Mechanical Properties and Brittle Behavior of Silica Aerogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of Synthesis and Process Parameters on the Mechanical Properties of Aerogels
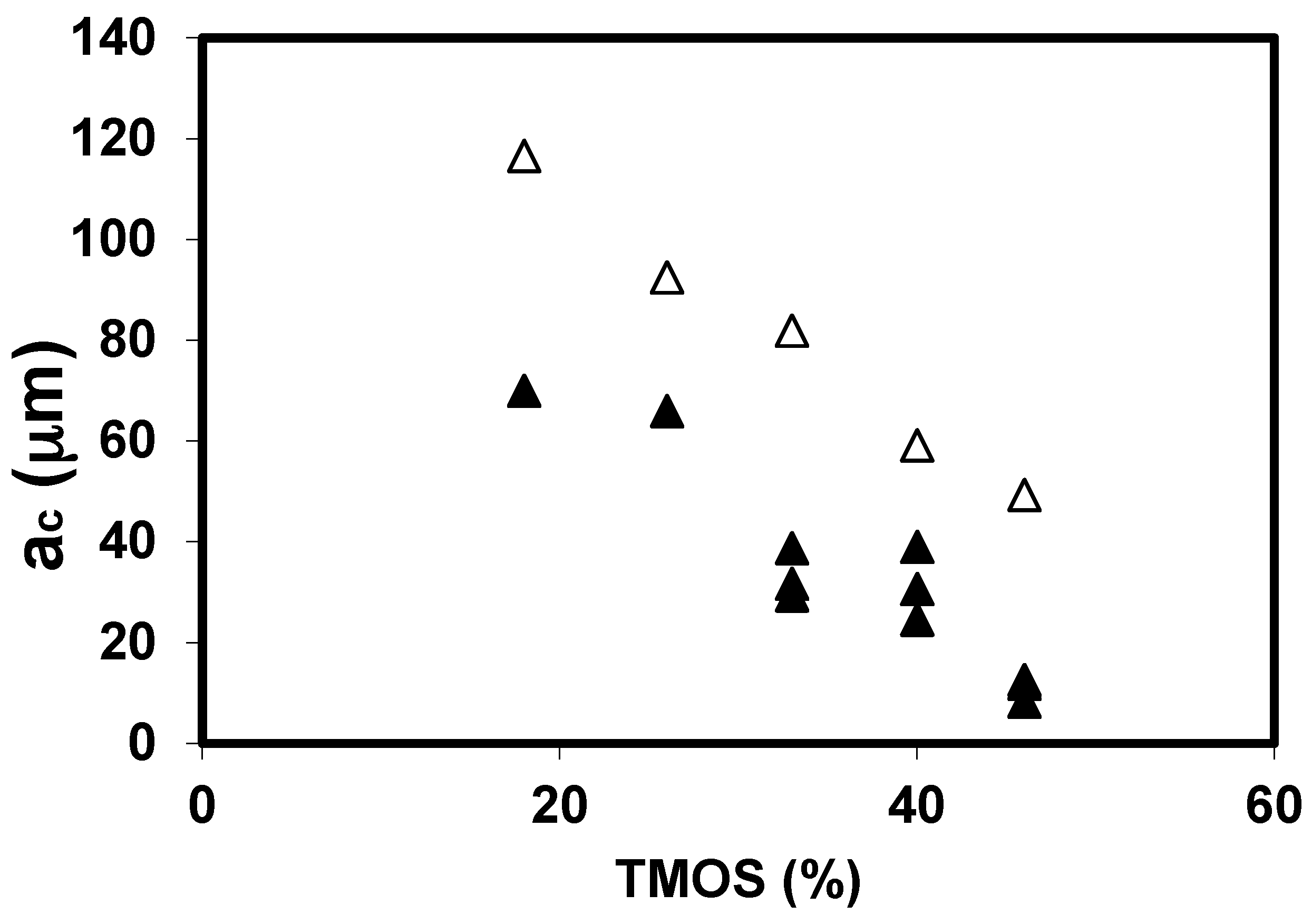
2.1.1. The Silane Concentration
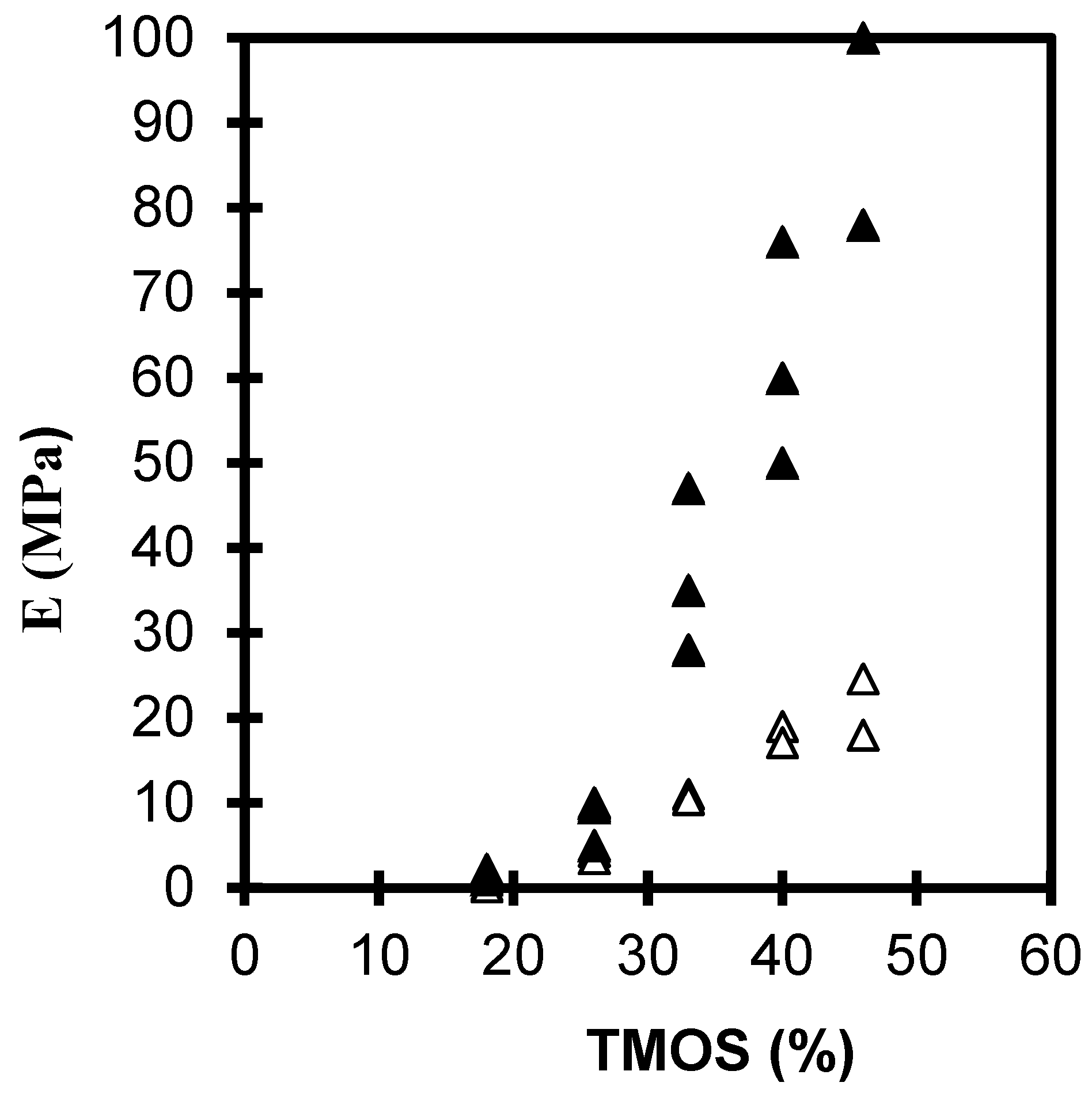
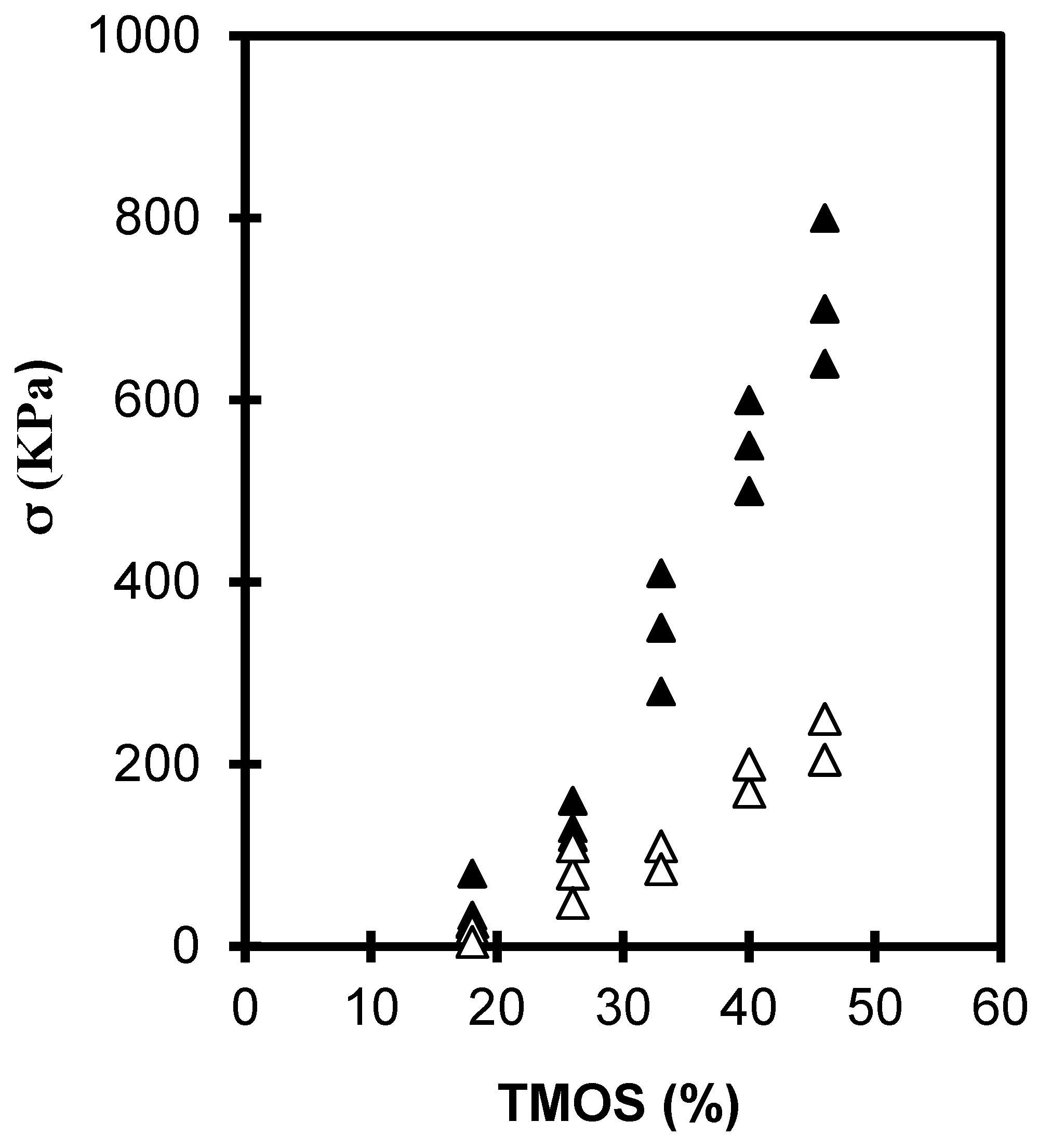
| TMOS (%) | Bulk Density, ρ (g·cm−3) | |
|---|---|---|
| Basic set | 18 | 0.1 |
| 26 | 0.14 | |
| 33 | 0.17 | |
| 40 | 0.21 | |
| 46 | 0.24 | |
| Neutral set | 18 | 0.11 |
| 26 | 0.15 | |
| 33 | 0.23 | |
| 40 | 0.28 | |
| 46 | 0.32 | |


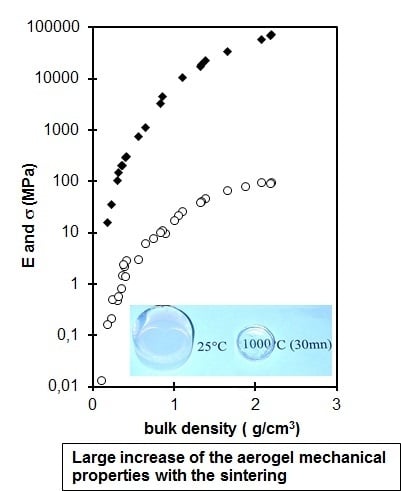
2.1.2. Sintering and Drying Process
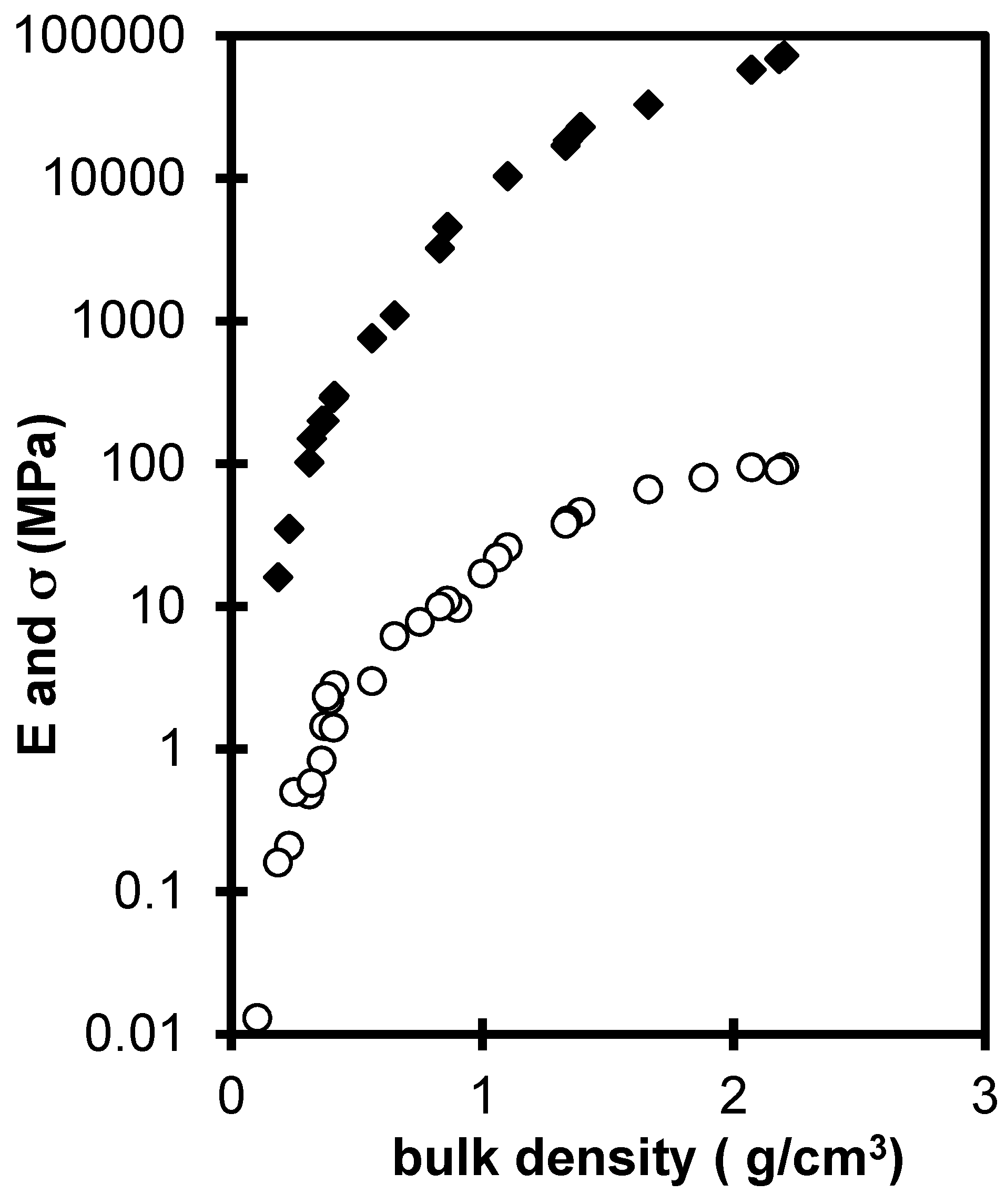
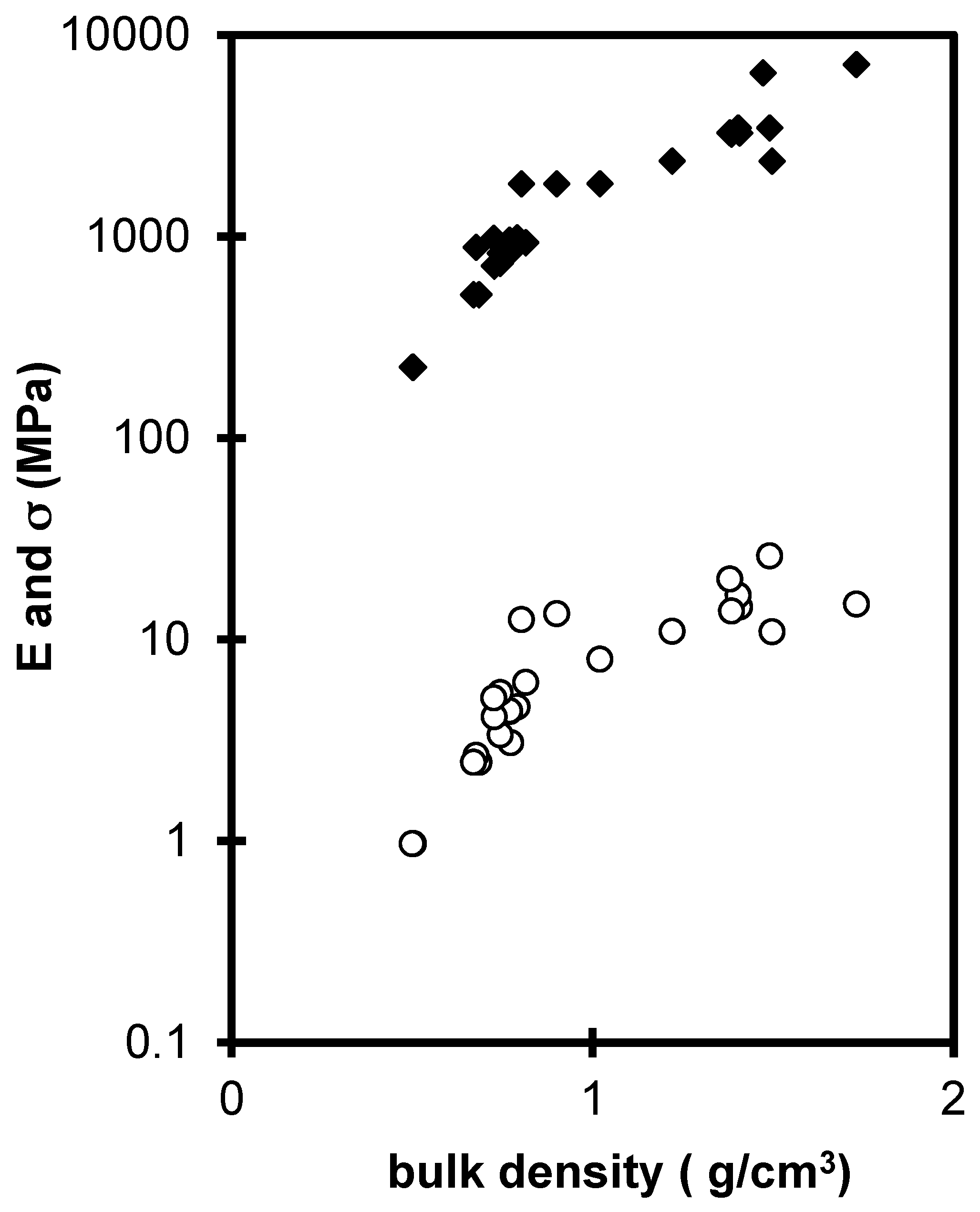
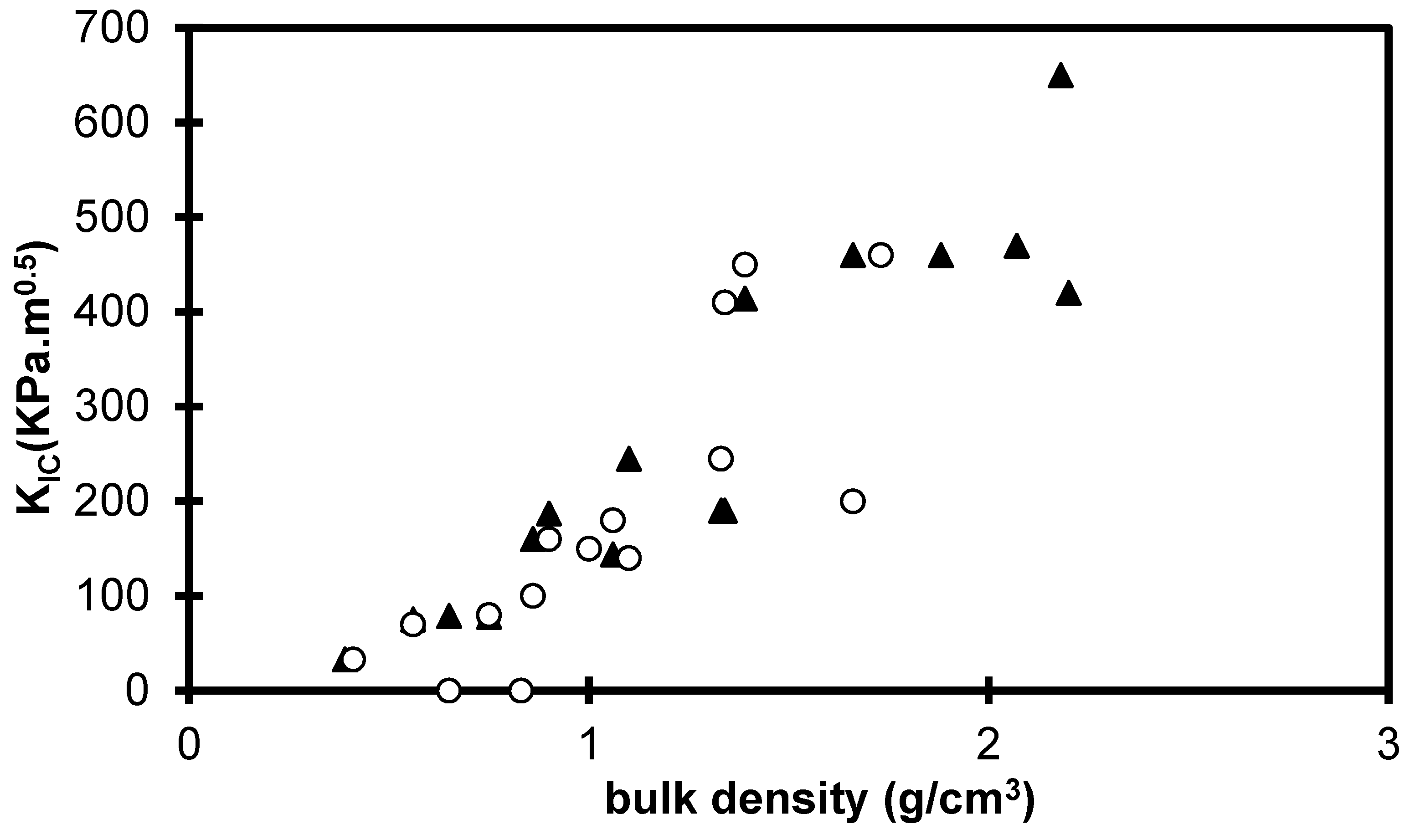
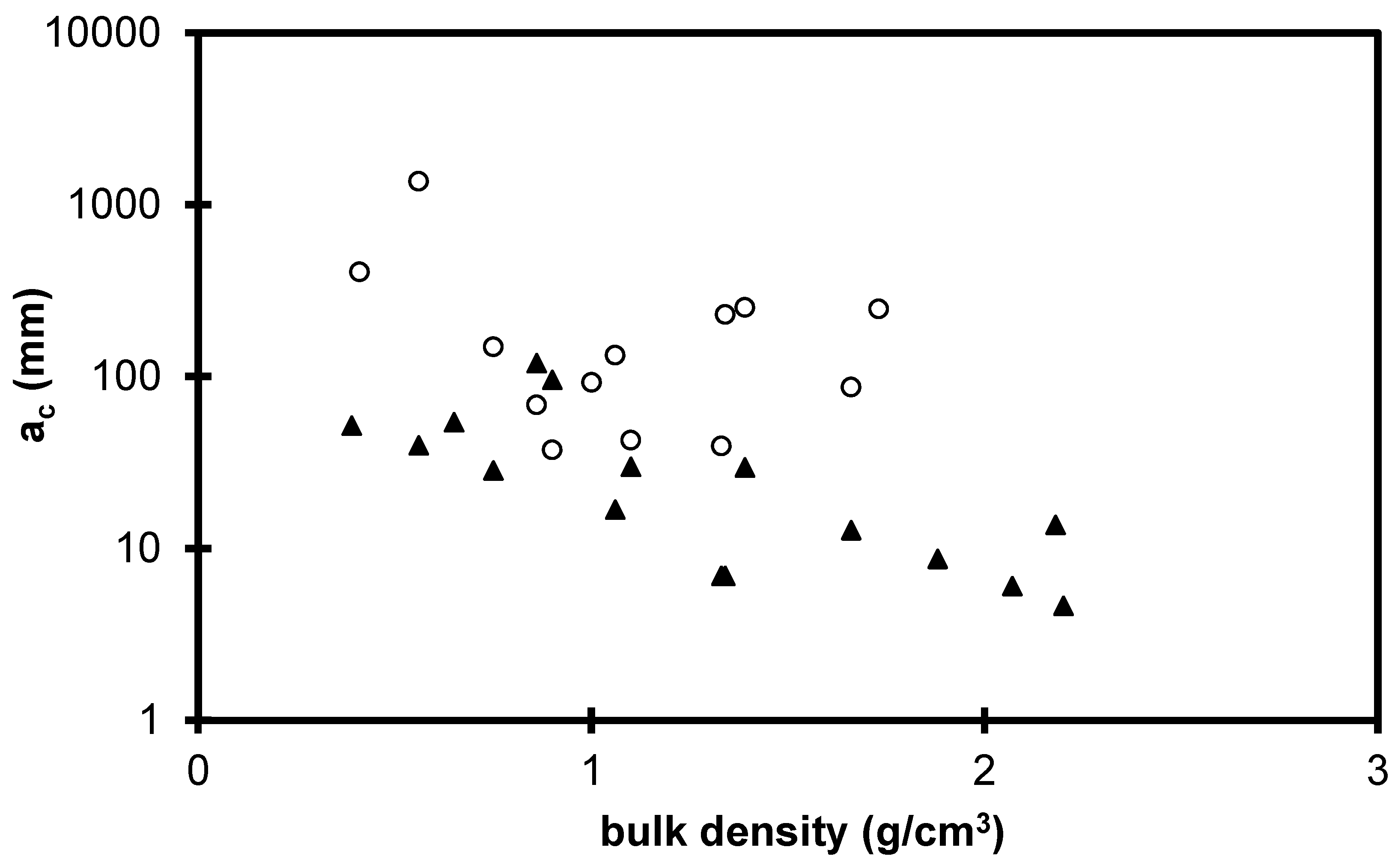
2.2. Influence of Environment on Fracture Mechanisms
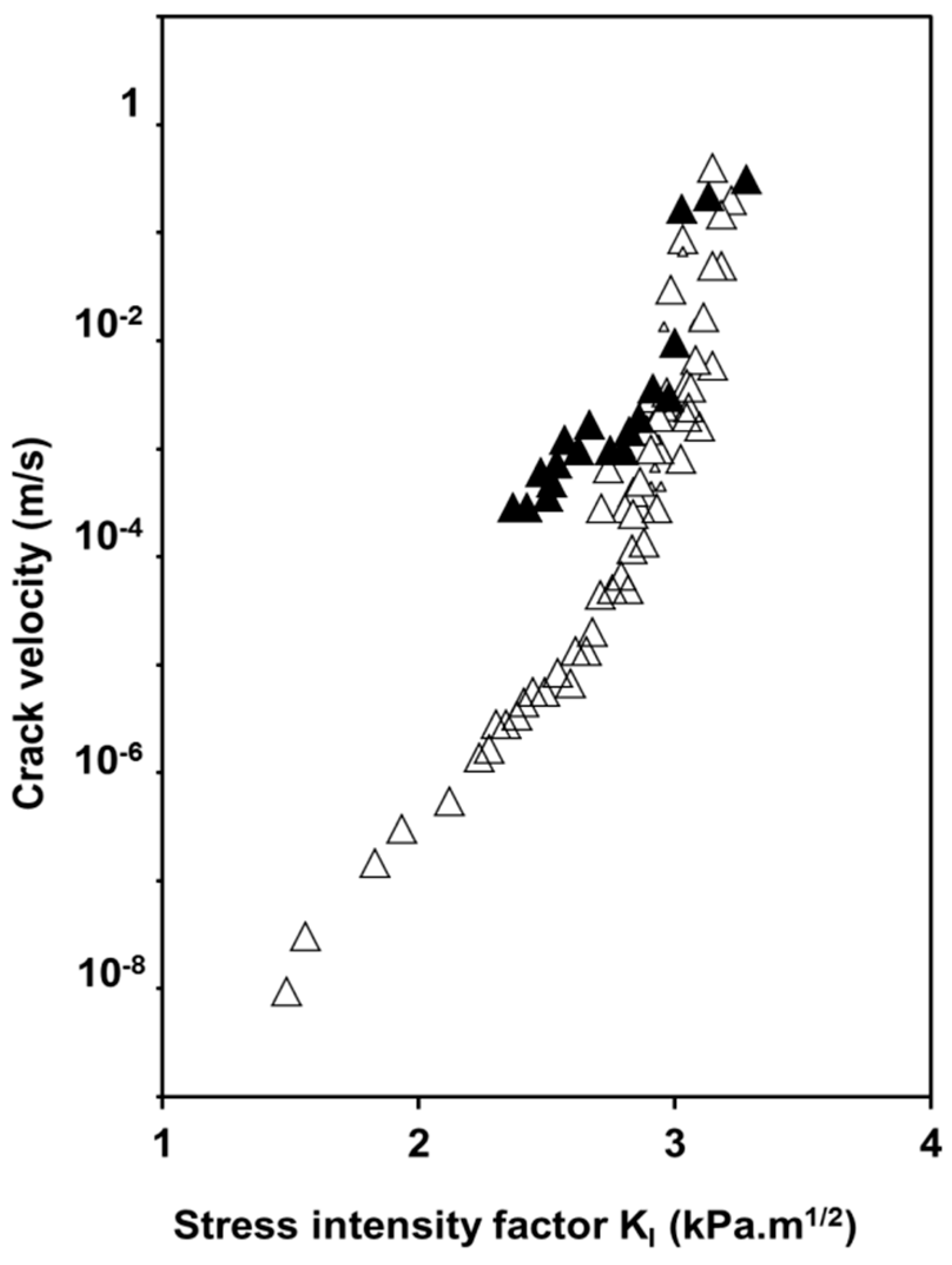
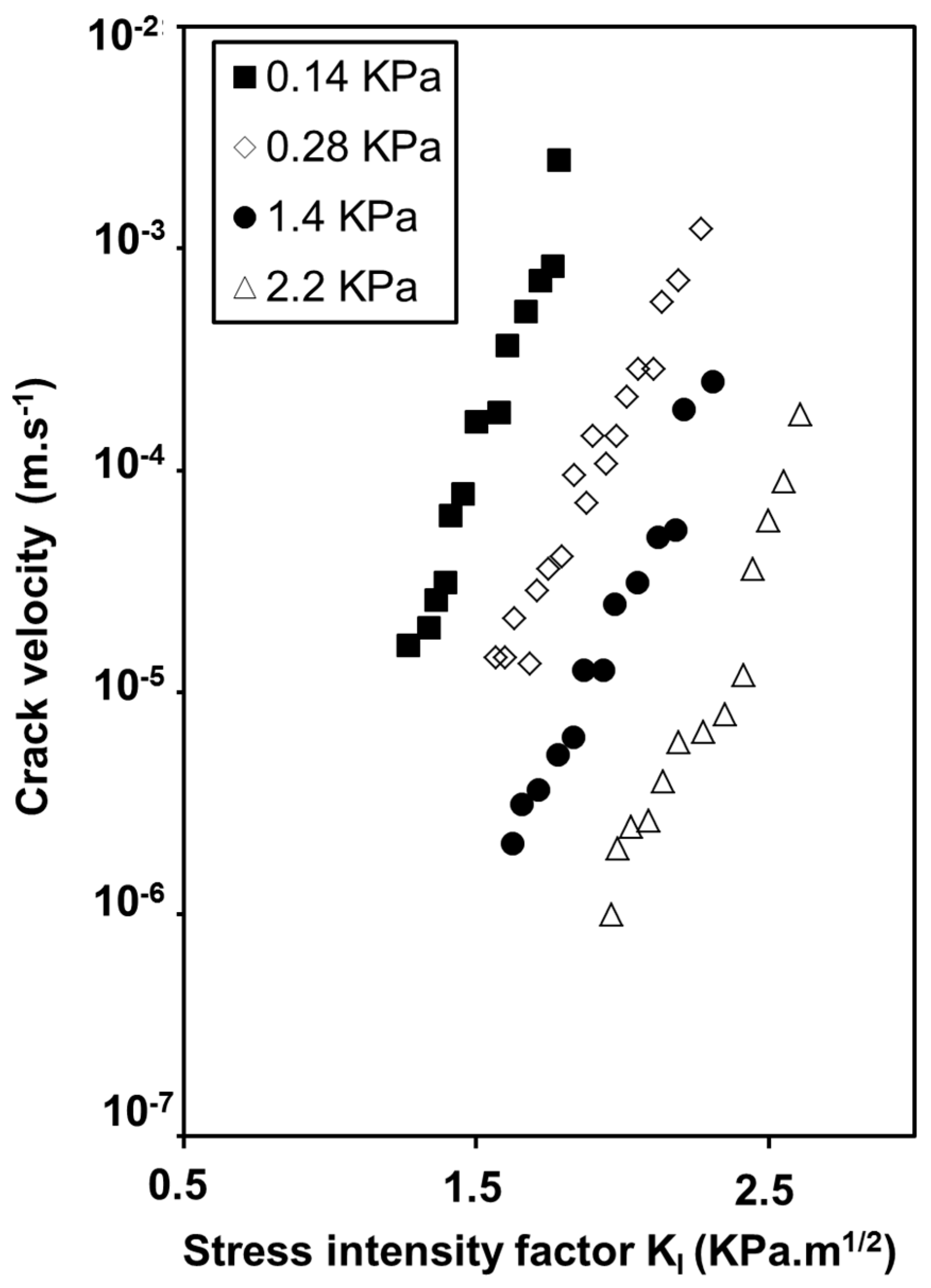
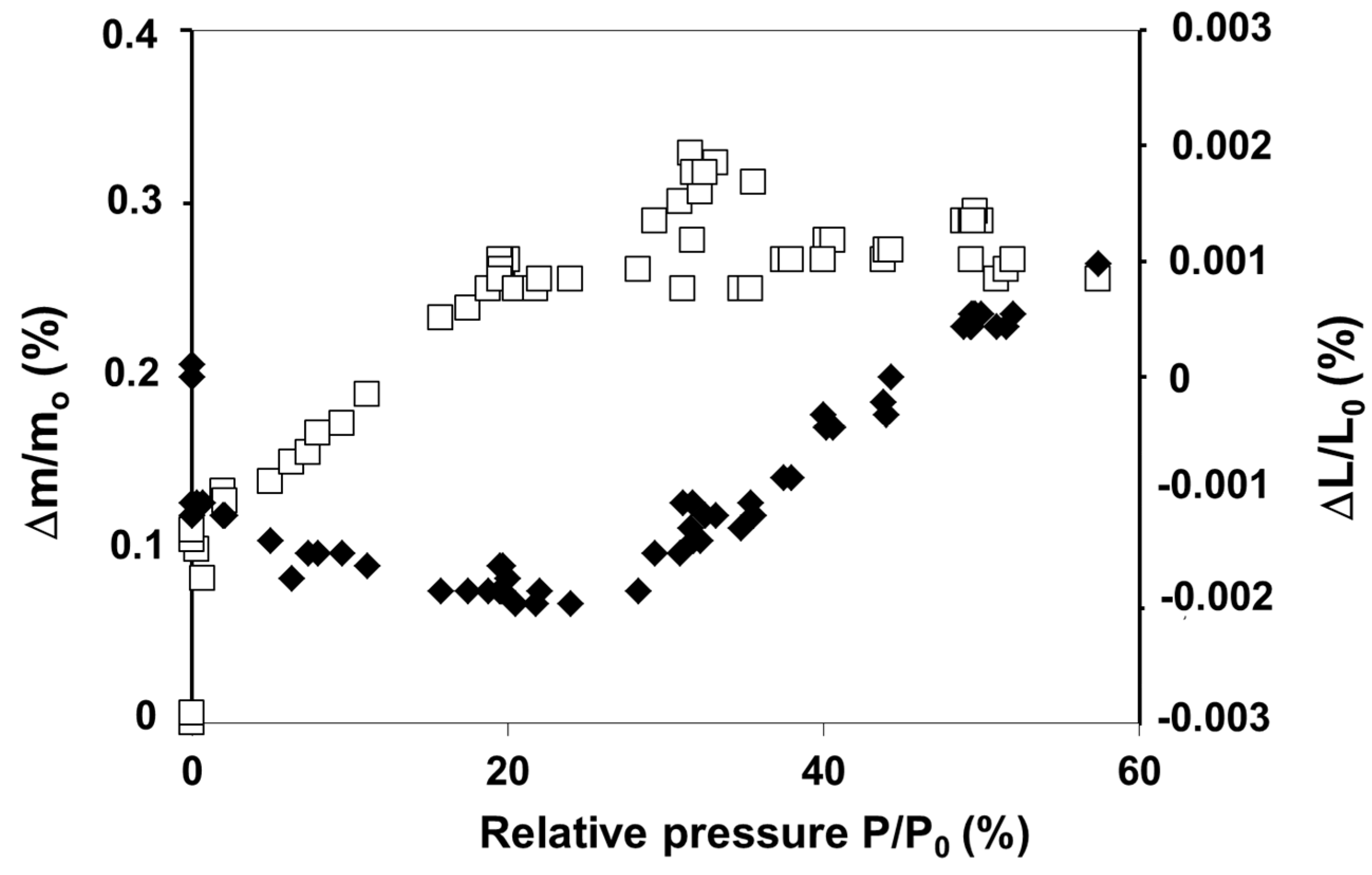

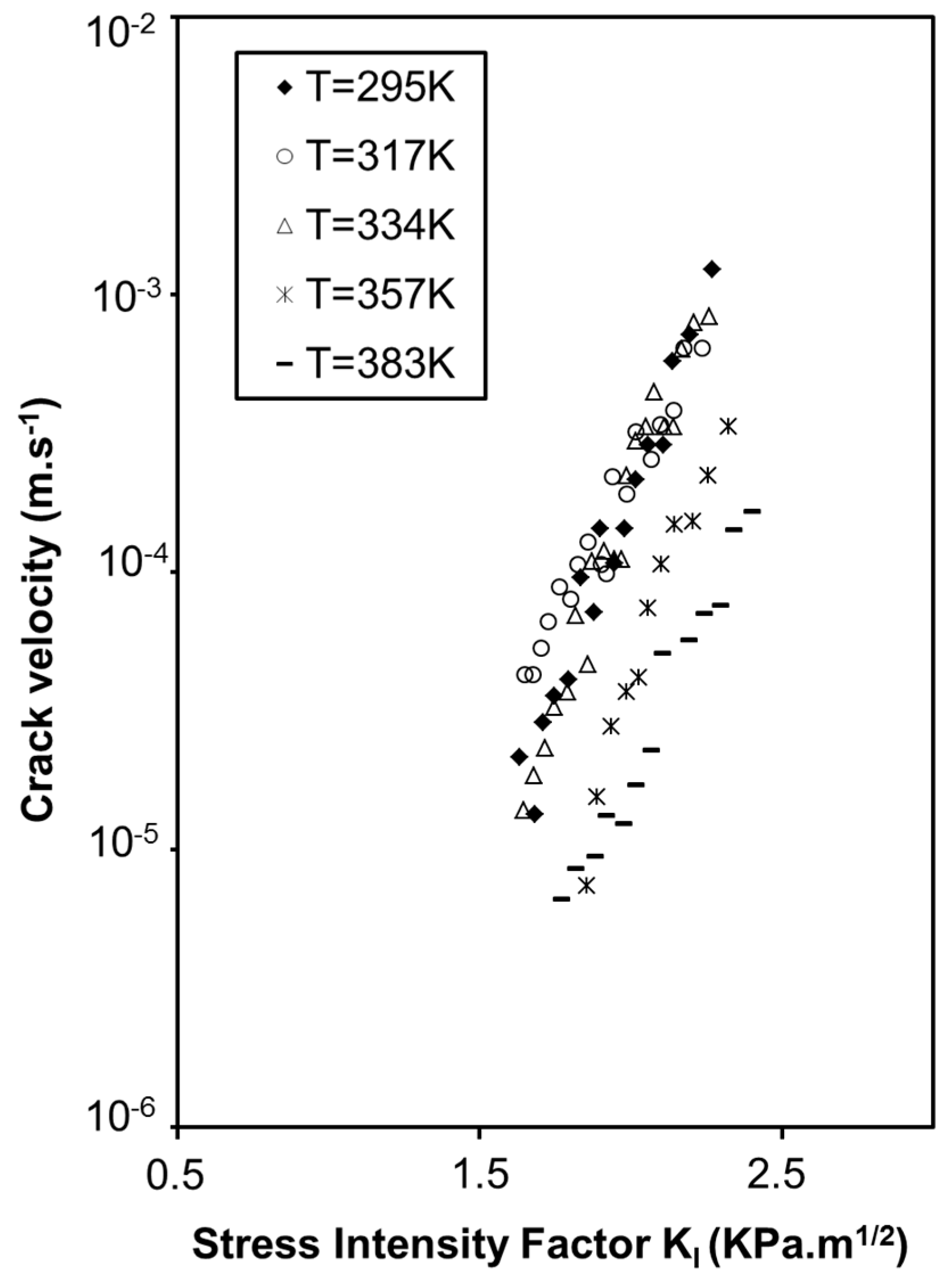


3. Conclusions
4. Experimental Section
4.1. Materials Synthesis
4.2. Mechanical Measurements

Conflicts of Interest
References
