2.3. Si-HPMC Solution
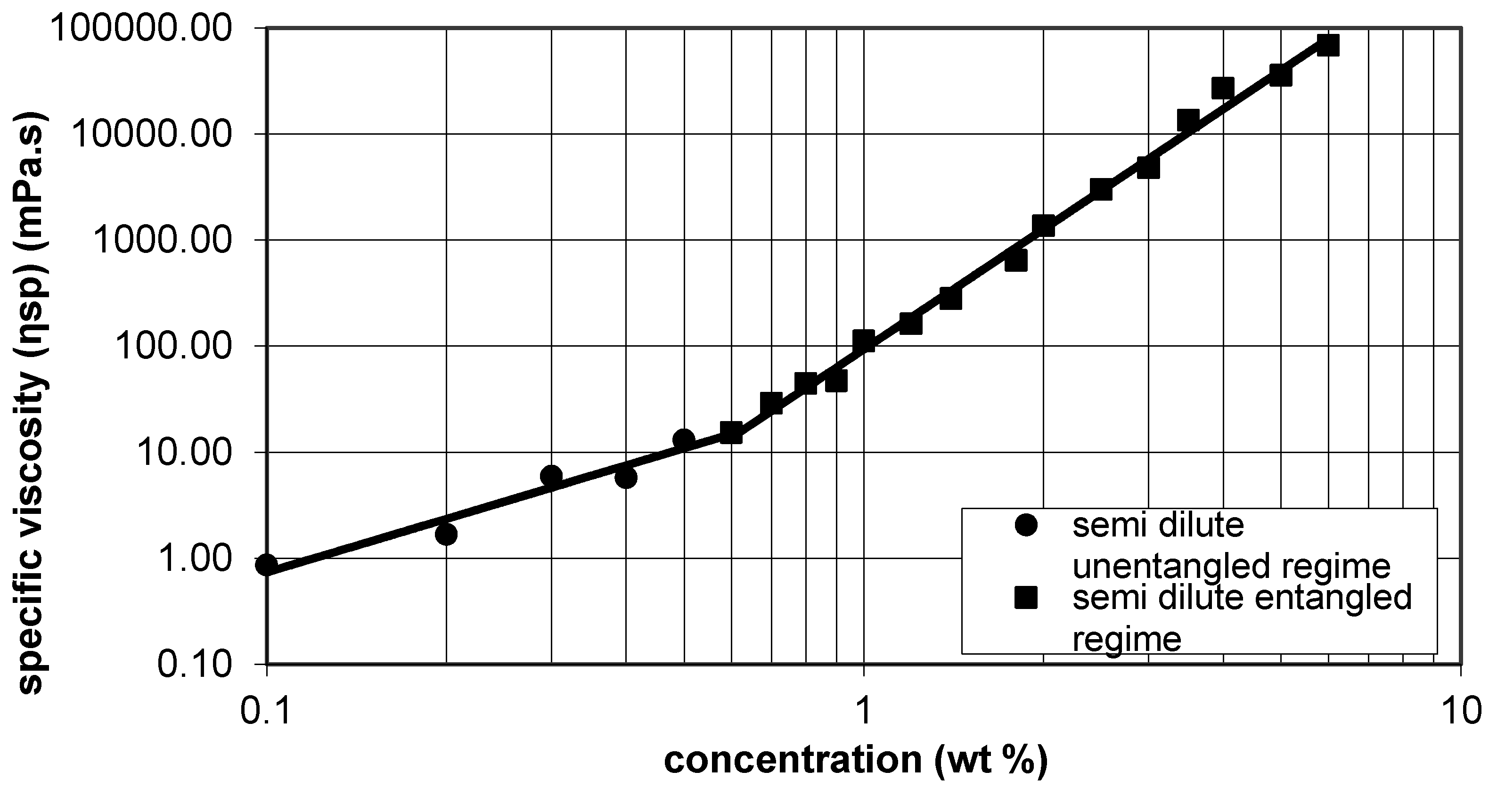
Si-HPMC is highly viscous in sodium hydroxide solution. Specific viscosity was used to characterize the outflow of the solution and compared to the literature to determine the range in which the solution could be electrospun. According to McKee
et al. the range of 2–2.5Ce (Ce is the entanglement concentration) is the minimal range of concentration for electrospinning [
11]. Neo
et al. confirmed this result, obtaining free bead fibers at a concentration of 2.1Ce for zein solution [
12]. According to
Figure 1, Ce has been determined as 0.6 %
wt/
v for Si-HPMC in 0.2 M·NaOH. Ce is the boundary concentration between semi dilute untangled and semi dilute entangled regimes. Indeed, the minimal range has been determined as (1.2; 1.8).
Figure 1.
Determination of Ce: specific viscosity as a function of Si-HPMC concentration [
11].
Figure 1.
Determination of Ce: specific viscosity as a function of Si-HPMC concentration [
11].
