Restoring Waning Production of Volatile Organic Compounds in the Endophytic Fungus Hypoxylon sp. (BS15)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
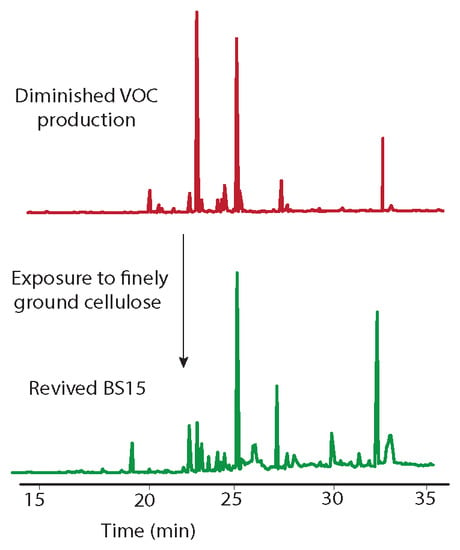
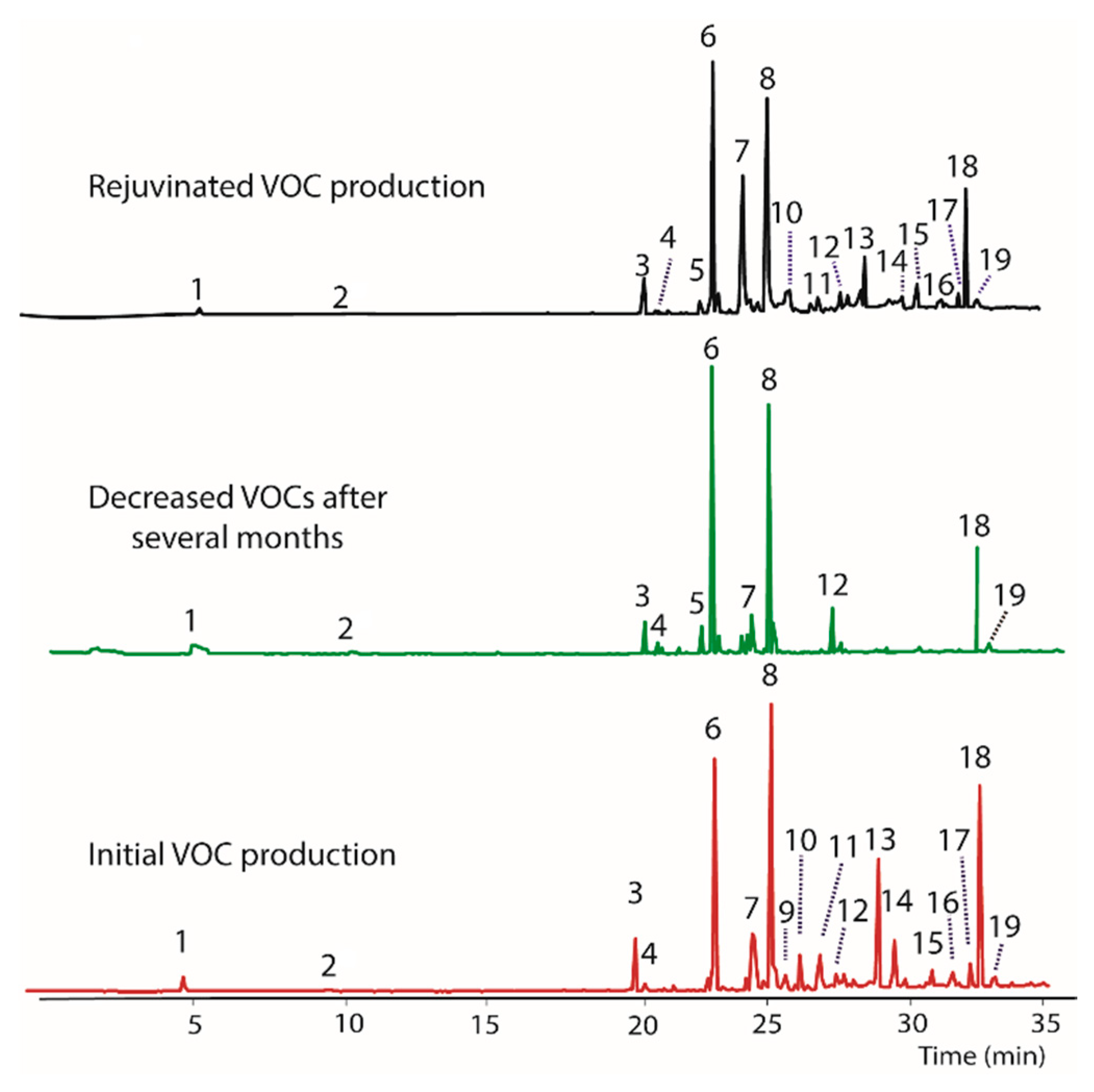
4. Decreased VOC Production in BS15 after Extended In Vitro Growth
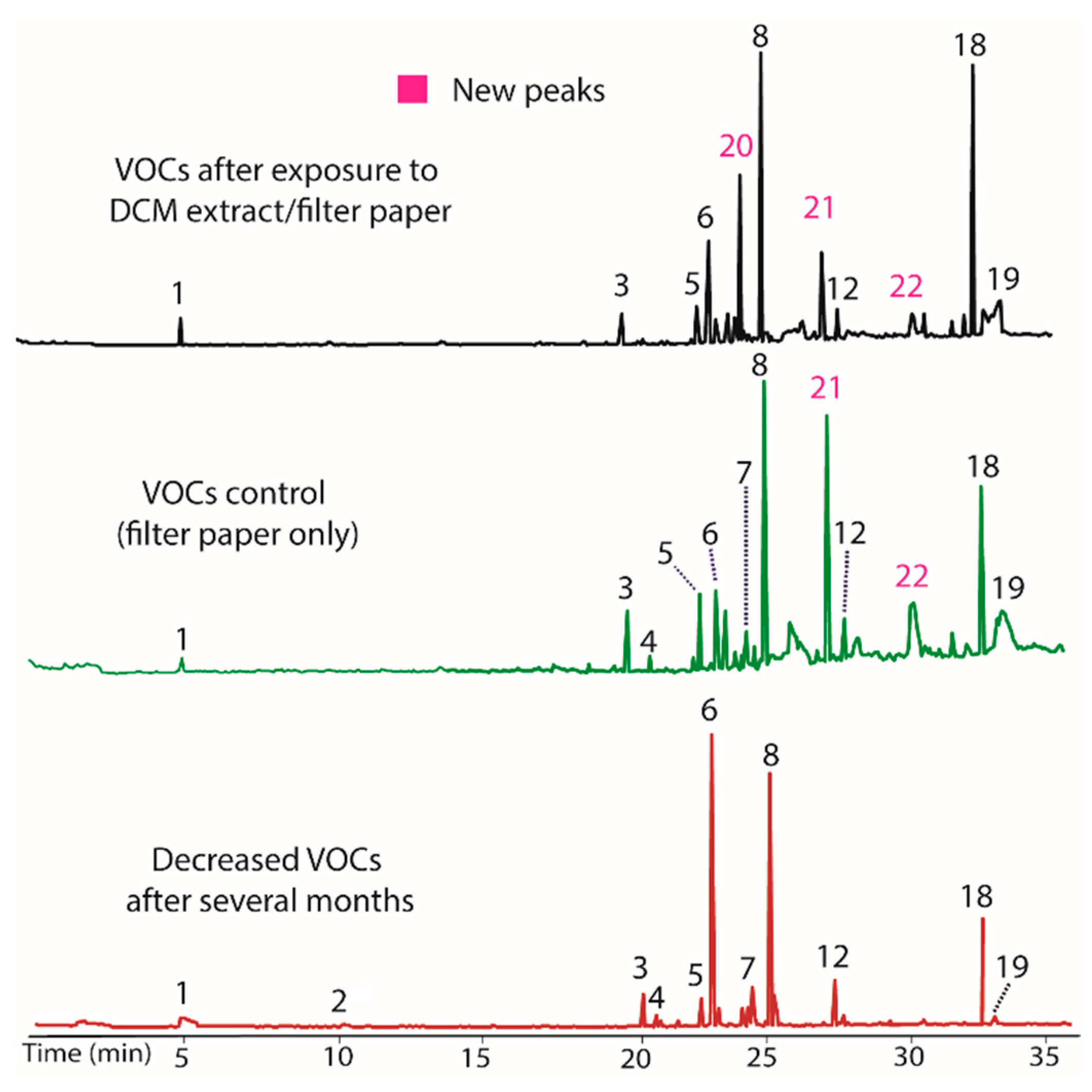
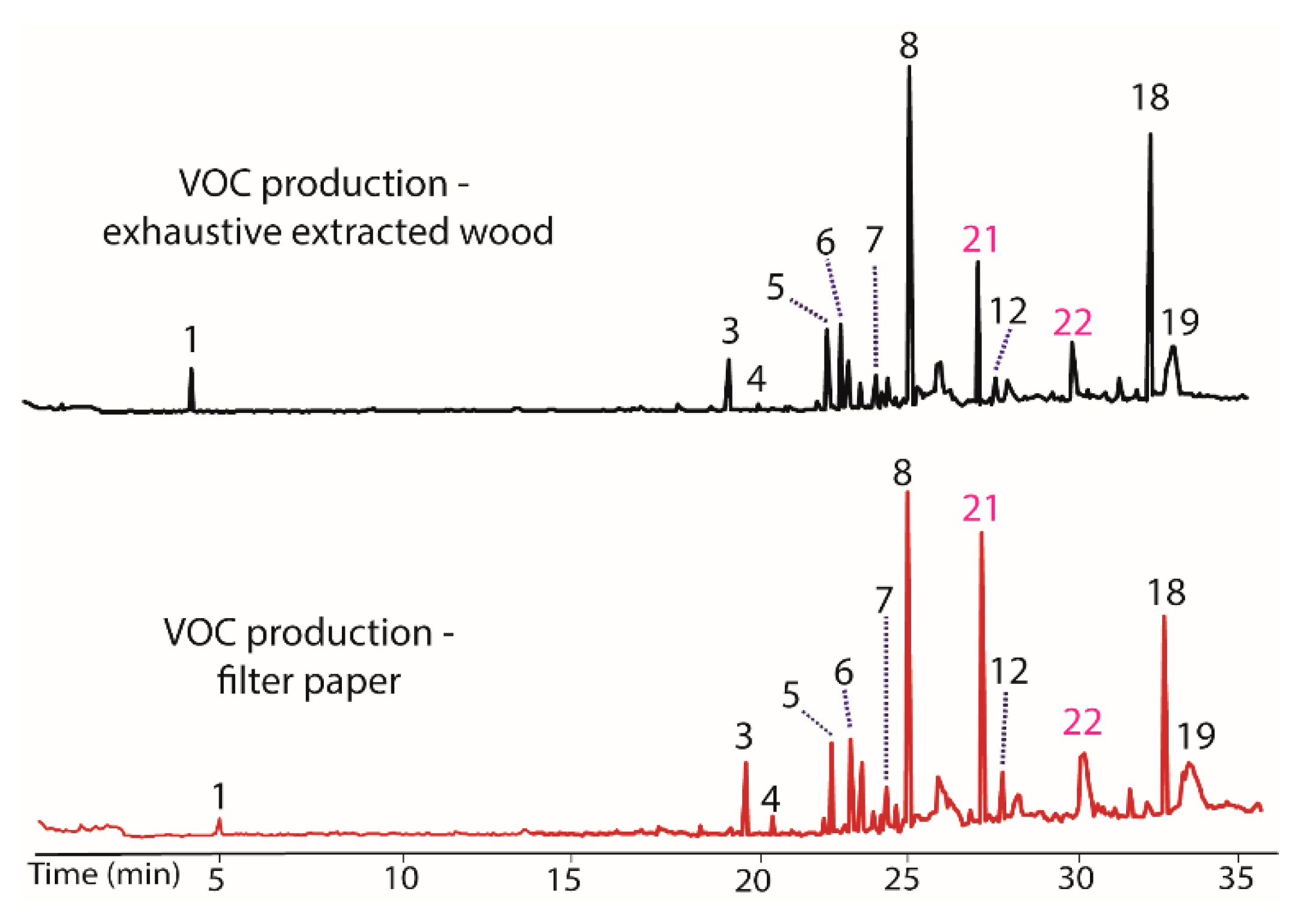
5. Assessing the Influence of DCM Extract/Filter Paper on VOC Production
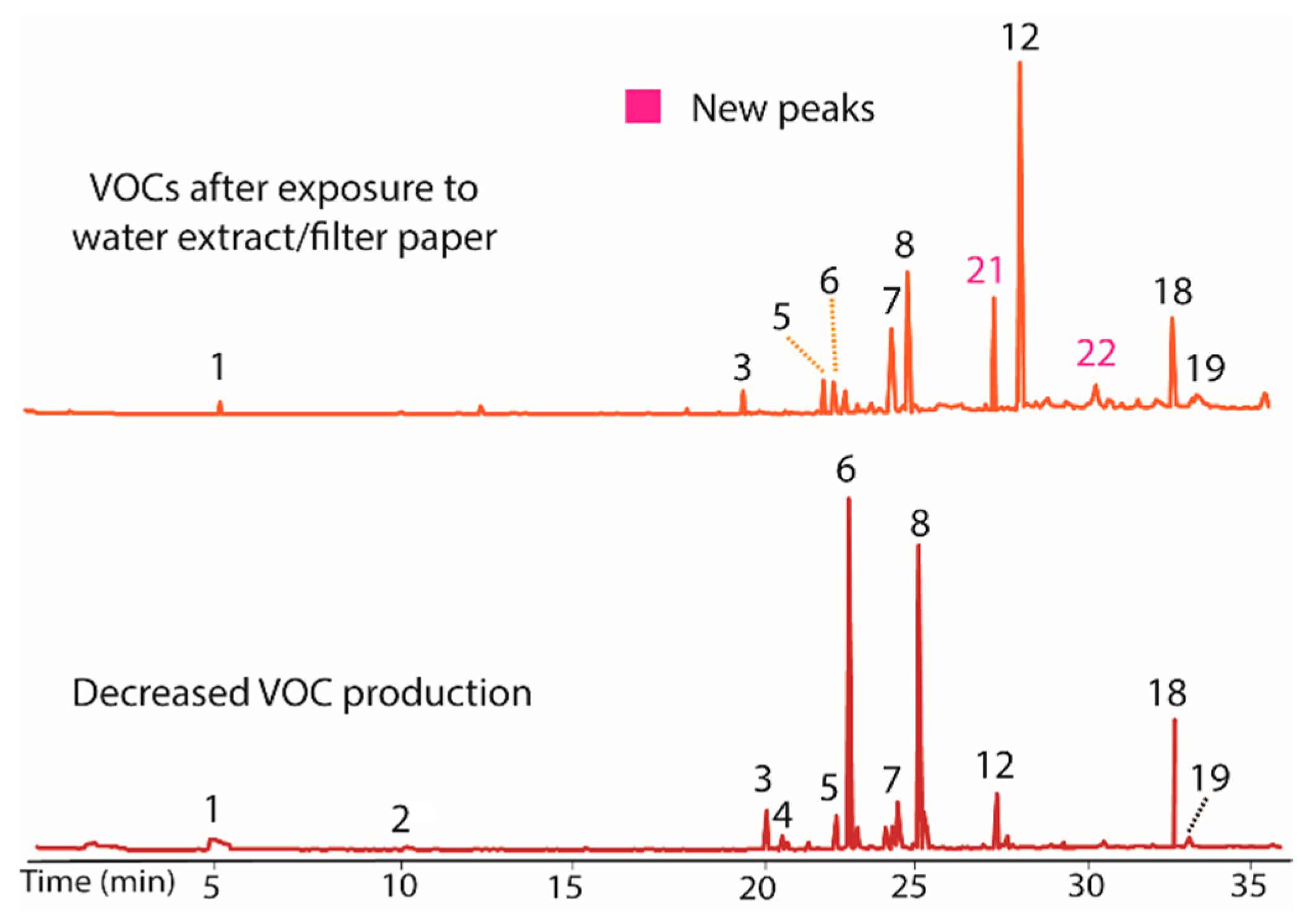
6. Evaluating the Influence of Methanol and Water Extracts on VOC Production in BS15
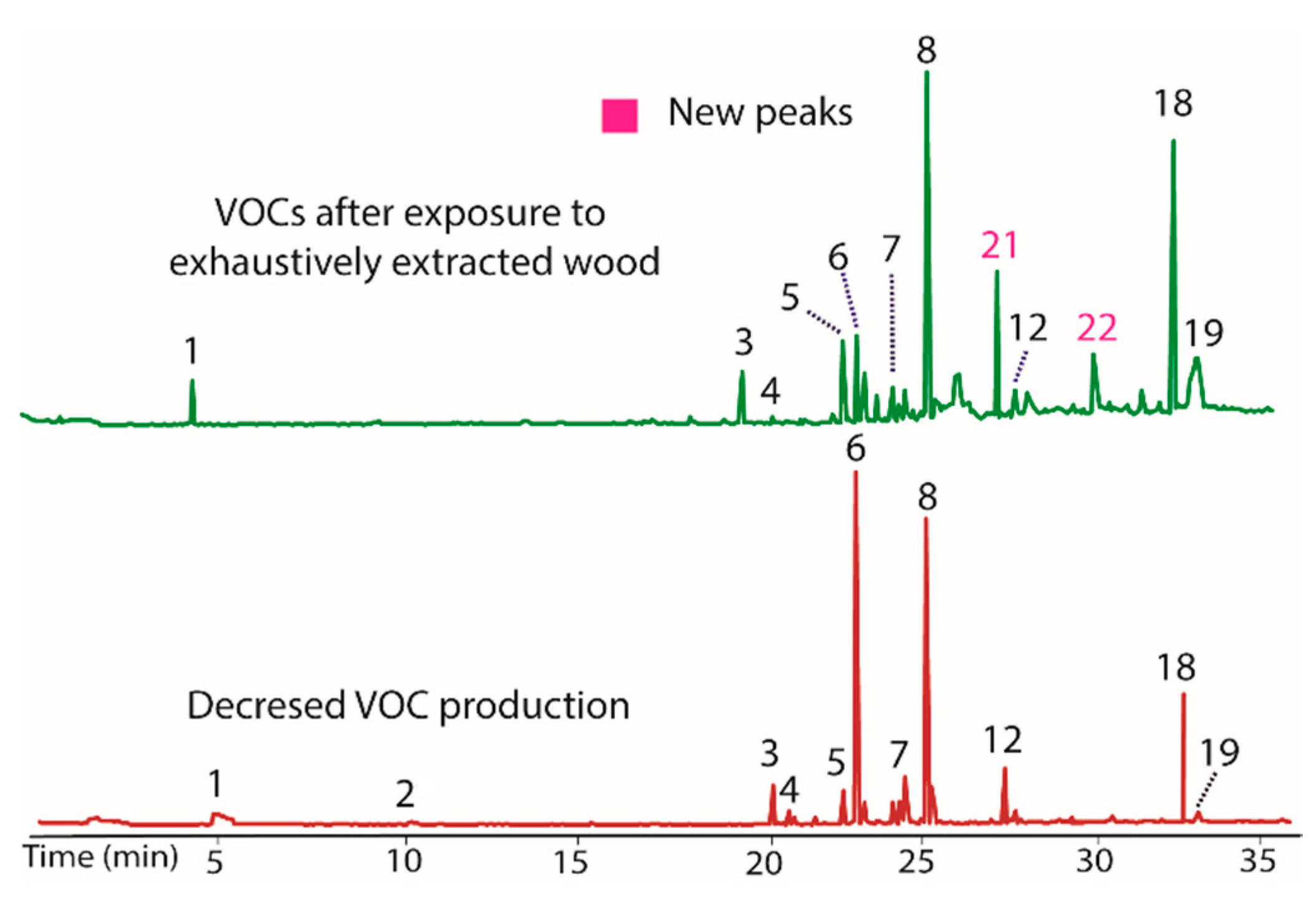
7. The Influence of Exhaustively Extracted T. distichum Wood on VOC Production in BS15
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.L.; Maccheroni, W., Jr.; Pereira, J.O.; Araújo, W.L.D. Endophytic microorganisms: A review on insect control and recent advances on tropical plants. Electron. J. Biotechnol. 2000, 3, 40–65. [Google Scholar] [CrossRef]
- Gao, F.K.; Dai, C.C.; Liu, X.Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010, 4, 1346–1351. [Google Scholar]
- Li, J.Y.; Harper, J.K.; Grant, D.M.; Tombe, B.O.; Bashyal, B.; Hess, W.M.; Strobel, G.A. Ambuic acid, a highly functionalized cyclohexenone with antifungal activity from Pestalotiopsis spp. and Monochaetia sp. Phytochemistry 2001, 56, 463–468. [Google Scholar] [CrossRef]
- Castillo, U.; Harper, J.K.; Strobel, G.A.; Sears, J.; Alesi, K.; Ford, E.; Lin, J.; Hunter, M.; Maranta, M.; Ge, H.; et al. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol. Lett. 2003, 224, 183–190. [Google Scholar] [CrossRef]