- Torquato, S. Random Heterogeneous Materials. Microstructure and Macroscopic Properties; Springer: New York, NY, USA, 2002. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids Structure and Properties; Pergamon press: Oxford, UK, 1988. [Google Scholar]
- Tokita, M.; Niki, R.; Hikichi, K. Percolation theory and elastic modulus of gel. J. Phys. Soc. Jpn. 1984, 53, 480–482. [Google Scholar] [CrossRef]
- Kantor, Y.; Webman, I. Elastic properties of random percolating systems. Phys. Rev. Lett. 1984, 52, 1891–1894. [Google Scholar] [CrossRef]
- Feng, S.; Sen, P. Percolation on elastic networks New exponent and threshold. Phys. Rev. Lett. 1984, 52, 216–219. [Google Scholar] [CrossRef]
- Emmerling, A.; Fricke, J. Scaling properties and structure of aerogels. J. Sol-Gel Sci. Technol. 1997, 8, 781–788. [Google Scholar] [CrossRef]
- Ma, H.S.; Prevost, J.H.; Jullien, R.; Scherer, G.W. Computer simulation of mechanical structure-property relationship of aerogels. J. Non-Cryst. Solids 2001, 285, 216–221. [Google Scholar] [CrossRef]
- Rice, R.W. Porosity of Ceramics Properties and Applications; Marcel Dekker Inc.: New York, NY, USA, 1998. [Google Scholar]
- Brinker, J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Gross, J.; Fricke, J. Ultrasonic velocity measurements in silica, carbon and organic aerogels. J. Non-Cryst. Solids 1992, 145, 217–222. [Google Scholar] [CrossRef]