Cross-linking of Si-HPMC solutions is a multi-factorial reaction. The solution-gel transition times vary in accordance with temperature, concentrations, and solvents. To obtain a sufficient quantity of fibers, an electrospinning duration of 1–2 h is necessary. As a consequence, the cross-linking has to be controlled and slower than 2 h. Under the conditions used in the laboratory for injection applications, the gelation time is approximately 40 min. In this case, a HEPES acid buffer is used to promote the neutralization of the solution and then the subsequent cross-linking. It has been observed that a solution with ethanol has a slower gelation (about 3 h), irrespective of an increase in temperature. There should be a competitive reaction between the silanol function grafted on the HPMC and ethanol.
2.4. Electrospinning of HPMC
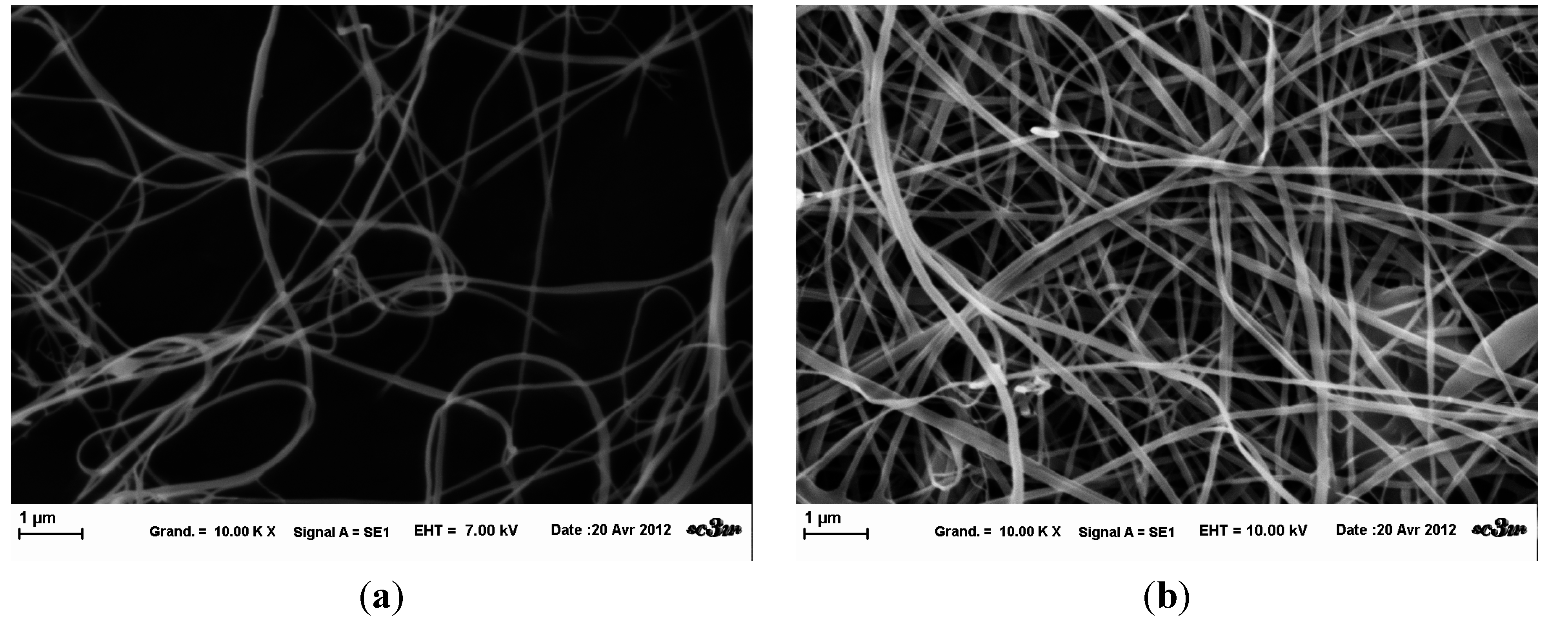
Initially, fibers were created according to the literature with a flow rate of 4.5mL/h. Variations of the solution were made to understand and control the process. Four solutions with different concentrations were electrospun. Samples were observed at same magnifications at SEM (
Figure 2). Differences in the quantity of fibers (
Figure 2b) could come from a difference of wrenching. Indeed, it is the only sample for which a continuous piece of mat was taken. Without any statistical measurement, fibers obtained from solution with 2%
wt/
v (
Figure 2a) were thinner than others. The 2.5 %
wt/
v solution fibers (
Figure 2b) had a well circular diameter, whereas those at higher concentration were more ribbon-like (
Figure 2c,d). These observations must be put into perspective because there were some variations in sample, due to poor evaporation of solvent resulting from drop projection (not shown here). The mean diameter of fibers from 2.86 %
wt/
v concentration was measured to be approximately 160 nm (
n = 40).
Figure 2.
SEM images of HPMC fibers for different concentration at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1 mm tip). (a): 2 % wt/v; (b): 2.5 % wt/v; (c): 2.86 % wt/v: (d): 3 % wt/v.
Figure 2.
SEM images of HPMC fibers for different concentration at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1 mm tip). (a): 2 % wt/v; (b): 2.5 % wt/v; (c): 2.86 % wt/v: (d): 3 % wt/v.