- Strobel, G.A.; Knighton, B.; Kluck, K.; Ren, Y.; Livinghouse, T.; Griffin, M.; Spakowicz, D.; Sears, J. The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072). Microbiology 2008, 154, 3319–3328. [Google Scholar] [CrossRef] [PubMed]
- Tamilvendhan, D.; Ilangovan, V.; Karthikeyan, R. Optimisation of engine operating parameters for eucalyptus oil mixed diesel fueled diesel engine using Taguchi method. ARPN J. Eng. Appl. Sci. 2011, 6, 14–22. [Google Scholar]
- Tarabet, L.; Loubar, K.; Lounici, M.S.; Hanchi, S.; Tazerout, M. Eucalyptus biodiesel as an alternative to diesel fuel: Preparation and tests on diesel engine. J. Biomed. Biotechnol. 2012, 2012, 235485. [Google Scholar] [CrossRef] [PubMed]
- Kazuo Sugito, K.S.T. Fuel Composition. U.S. Patent No. 4,297,109, 27 October 1981. [Google Scholar]
- Tomsheck, A.R.; Strobel, G.A.; Booth, E.; Geary, B.; Spakowicz, D.; Knighton, B.; Floerchinger, C.; Sears, J.; Liarzi, O.; Ezra, D. Hypoxylon sp., an endophyte of Persea indica, producing 1,8-cineole and other bioactive volatiles with fuel potential. Microb. Ecol. 2010, 60, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G. Methods of discovery and techniques to study endophytic fungi producing fuel-related hydrocarbons. Nat. Prod. Rep. 2014, 31, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yin, G.; Inamdar, A.A.; Luo, J.; Zhang, N.; Yang, I.; Buckley, B.; Bennett, J.W. Volatile organic compounds emitted by filamentous fungi isolated from flooded homes after Hurricane Sandy show toxicity in a Drosophila bioassay. Indoor Air 2017, 27, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S.; Azad, S.A.; Abu Bakar, F.; Naher, L.; Vijay Kumar, S. Separation and identification of hydrocarbons and other volatile compounds from cultures of Aspergillus niger by GC–MS using two different capillary columns and solvents. J. Saudi Chem. Soc. 2015, 19, 243–256. [Google Scholar] [CrossRef]