- Nicolaon, G.A.; Teichner, S.J. New preparation process for silica xerogels and aerogels and their textural properties. Bull. Soc. Chim. France 1968, 5, 1900–1906. [Google Scholar]
- Fricke, J. Aerogels and their applications: Aerogels and their applications Aerogels and their applications. J. Non-Cryst. Solids 1992, 147–148, 356–362. [Google Scholar] [CrossRef]
- Schaeffer, D.W.; Keefer, K.D. Structure of Random Porous Materials: Silica Aerogel. Phys. Rev. Lett. 1986, 56, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Woignier, T.; Phalippou, J.; Pelous, J.; Courtens, E. Different kinds of fractal structures in silica aerogels. J. Non-Cryst. Solids 1990, 121, 198–201. [Google Scholar] [CrossRef]
- Calemczuck, R.; de Goer, A.M.; Salce, B.; Maynard, R.; Zarembowitch, A. Low temperature properties of silica aerogels. Europhys. Lett. 1987, 3, 1205–1211. [Google Scholar] [CrossRef]
- Gross, J.; Reichenauer, G.; Fricke, J. Mechanical Properties of SiO2 Aerogels. J. Phys. D Appl. Phys. 1988, 21, 1447–1451. [Google Scholar] [CrossRef]
- Lemay, J.D.; Tillotson, T.M.; Hrubesch, H.W.; Pekala, R.W. Microstructural Dependence of Aerogel Mechanical Properties. Mater. Res. Soc. Symp. Proc. 1990, 180, 321–328. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J. Mechanical strength of silica aerogels. J. Non-Cryst. Solids 1988, 100, 404–408. [Google Scholar] [CrossRef]