2.5. Analogy Between HPMC and Si-HPMC
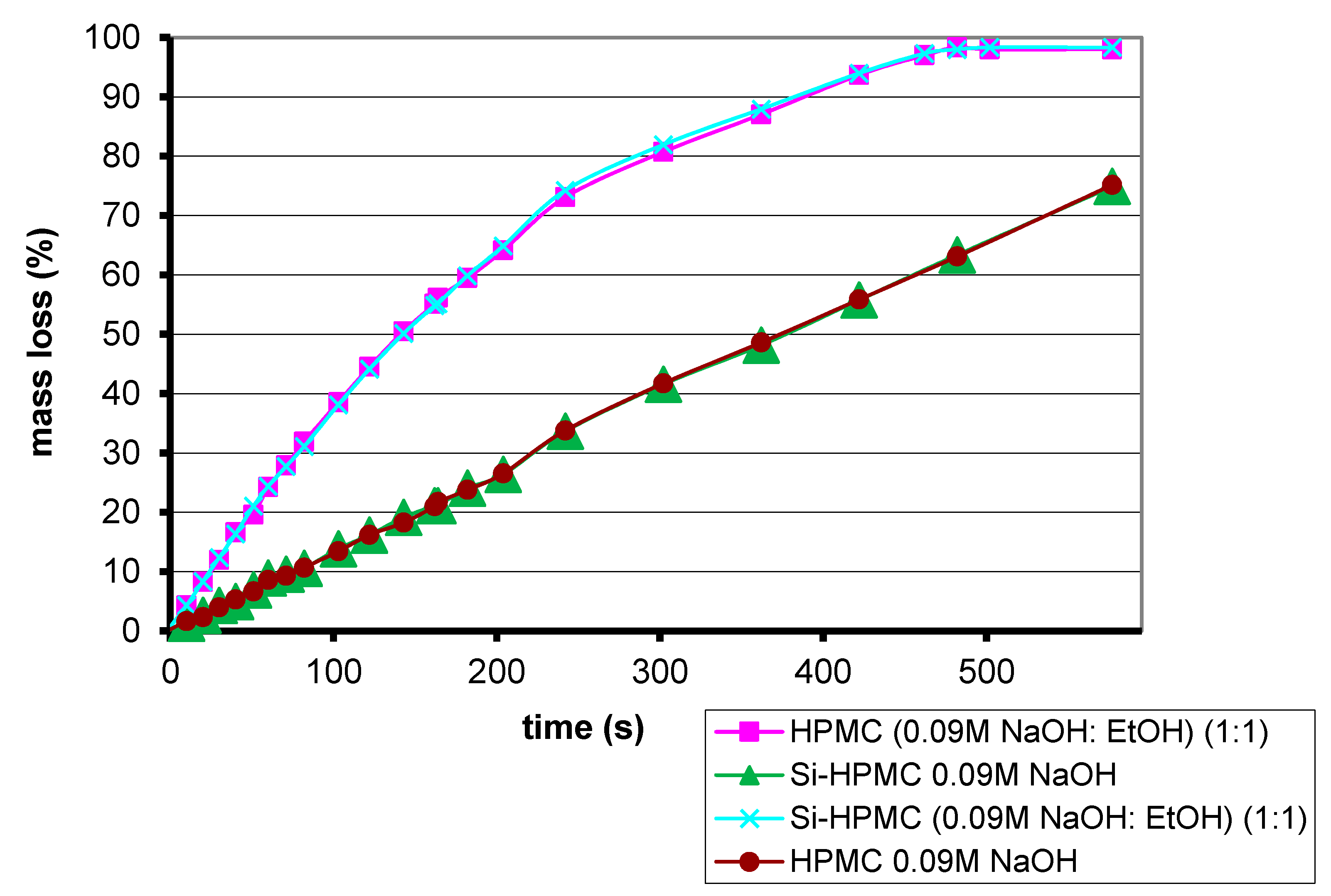
Electrospinning has never been applied to Si-HPMC, an analogy with HPMC was required to set up initial conditions. Comparisons between Si-HPMC and HPMC enable an understanding of which parameters have change to manage fiber preparation. The evaporation of solvent is similar for both solutions of polymers (
Figure 3). Four samples were incubated at 37 °C. Ethanol evaporates faster than sodium hydroxide solution. No differences were observed between Si-HPMC and HPMC solutions, which mean that silanization does not influence or prevent solvent evaporation.
Figure 3.
Percentage mass loss through evaporation for 2% wt/v polymer solutions.
Figure 3.
Percentage mass loss through evaporation for 2% wt/v polymer solutions.
The influence of non-modifiable parameters was investigated on HPMC solutions in order to study the electrospinning technique through a step by step process. The influence of sodium hydroxide has been studied on HPMC solutions. HPMC dissolved in 0.09 M·NaOH: ethanol mixture (1:1; 1:0.66; wt:wt) has been successfully electrospun at different concentrations and for different processing parameters. The ions (Na+ and OH−) had no critical influence.