- Fiers, M.; Lognay, G.; Fauconnier, M.-L.; Jijakli, M.H. Volatile compound-mediated interactions between barley and pathogenic fungi in the soil. PLoS ONE 2013, 8, e66805. [Google Scholar] [CrossRef] [PubMed]
- Sav Savelieva, E.I.; Gustyleva, L.K.; Kessenikh, E.D.; Khlebnikova, N.S.; Leffingwell, J.; Gavrilova, O.P.; Gagkaeva, T.Y. Study of the vapor phase over Fusarium fungi cultured on various substrates. Chem. Biodivers. 2016, 13, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A. Bioprospecting—Fuels from fungi. Biotechnol. Lett. 2015, 37, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G. Muscodor species—Endophytes with biological promise. Phytochem. Rev. 2011, 10, 165–172. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Strobel, G.A.; Moore, E.; Robison, R.; Sears, J. Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microbiology 2010, 156, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Meshram, V.; Kapoor, N.; Saxena, S. Muscodor kashayum sp. nov.—A new volatile anti-microbial producing endophytic fungus. Mycology 2013, 4, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Meshram, V.; Saxena, S.; Kapoor, N. Muscodor strobelii, a new endophytic species from South India. Mycotaxon 2014, 128, 93–104. [Google Scholar] [CrossRef]
- Saxena, S.; Meshram, V.; Kapoor, N. Muscodor darjeelingensis, a new endophytic fungus of Cinnamomum camphora collected from northeastern Himalayas. Sydowia 2014, 66, 55–67. [Google Scholar]