- Scherer, G.W. Crack tip stress in gels. J. Non-Cryst. Solids 1992, 144, 210–214. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J.; Hdach, H.; Larnac, G.; Pernot, F.; Scherer, G.W. Evolution of mechanical properties during the alcogel-aerogel-glass process. J. Non-Cryst. Solids 1992, 147–148, 672–680. [Google Scholar] [CrossRef]
- Zarzycki, J. Critical stress intensity factors of wet gels. J. Non-Cryst. Solids 1988, 100, 359–363. [Google Scholar] [CrossRef]
- Evans, A.G. Slow crack in brittle materials under dynamic loading conditions. Int. J. Fract. 1974, 10, 251–261. [Google Scholar] [CrossRef]
- Chermant, J.L.; Osterstock, F.; Vadam, G. Etude critique de la mesure de Kic dans le cas de quelques matériaux verriers. Verres Refract. 1980, 34, 624–636. [Google Scholar]
- Evans, A.G.; Tappin, G. Effects of microstructure on the stress propagate inherent flaws. Proc. Br. Ceram. Soc. 1972, 23, 275–296. [Google Scholar]
- Phalippou, J.; Ayral, A.; Woignier, T.; Pauthe, M.; Quinson, J.F.; Lechatelut, A. Fractal Geometry of Silica Alcogels from Thermoporometry Experiments. Europhys. Lett. 1991, 14, 249–255. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J.; Prassas, M. Glasses from aerogels II. J. Mater. Sci. 1990, 25, 3118–3126. [Google Scholar] [CrossRef]