To prepare sol-gel electrospinning, the influence of the HEPES based buffer was studied. A 4.3%
wt/
v HPMC (0.09 M NaOH: EtOH) (1:1) (
wt:
wt) was neutralized with HEPES buffer (volume bending 1v0.5) and electrospun at varying process parameters (
Table 1). In both attempts, the same fiber orientation described by Frenot
et al. was observed [
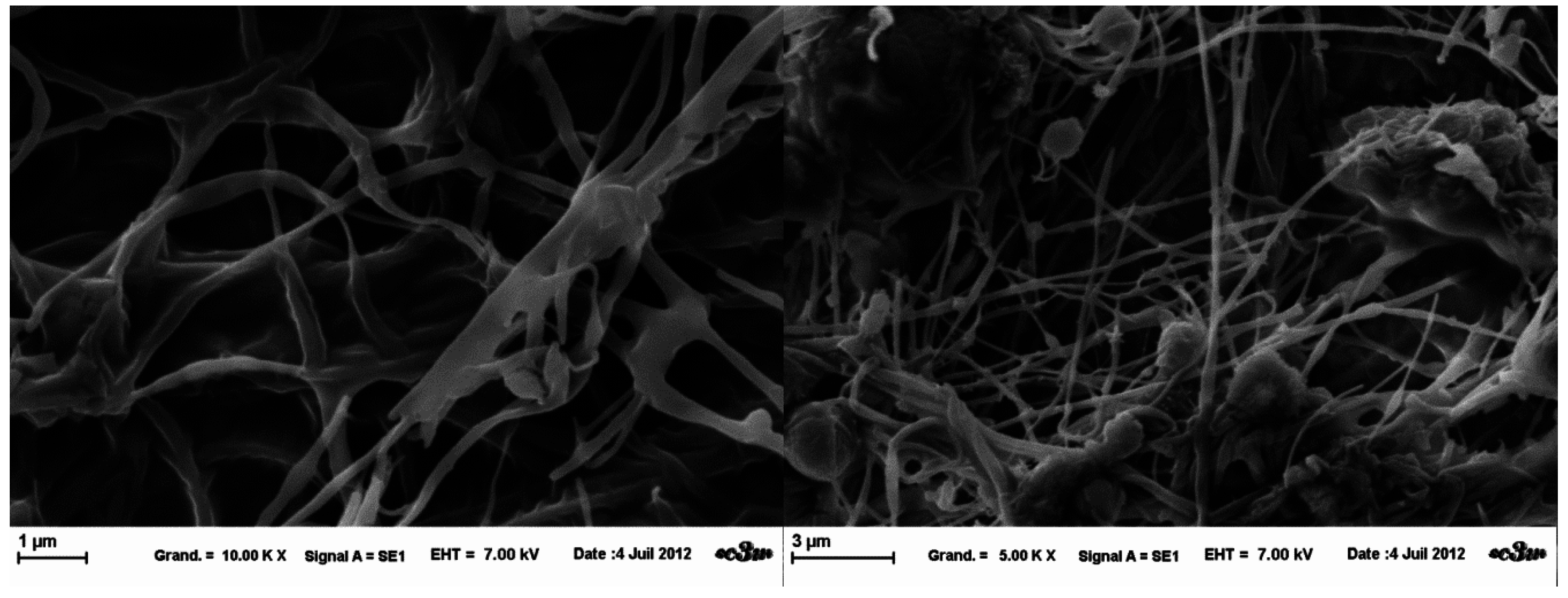
13]. The fibers fixed on the collector only by one extremity after projection, stood straight up in the electrical field. SEM images revealed the creation of micron fibers (
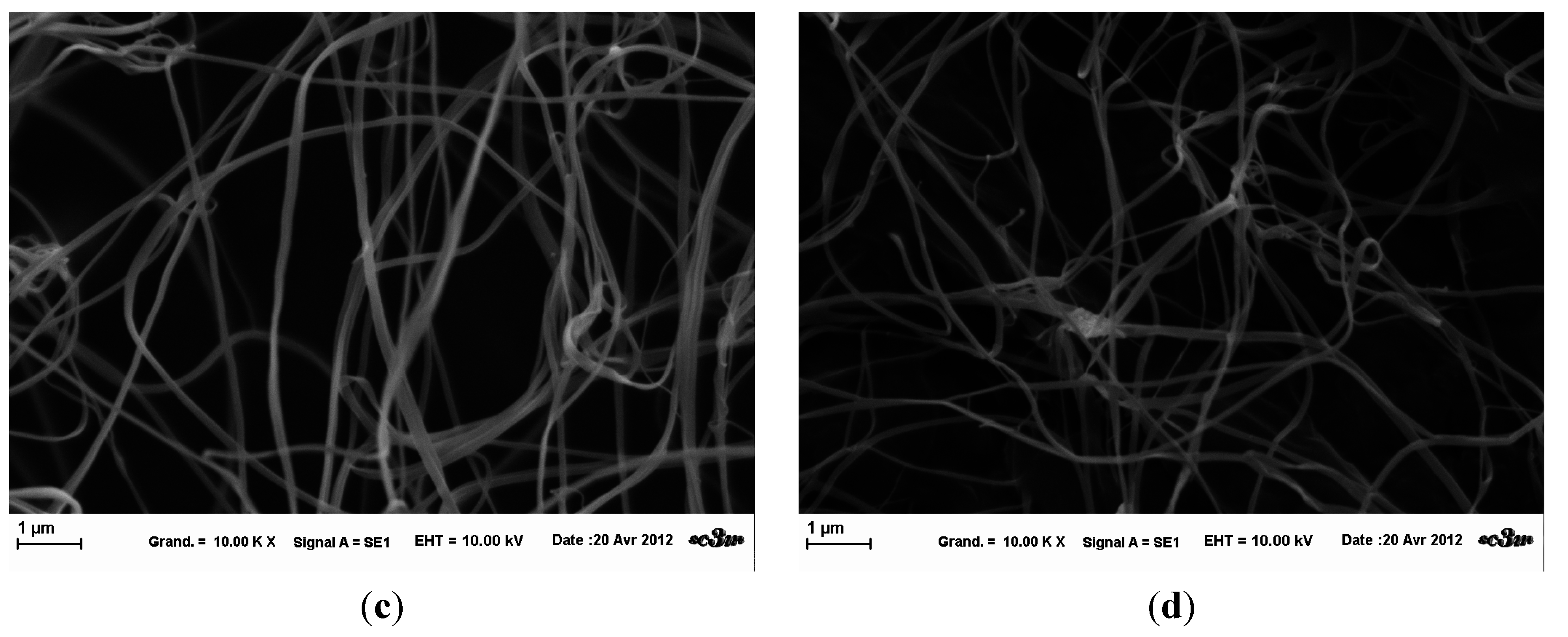
Figure 4). However, a lot of salt inclusions and beads were present within the fiber network. There are beaded fibers and ribbon-like fibers, which show the variability of the fibers obtained in these conditions.
Figure 4.
Influence of HEPES buffer on HPMC electrospinning at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1 mm tip). (a) at magnification 2.5 k×; (b) at magnification 5 k×; (c) at magnification 2,5 k×; (d) at magnification 5 k×.
Figure 4.
Influence of HEPES buffer on HPMC electrospinning at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1 mm tip). (a) at magnification 2.5 k×; (b) at magnification 5 k×; (c) at magnification 2,5 k×; (d) at magnification 5 k×.
Table 1.
Electrospinning parameters to study HEPES buffer influence.
Table 1.
Electrospinning parameters to study HEPES buffer influence.
| HPMC [c] % (wt/wt) | Solution and Solvent | Tension (kV) | Flow Rate (mL/h) | Capillary Tip (mm) | Gap Distance (cm) | Duration (mn) | Observations Figure 4 |