- Saxena, S.; Meshram, V.; Kapoor, N. Muscodor tigerii sp. nov.-volatile antibiotic producing endophytic fungus from the Northeastern Himalayas. Ann. Microbiol. 2015, 65, 47–57. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Bussaban, B.; Hyde, K.D.; Matsui, K.; Lumyong, S. Molecular and morphological evidence support four new species in the genus Muscodor from northern Thailand. Ann. Microbiol. 2013, 63, 1341–1351. [Google Scholar] [CrossRef]
- Kudalkar, P.; Strobel, G.; Riyaz-Ul-Hassan, S.; Geary, B.; Sears, J. Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience 2012, 53, 319–325. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, G.P.; Mao, L.J.; Komon-Zelazowska, M.; Yuan, Z.L.; Lin, F.C.; Druzhinina, I.S.; Kubicek, C.P. Muscodor fengyangensis sp. nov. from southeast China: Morphology, physiology and production of volatile compounds. Fungal Biol. 2010, 114, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Strobel, G.A.; Booth, E.; Geary, B.; Sears, J.; Spakowicz, D.; Busse, S. An endophytic Myrothecium inundatum producing volatile organic compounds. Mycosphere 2010, 1, 229–240. [Google Scholar]
- Samaga, P.V.; Rai, V.R.; Rai, K.M.L. Bionectria ochroleuca NOTL33—An endophytic fungus from Nothapodytes foetida producing antimicrobial and free radical scavenging metabolites. Ann. Microbiol. 2014, 64, 275–285. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Strobel, G.A.; Knighton, B.; Geary, B.; Sears, J.; Ezra, D. An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb. Ecol. 2011, 61, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.; Pedrini, N.; Juárez, M.P.; Dal Bello, G.M. Volatile organic compounds released by the entomopathogenic fungus Beauveria bassiana. Microbiol. Res. 2008, 163, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.A.; Spakowicz, D.J.; Gianoulis, T.A.; Strobel, S.A. Volatile organic compound production by organisms in the genus Ascocoryne and a re-evaluation of myco-diesel production by NRRL 50072. Microbiology 2010, 156, 3814–3829. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Singh, S.K.; Riyaz-Ul-Hassan, S.; Mitchell, A.M.; Geary, B.; Sears, J. An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol. Lett. 2011, 320, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ul-Hassan, S.R.; Strobel, G.A.; Booth, E.; Knighton, B.; Floerchinger, C.; Sears, J. Modulation of volatile organic compound formation in the Mycodiesel-producing endophyte Hypoxylon sp CI-4. Microbiology 2012, 158, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Nigg, J.; Strobel, G.; Knighton, W.B.; Hilmer, J.; Geary, B.; Riyaz-Ul-Hassan, S.; Harper, J.K.; Valenti, D.; Wang, Y. Functionalized para-substituted benzenes as 1,8-cineole production modulators in an endophytic Nodulisporium sp. Microbiology 2014, 160, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Dirkse, E.; Sears, J.; Markworth, C. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 2001, 147, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.R.; Shockley, K.R.; Conners, S.B.; Scott, K.L.; Wolfinger, R.D.; Kelley, R.M. Carbohydrate-induced differential gene expression patterns in the hyperthermophilic bacterium Thremotoga maritima. J. Biol. Chem. 2003, 278, 7540–7552. [Google Scholar] [CrossRef] [PubMed]

| Species | Site Isolation | Extraction Method a | No. of VOCs Detected | Reference |
|---|---|---|---|---|
| Aspergillus fumigatus | New Jersey, USA | SPME | >10 | [13] |
| A. niger | New Jersey, USA | SPME | [13] | |
| A. tubingensis | New Jersey, USA | SPME | [13] | |