- Scherer, G.W. Sintering of Low Density Glasses: I. Theory. J. Am. Ceram. Soc. 1977, 60, 236–239. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J.; Quinson, J.F.; Pauthe, M.; Repellin-Lacroix, M.; Scherer, G.W. The sintering of silica aerogels studied by thermoporometry. J. Sol-Gel Sci. Technol. 1994, 2, 277–281. [Google Scholar] [CrossRef]
- Scherer, G.W.; Smith, D.M.; Qiu, X.; Anderson, J.M. Compression of aerogels. J. Non-Cryst. Solids 1995, 186, 316–320. [Google Scholar] [CrossRef]
- Pirard, R.; Blacher, R.; Brouers, S.; Pirard, J.P. Interpretation of mercury porosimetry applied to aerogels. J. Mater. Res. 1995, 10, 2114–2119. [Google Scholar] [CrossRef]
- Duffours, L.; Woignier, T.; Phalippou, J. Plasticity of aerogels under isostatic pressure. J. Non-Cryst. Solids 1995, 186, 321–327. [Google Scholar] [CrossRef]
- Despetis, F.; Etienne, P.; Phalippou, J. Crack speed in Ultraporous brittle amorphous material. Phys. Chem. Glasses 2000, 41, 104–106. [Google Scholar]
- Despetis, F.; Etienne, P.; Etienne-Calas, S. Subcritical crack growth in silica aerogel. J. Non-Cryst. Solids 2004, 344, 22–25. [Google Scholar] [CrossRef]
- Crichton, S.N.; Tomozawa, M.; Hayden, J.S.; Suratwala, T.I.; Campbell, J.H. Subcritical crack growth in a phosphate laser glass. J. Am.Ceram. Soc. 1999, 82, 3097–3104. [Google Scholar] [CrossRef]
- Despetis, F.; Calas, S.; Etienne, P.; Phalippou, J. Effect of oxidation treatment on the crack propagation rate of aerogels. J. Non-Cryst. Solids 2001, 285, 251–255. [Google Scholar] [CrossRef]
- Suratwala, T.I.; Steele, R.A. Anomalous temperature dependence of sub-critical crack growth in silica glass. J. Non-Cryst. Solids 2003, 16, 174–182. [Google Scholar] [CrossRef]
- Woignier, T.; Scherer, G.W.; Alaoui, A. Stress in Aerogel during Depressurization of Autoclave: II. Silica Gel. J. Sol-Gel Sci. Technol. 1994, 3, 141–150. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress in Aerogel during Depressurization of Autoclave: I. Theory. J. Sol-Gel Sci. Technol. 1994, 3, 127–139. [Google Scholar] [CrossRef]
- Hibino, Y.; Sakaguchi, S.; Tajima, Y. Crack growth in silica glass under dynamic loading. J. Am. Ceram. Soc. 1984, 67, 64–68. [Google Scholar] [CrossRef]
- Abe, K.; Glaesemann, G.S.; Gulati, S.T.; Hanson, T.A. Application of a phenomenological fatigue model to optical fibers. Opt. Eng. 1991, 30, 728–732. [Google Scholar] [CrossRef]
- Etienne, P.; Phalippou, J.; Woignier, T.; Alaoui, A. Slow crack growth in aerogels. J. Non-Cryst. Solids 1995, 188, 19–26. [Google Scholar] [CrossRef]
- Prassas, M.; Woignier, T.; Phalippou, J. Glasses from aerogels I. J. Mater. Sci. 1990, 25, 3110–3117. [Google Scholar]
- Janssen, C. Specimen for fracture mechanics studies on glass. Ceram. Soc. Jpn. 1974, 12. [Google Scholar] [CrossRef]
- Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar]
- Sullivan, J.D.; Lauzon, P.H. Experimental probability estimators for Weibull plots. J. Mater. Sci. Lett. 1986, 5, 1245–1247. [Google Scholar] [CrossRef]
- Wiederhorn, M. A Chemical Interpretation of Static Fatigue. J. Am. Ceram. Soc. 1972, 55, 81–85. [Google Scholar] [CrossRef]
- Michalske, T.A.; Freiman, S.W. A molecular mechanism for stress corrosion in vitreous silica. J. Am. Ceram. Soc. 1983, 66, 284–288. [Google Scholar] [CrossRef]
- Kerkhof, F. Bruchvorgänge in Gläsern, Verlag der Deutschen Glastechnischen Gesellschaft, Frankfurt (Main). 1970; 217–223. [Google Scholar]
- Courtens, E.; Pelous, J.; Phalippou, J.; Vacher, R.; Woignier, T. Brillouin-scattering measurements of phonon-fracton crossover in silica aerogels. Phys. Rev. Lett. 1987, 58, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Charles, R.J.; Hillig, W.B. Proceedings of Symposium on Mechanical Strenght of Glass and Ways of Improving it, Florence, Italy, 25–29 September 1961; Edited by Union Scientifique Continentale du Verre: Charleroi, Belgium, 1962; pp. 511–527.
- Wiederhorn, S.M. Fracture Mechanics of Ceramics; Bradt, R.C., Hasselman, D.P.H., Lange, F.F., Eds.; Plenum Press: New York, NY, USA, 1974; Volume 2, p. 613. [Google Scholar]
- Ritter, J.E. Fracture Mechanics of Ceramics; Bradt, R.C., Hasselman, D.P.H., Lange, F.F., Eds.; Plenum Press: New York, NY, USA, 1978; Volume 4, p. 667. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woignier, T.; Primera, J.; Alaoui, A.; Etienne, P.; Despestis, F.; Calas-Etienne, S. Mechanical Properties and Brittle Behavior of Silica Aerogels. Gels 2015, 1, 256-275. https://doi.org/10.3390/gels1020256
Woignier T, Primera J, Alaoui A, Etienne P, Despestis F, Calas-Etienne S. Mechanical Properties and Brittle Behavior of Silica Aerogels. Gels. 2015; 1(2):256-275. https://doi.org/10.3390/gels1020256
Chicago/Turabian StyleWoignier, Thierry, Juan Primera, Adil Alaoui, Pascal Etienne, Florence Despestis, and Sylvie Calas-Etienne. 2015. "Mechanical Properties and Brittle Behavior of Silica Aerogels" Gels 1, no. 2: 256-275. https://doi.org/10.3390/gels1020256