|---|
| 2.9 | 4.3 % wt/v HPMC (0.09M NaOH: EtOH) (1:1) (wt:wt) neutralized 1v0.25. Final pH 6.5. | 35 | 4.5 | 1 | 20 | 5 | Photo a and b |
| 2.9 | 30 | 3.0 | 0.8 | 14 | 30 | Photo c and d |
2.6. Electrospinning of HPMC/Si-HPMC Mixtures
Si-HPMC electrospinning parameters were based on those studied previously. In brief, injection flow rate was at 3.8 mL/h, needle diameter 0.6 mm, 15 cm2 aluminum foil acted as a collector and a gap distance of 15 cm.
The mixture of HPMC (Methocel™ E4M) and Si-HPMC will be subsequently referred to as “Template Solutions”. Three different template solutions were successfully electrospun, each containing different proportions of each component (
Table 2). The presence of nanofibers containing Si-HPMC was verified via Scanning Electron Microscopy (SEM) observation and Energy Dispersive Analysis (EDX).
It can be observed that a continuous jet projection cannot take place below a certain voltage applied to the generator. If the applied voltage is lower than this limit, there was only projections of droplets (electrospraying). In fact, the jet undergoes a capillary breakdown during the bending instabilities due to its surface tension. If the applied voltage is increased, there will be continuous projections with few droplet projections. During electrospinning, formation of vertical fibers up to a few centimeters long was observed on the collector screen. These fibers collapsed back to the collector, once the high tension is switched off.
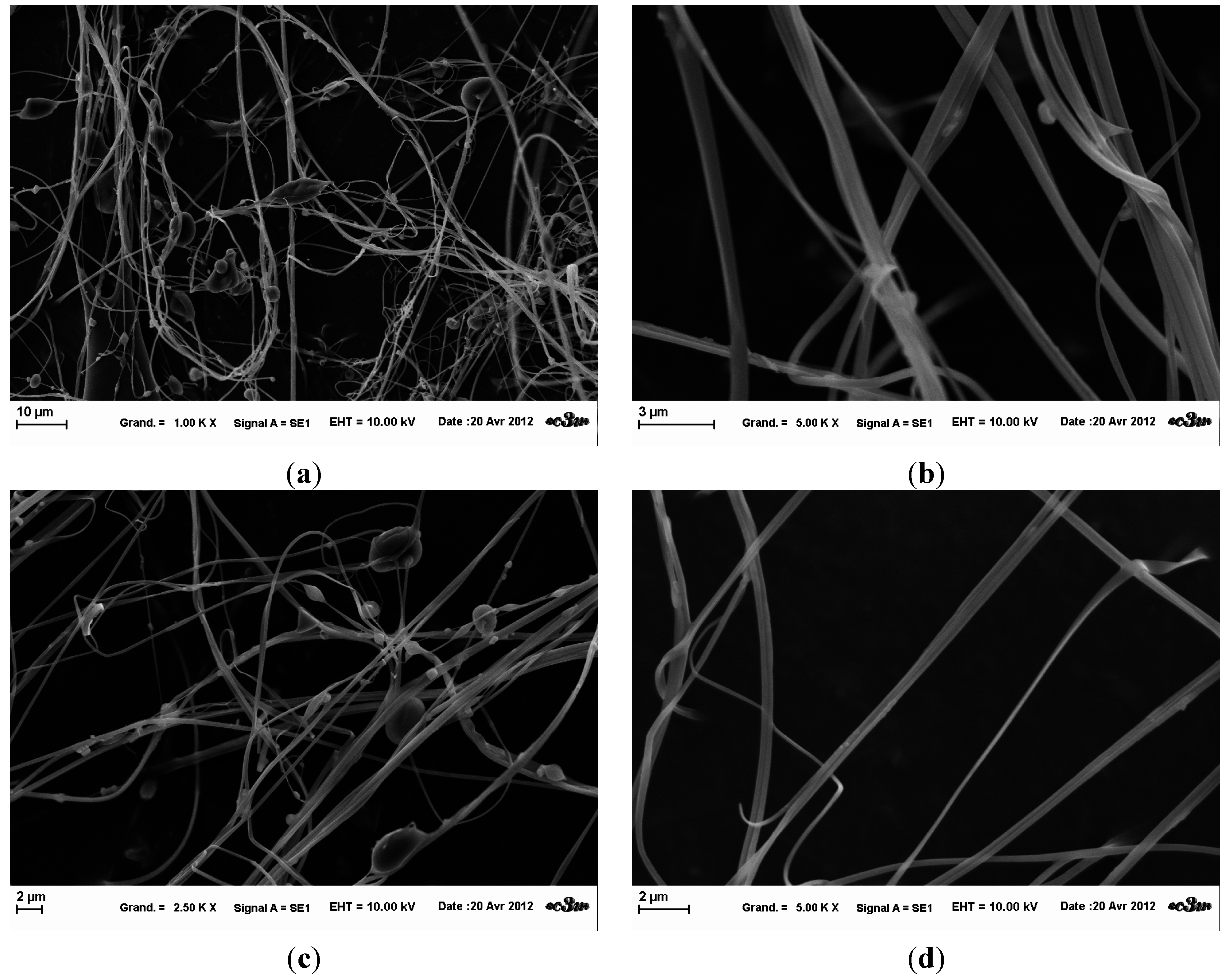
The fibers obtained from the three template solutions presented a very heterogeneous structure (
Figure 5,
Figure 6 and
Figure 7). Through SEM observations, it was noted that there is coexistence of fibers of different diameters (100 nm–500 nm), and also a beaded-structure.
Figure 5.
SEM images of template A nanofibers obtained at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1mm tip), at 5 k× magnification (left) and 10 k× (right).
Figure 5.
SEM images of template A nanofibers obtained at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1mm tip), at 5 k× magnification (left) and 10 k× (right).
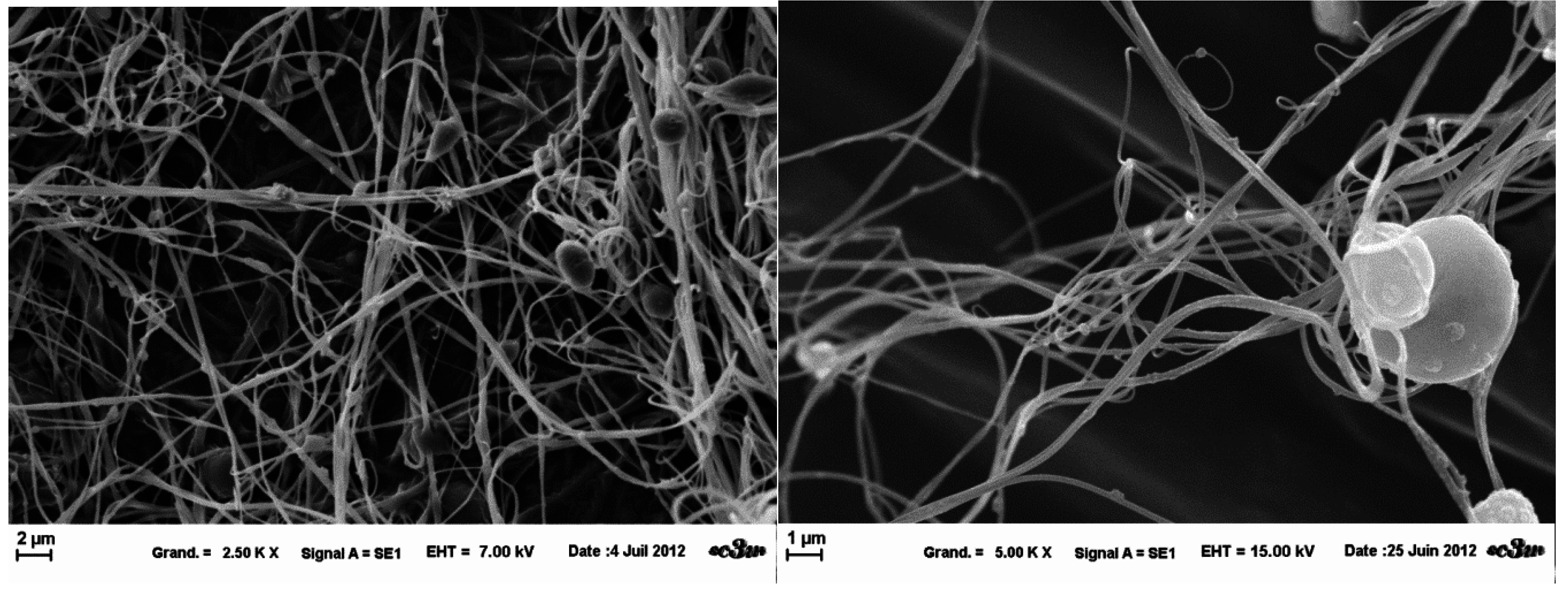
Figure 6.
SEM images of template B nanofibers obtained at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1 mm tip), at 2.5 k× magnification (left) and 5 k× (right).
Figure 6.
SEM images of template B nanofibers obtained at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1 mm tip), at 2.5 k× magnification (left) and 5 k× (right).
Figure 7.
SEM images of template A nanofibers obtained at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1mm tip), at 1 k× magnification (left) and 10 k× (right).
Figure 7.
SEM images of template A nanofibers obtained at fixed parameters (35 kV, 4.5 mL/H, 20 cm distance and 1mm tip), at 1 k× magnification (left) and 10 k× (right).
2.7. EDX Analyzes
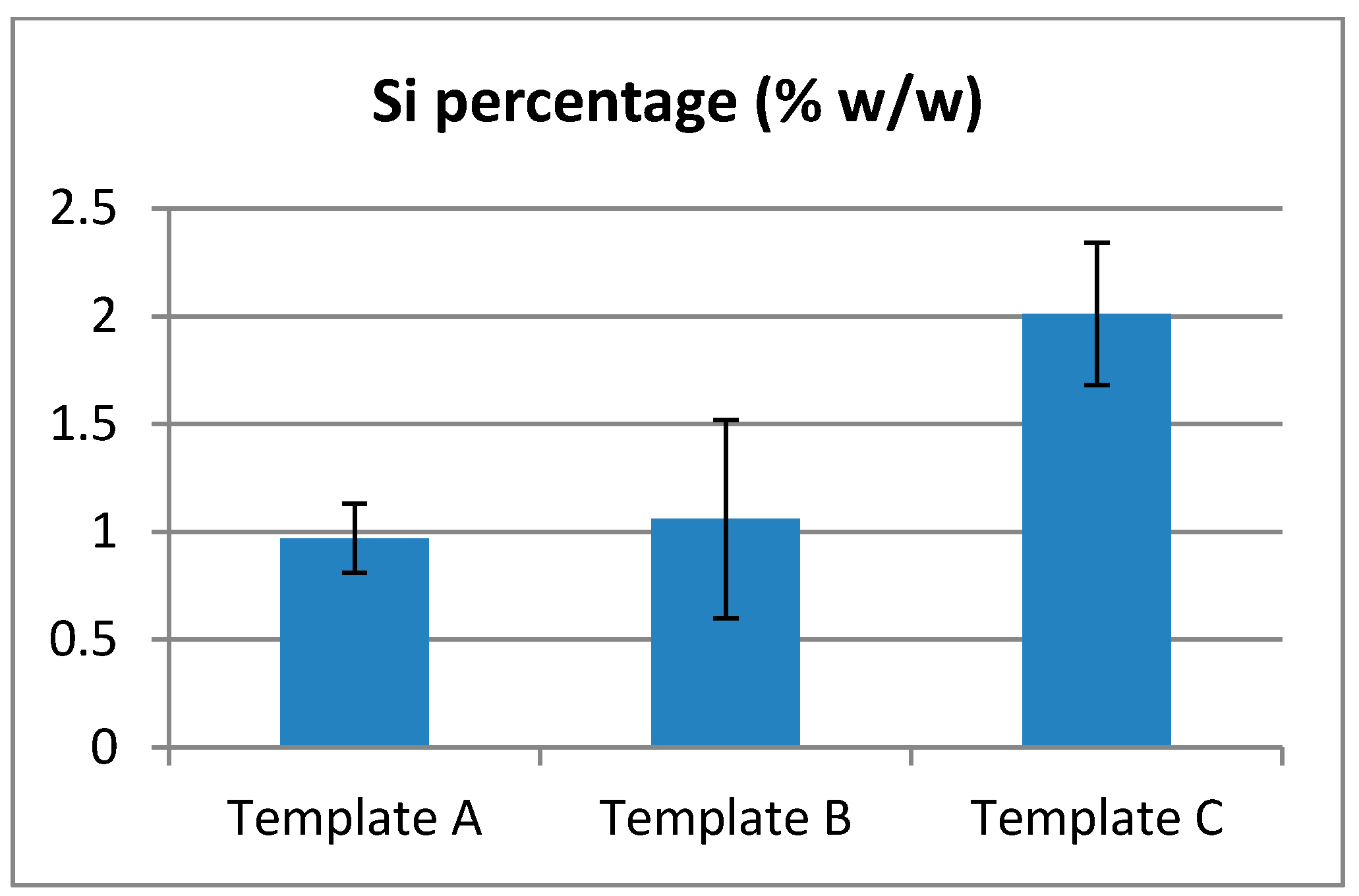
EDX Analyzes showed the presence of Silicon amongst the fibers collected (
Figure 8). However, due to the large discrepancies in the data, no conclusive quantitative results can be made about the quantity or repartition of Si. It is possible to only conclude that the collected fibers contain Si-HPMC.
According to
Fatimi et al., Si-HPMC dissolution strongly depends upon sodium hydroxide concentration [
14]. However, it does not change the maximal concentration of polymer that is approximately 5–6 %
wt/
v. Only solutions with low concentrations are obtained leading to high viscosity, thus limiting their use for electrospinning. Inhomogeneity was also observed when creating co-solvent solutions during dilution, particularly when the amount of solvent was higher than the 0.09 M NaOH volume. An example was shown when 2.5 %
wt/
v Si-HPMC (0.09 M NaOH:THF) (1:1.5) (
wt:
wt) formed a solution that included more viscous aggregates.
Figure 8.
Rate of Silicon detected on electrospun fibers.
Figure 8.
Rate of Silicon detected on electrospun fibers.
Electrospinning procedures were reproduced as described in the literature. In terms of HPMC, the influence of process parameters were not as important as previously described [
15]. It has been found that a range of parameters enables fiber fabrication with little influence on morphology. Beaded fibers, ribbon-like fibers and small fiber bridges were observed at the same time. However, a limiting parameter for HPMC was the concentration. If the quantity of polymer was low (less than 2 %
wt/
v for HPMC), fibers were not created because of lack of interactions between the macromolecules. On the contrary, if the quantity of polymer was too high (more than 3.5 %
wt/
v), the viscosity was too high, preventing injectability and thus preventing the fiber formation.
Macroscopic mat appearance seems to differ in homogeneity. If drops did not evaporate, their projection on the collector partially dissolves the mat. Thus, the evaporation of the solvent controlled the appearance of the mat. Despite optimal conditions, in certain instances, the macroscopic appearance of the mat was not controlled. This could be due to drying of the solution at the needle tip. As the Taylor’s cone elongates and solution builds up at the needle tip, drop formations are promoted. The size of the aluminum foil used as a collector appears to help the formation of a steady mat. Consequently, more polymer needs to be electrospun for retrieval of the mat, this increases the time of the electrospinning experiments.
The main limitations observed were that the polymer concentration and the ability of the solution to dry. The high molecular weight of HPMC and SI-HPMC limits the maximum concentration and consequently the size range of the electrospun fibers. Indeed, only nanometer size fibers could be spun, which fits perfectly within the objectives of the study. However, the ability to dry represents an issue that has to be overcome. Fibers cannot be spun without controlling this parameter, as it depends upon the capability of the polymer to retain water within its backbone and it can be used to modulate specific parameters of the electrospinning device (e.g., tension and gap distance). The hydrophilicity of the polymer and the presence of ionic head groups and salts help to retain water and induce a decrease of the applied tension because of the solution conductivity properties that are increased by the presence of charges. However, despite the lack of homogeneity within beaded fiber mats, due to salt inclusions, no influence is expected on the future application. Indeed, once formulated within a hydrogel, containing up to 98% of water, the salt inclusions are expected to dissolve and only the cross-linked fibers will remain.
A typical organization of HPMC fibers was obtained with the solution containing HEPES buffer. Rather than being projected on the grounded collector as an unwoven mat, fibers stood straight up in the electrical field. In comparison with same observation by Frenot et al. for enzymatically treated cellulose, we could conclude that Cl− ions are responsible for this conformation. These anions are attracted by the high positive charge at the tip of the nozzle. Further investigations should be done to study the influence of conformation on fiber morphology.
Our strategy was to use the HPMC E4M as template because its spinnability has been demonstrated and the objectives are to create insoluble and cross-linkable fibers. This study has demonstrated that this strategy is applicable and encourages further insight. Indeed, we have successfully prepared electrospun fibers with template solution regarding the HPMC/Si-HPMC ratio.
Electrospinning is a very complex technique dependent upon many parameters such as the polymer, the polar head groups within the polymer backbone, the concentration, the solvent and co-solvent and the presence of other molecules (salts). Their influence also differ according to the technical parameters (e.g., voltage, temperature, collector-tip distance, humidity, and injection speed). Regarding the data obtained throughout this study, it is worth noting that all of these parameters did not interfere with the electrospinnability on their own but more as one multifactorial parameter. Indeed, solvent, co-solvent, and salts present are important for dryness, fiber quality (e.g., salt inclusions), the polymer structure and more importantly the presence of polar head groups within its structure are key for the entanglement properties at fiber formation.