| A. niger | Malaysia | LLE | >295 | [14] |
| Fusarium armeniacum | New Jersey, USA | SPME | >10 | [13] |
| F. graminearum | New Jersey, USA | SPME | [13] | |
| F. oxysporum | New Jersey, USA | SPME | [13] | |
| F. proliferatum | New Jersey, USA | SPME | [13] | |
| F. culmorum | Belgium | SPME | >10 | [15] |
| F. langsethiae | Russia | SPME | >40 | [16] |
| F. sibiricum | Russia | SPME | [16] | |
| F. poae | Russia | SPME | [16] | |
| F. sporotrichioides | Russia | SPME | [16] | |
| Metarhizium anisopliae | New Jersey, USA | SPME | >5 | [13] |
| Mucor racemsus | New Jersey, USA | SPME | >10 | [13] |
| Penicillium chrysogenum | New Jersey, USA | SPME | >10 | [13] |
| P. citreonigrum | New Jersey, USA | SPME | [13] | |
| P. commune | New Jersey, USA | SPME | [13] | |
| P. corylophilum | New Jersey, USA | SPME | >10 | [13] |
| P. crustosum | New Jersey, USA | SPME | [13] | |
| P. glabrum | New Jersey, USA | SPME | [13] | |
| P. pinophilum | New Jersey, USA | SPME | [13] | |
| P. polonicum | New Jersey, USA | SPME | [13] | |
| P. sclerotiorum | New Jersey, USA | SPME | [13] | |
| P. steckii | New Jersey, USA | SPME | [13] | |
| P. sumatrense | New Jersey, USA | SPME | [13] | |
| Nodulisporium | Canary Islands, Ecuador, Thailand, Nicaragua, South Australia, Colombia, and Wetlands of Florida | SPME | >40 | [17] |
| Muscodor albus | Honduras, Thailand, and Ecuador | SPME | >20 | [18] |
| M. crispans | Bolivian Amazon basin | SPME | >15 | [19] |
| M. kashayum | India | SPME | >20 | [20] |
| M. strobelii | India | SPME | >14 | [21] |
| M. darjeelingensis | India | SPME | >20 | [22] |
| M. tigerii | India | SPME | >20 | [23] |
| M. suthepensis | Thailand | SPME | >25 | [24] |
