An In Vitro Model for Candida albicans–Streptococcus gordonii Biofilms on Titanium Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Composition and Preparation of Basal Medium Mucin (BMM) Synthetic Saliva Medium
2.3. Antimicrobial Drugs
2.4. Titanium Preparation, Cleaning, and Sterilization
2.5. Biofilm Formation on Titanium Discs
2.6. Kinetic Studies on the Formation of Mixed Biofilms on Titanium
2.7. Scanning Electron Microscopy
2.8. Confocal Laser Scanning Microscopy
2.9. Antimicrobial Susceptibility Testing of Dual-Species Biofilms Formed on Titanium
2.10. Statistics
3. Results
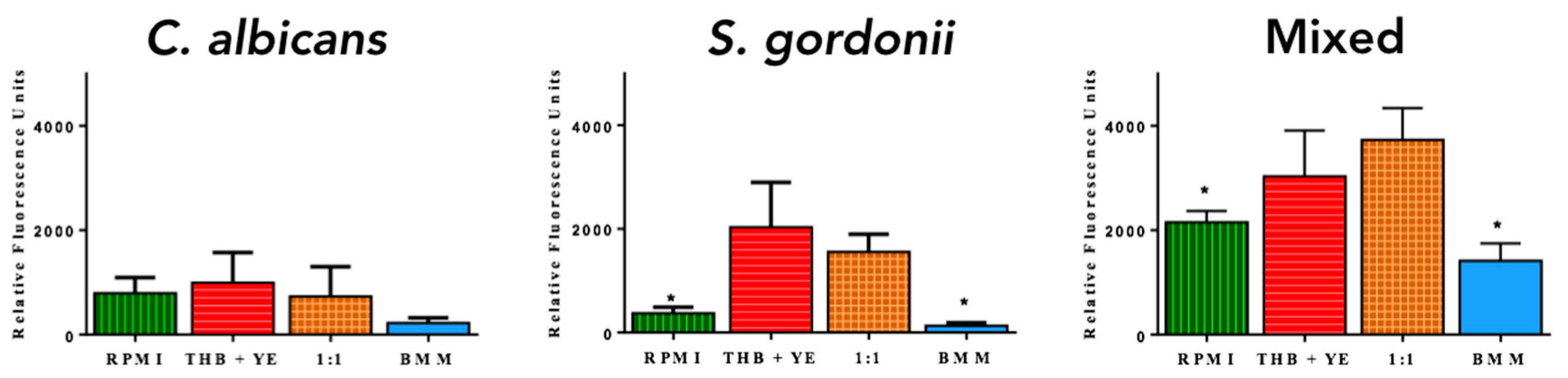
3.1. Development of C. albicans and S. gordonii Single- and Dual-Species Biofilms on Titanium Discs
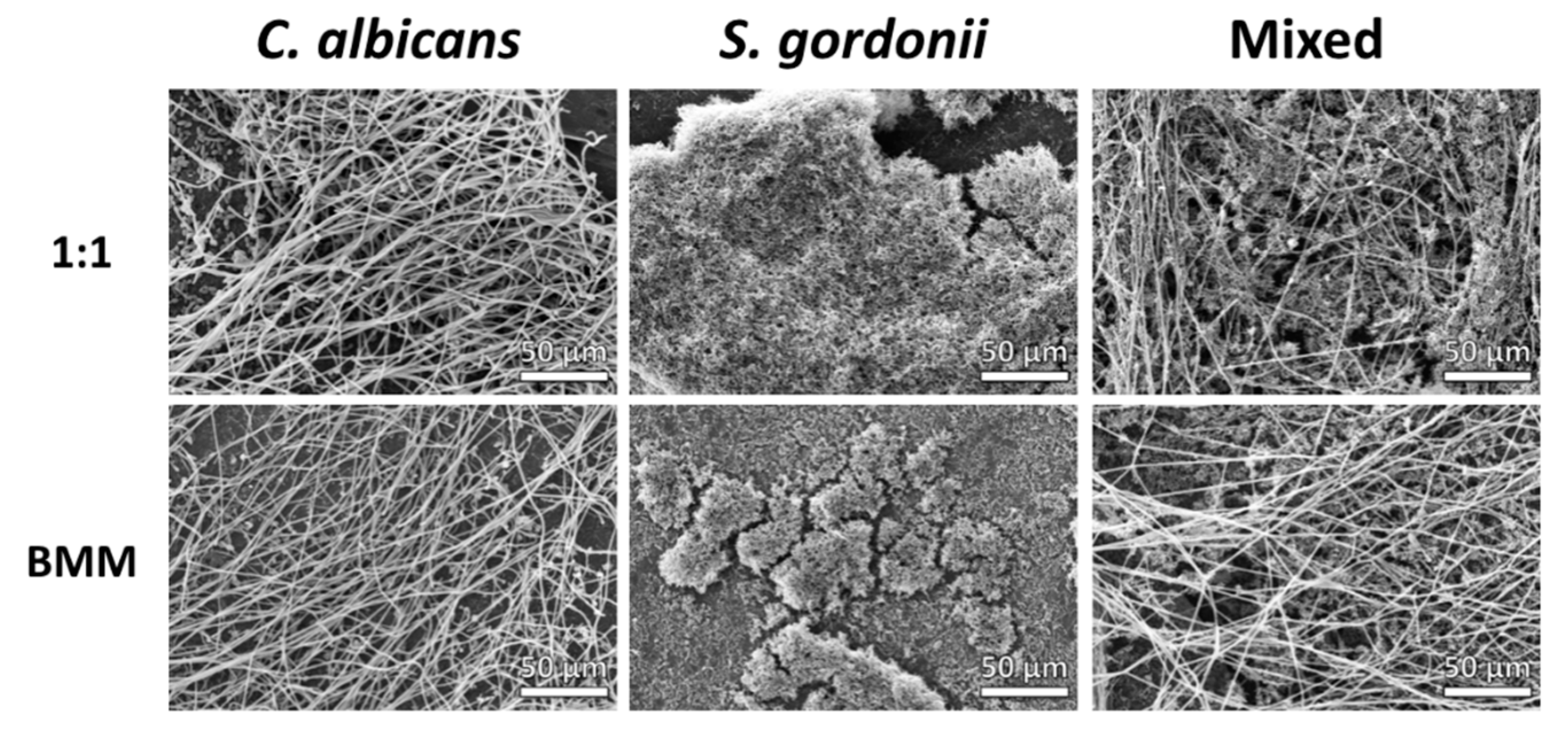
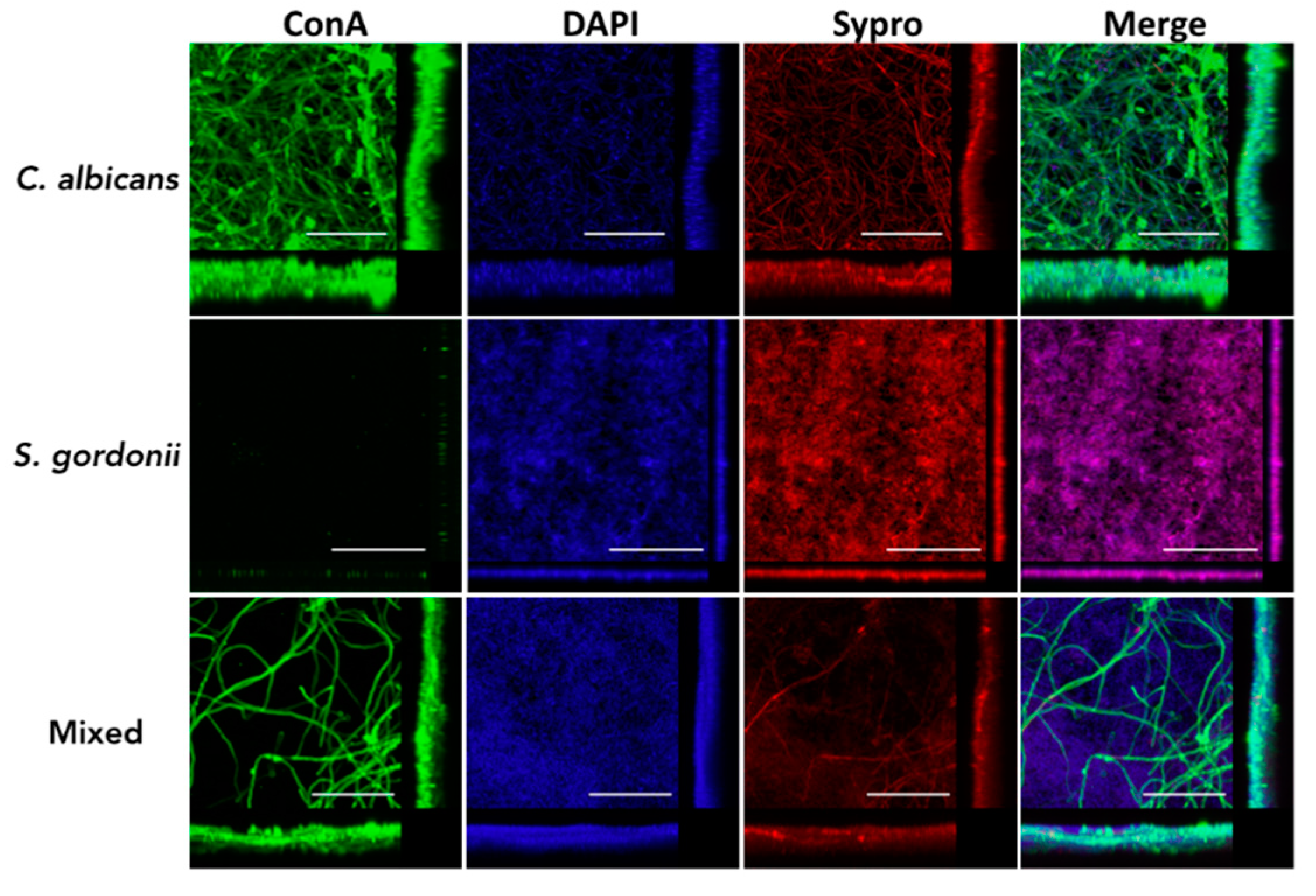
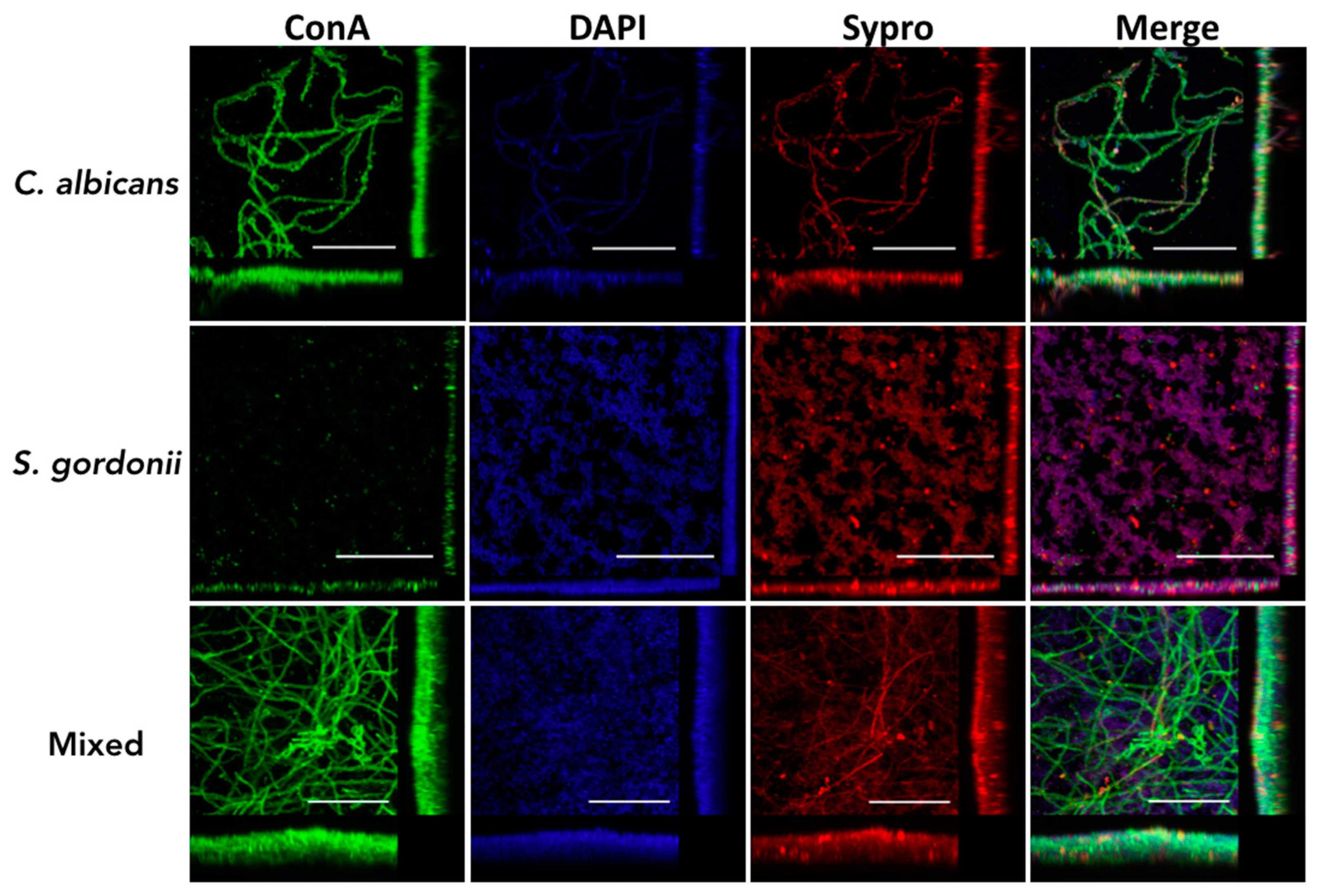
3.2. Structural Characteristics of C. albicans and S. gordonii Single- and Dual-Species Biofilms Formed on Titanium Discs
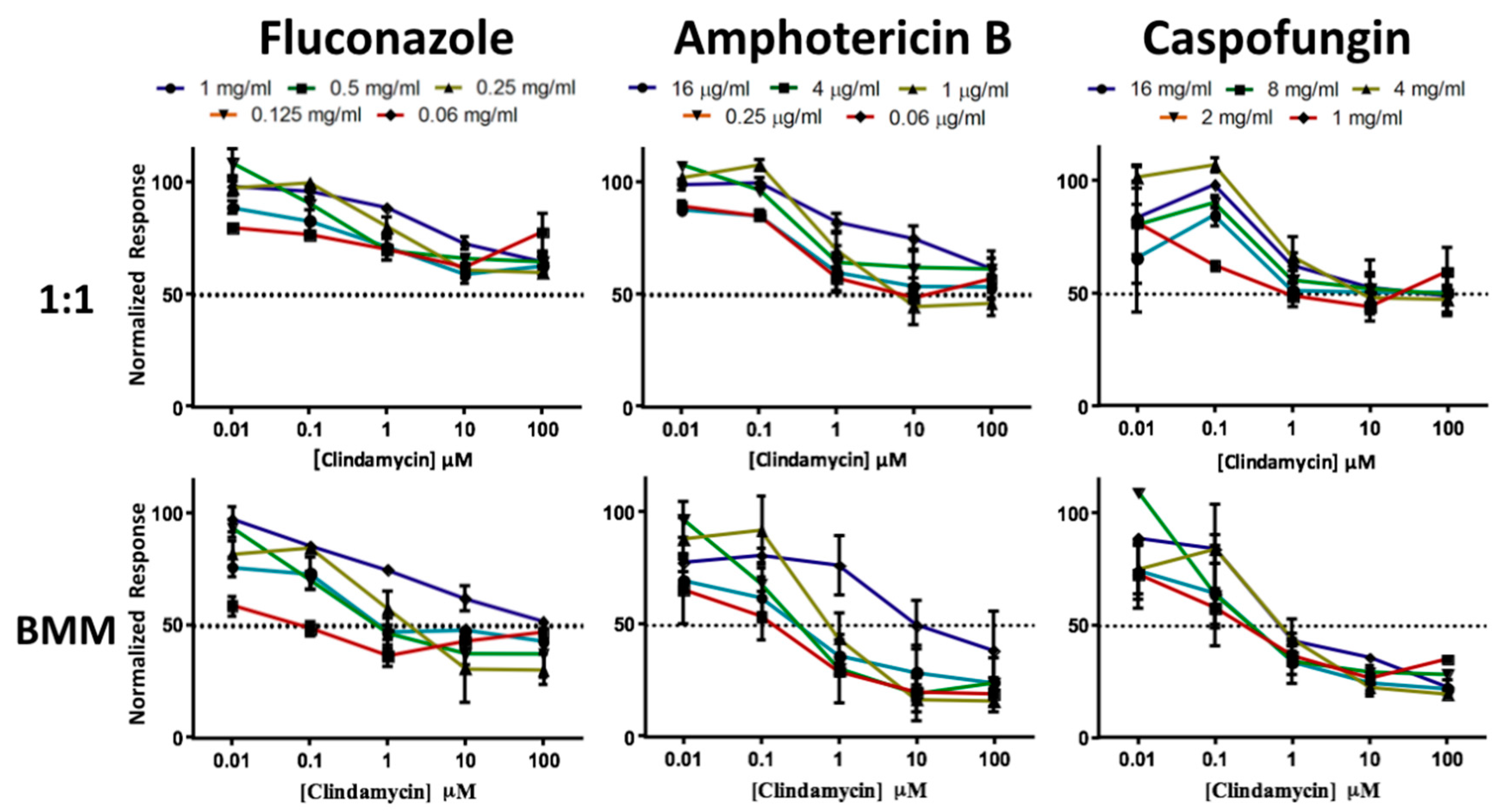
3.3. Dual-Species Biofilms of C. albicans and S. gordonii Formed on Titanium Display High Levels of Resistance to Antimicrobial Therapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 27, 1269–1277. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Actis, L.; Gaviria, L.; Guda, T.; Ong, J.L. Antimicrobial surfaces for craniofacial implants: State of the art. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Rutger Persson, G. Infection at titanium implants with or without a clinical diagnosis of inflammation. Clin. Oral Implants Res. 2007, 18, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Antoci, V., Jr.; Adams, C.S.; Parvizi, J.; Davidson, H.M.; Composto, R.J.; Freeman, T.A.; Wickstrom, E.; Ducheyne, P.; Jungkind, D.; Shapiro, I.M.; et al. The inhibition of staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials 2008, 29, 4684–4690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2009, 24, 616–626. [Google Scholar] [PubMed]
- Tawakoli, P.N.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Investig. 2013, 17, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Covani, U.; Marconcini, S.; Crespi, R.; