidal disks. Phys. Rev. E 1998, 57, 1962–1970. [Google Scholar] [CrossRef]
- Li, L.; Harnau, L.; Rosenfeldt, S.; Ballauff, M. Effective interaction of charged platelets in aqueous solution: Investigations of colloid laponite suspensions by static light scattering and small-angle X-ray scattering. Phys. Rev. E 2005, 72, 051504. [Google Scholar] [CrossRef] [PubMed]
- Pignon, F.; Magnin, A.; Piau, J.M.; Cabane, B.; Lindner, P.; Diat, O. Yield stress thixotropic clay suspension: Investigation of structure by light, neutron, and X-ray scattering. Phys. Rev. E 1997, 56, 3281–3289. [Google Scholar] [CrossRef]
- Saunders, J.M.; Goodwin, J.W.; Richardson, R.M.; Vincent, B. A small-angle X-ray scattering study of the structure of aqueous laponite dispersions. J. Phys. Chem. B 1999, 103, 9211–9218. [Google Scholar] [CrossRef]
- Shang, C.; Rice, J.A. Invalidity of deriving interparticle distance in clay-water systems using the experimental structure factor maximum obtained by small-angle scattering. J. Colloid Interface Sci. 2005, 283, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Cosgrove, T. A small-angle neutron scattering study of adsorbed poly(ethylene oxide) on laponite. Langmuir 2004, 20, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Glatter, O.; Kratky, O. Small Angle X-ray Scattering; Academic Press: London, UK, 1982. [Google Scholar]
- Miyazaki, S.; Endo, H.; Karino, T.; Haraguchi, K.; Shibayama, M. Gelation mechanism of poly(n-isopropylacrylamide)-clay nanocomposite gels. Macromolecules 2007, 40, 4287–4295. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D: An Introduction and Overview. ESRF Int. Rep. ESRF97HA02T 1997, 68. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeno, H.; Kimura, Y.; Nakamura, W. Mechanical, Swelling, and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels. Gels 2017, 3, 10. https://doi.org/10.3390/gels3010010
Takeno H, Kimura Y, Nakamura W. Mechanical, Swelling, and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels. Gels. 2017; 3(1):10. https://doi.org/10.3390/gels3010010
Chicago/Turabian StyleTakeno, Hiroyuki, Yuri Kimura, and Wataru Nakamura. 2017. "Mechanical, Swelling, and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels" Gels 3, no. 1: 10. https://doi.org/10.3390/gels3010010