| M. musae | Thailand | SPME | >15 | [24] |
| M. oryzae | Thailand | SPME | >15 | [24] |
| M. equiseti | Thailand | SPME | >15 | [24] |
| M. sutura | Colombia | SPME | >20 | [25] |
| M. fengyangensis | China | SPME | >20 | [26] |
| Myrothecium inunduatum | India | SPME | >30 | [27] |
| Bionectria ochroleuca | India | LLE | >5 | [28] |
| Ampelomyces | Japan | SPME | >5 | [29] |
| Phoma | Japan | SPME | >5 | [29] |
| Cladosporium | Japan | SPME | <5 | [29] |
| Phomopsis | Ecuador | SPME | >10 | [30] |
| Gliocladium roseum | Northern Patagonia | SPME | >40 | [7] |
| Beauveria bassiana | Montana, USA | SPME | 6 | [31] |
| Ascocoryne sarcoides | Northern Patagonia, UK, Germany, France, Norway, and Canada | SPME | >100 | [32] |
| A. cylichnium | Norway, Switzerland | SPME | [32] | |
| A. solitaria | Netherlands | SPME | [32] | |
| Schizophylum commune | Chile | SPME | 10 | [33] |
| Hypoxylon | Thailand, Spain | SPME | >15 | [34] |
| Peak | R.T. (min) | Tentative Identify | Mol. Mass |
|---|---|---|---|
| 1 | 4.75 | unknown | 70 |
| 2a | 9.41 | 1,8-cineole | 154 |
| 3 | 19.15 | unknown | 142 |
| 4 | 19.78 | unknown | 120 |
| 5 | 21.84 | unknown | 126 |
| 6a | 22.35 | Phenyl ethyl alcohol | 122 |
| 7a | 23.85 | 2,3-naphthalenediamine | 158 |
| 8 | 24.51 | unknown | 182 |
| 9 | 24.75 | Unknown | 184 |
| 10 | 25.87 | unknown | 220 |
| 11a | 26.58 | Phenylacetic acid | 136 |
| 12 | 27.62 | Unknown | 298 |
| 13a | 28.72 | Diethyl phthalate | 222 |
| 14 | 29.52 | unknown | 297 |
| 15 | 30.72 | unknown | 213 |
| 16 | 31.47 | unknown | 334 |
| 17 | 32.15 | unknown | 213 |
| 18 | 32.63 | unknown | 192 |
| 19 | 33.27 | unknown | 314 |
| Peak | R.T. (min) | Tentative Identify | Mol. Mass |
|---|---|---|---|
| 1 | 4.75 | Unknown | 70 |
| 2 | 9.41 | 1,8-Cineole | 154 |
| 3 | 19.15 | Unknown | 142 |
| 4 | 19.78 | Unknown | 120 |
| 5 | 21.84 | Unknown | 126 |
| 6 | 22.35 | Phenyl ethyl alcohol | 122 |
| 7 | 23.85 | 2,3-Naphthalenediamine | 158 |
| 8 | 24.51 | Unknown | 182 |
| 9 | 24.75 | Unknown | 184 |
| 10 | 25.87 | Unknown | 220 |
| 11 | 26.58 | Phenylacetic acid | 136 |
| 12 | 27.62 | Unknown | 298 |
| 13 | 28.72 | Diethyl phthalate | 222 |
| 14 | 29.52 | Unknown | 297 |
| 15 | 30.72 | Unknown | 213 |
| 16 | 31.47 | Unknown | 334 |
| 17 | 32.15 | Unknown | 213 |
| 18 | 32.63 | Unknown | 192 |
| 19 | 33.27 | Unknown | 314 |
| 20a | 23.76 | 3-methyl-2,5-furandione | 112 |
| 21 | 26.85 | Unknown | 216 |
| 22a | 29.82 | 4,4′-thiobis-benzeneamine | 154 |
| Extract | Increased Production | Decreased Production | New Compounds Produced |
|---|---|---|---|
| DCM | 18 | 6 | 20, 21, 22 |
| Methanol | None | None | 21, 22 |
| Water | 7, 12, 18 | 6 | 21, 22 |
| Wood, extracted | 5, 18 | 7, 12 | 21, 22 |
| Filter paper | 5, 18 | 7, 12 | 21, 22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Harper, J.K. Restoring Waning Production of Volatile Organic Compounds in the Endophytic Fungus Hypoxylon sp. (BS15). J. Fungi 2018, 4, 69. https://doi.org/10.3390/jof4020069
Wang Y, Harper JK. Restoring Waning Production of Volatile Organic Compounds in the Endophytic Fungus Hypoxylon sp. (BS15). Journal of Fungi. 2018; 4(2):69. https://doi.org/10.3390/jof4020069
Chicago/Turabian StyleWang, Yuemin, and James K. Harper. 2018. "Restoring Waning Production of Volatile Organic Compounds in the Endophytic Fungus Hypoxylon sp. (BS15)" Journal of Fungi 4, no. 2: 69. https://doi.org/10.3390/jof4020069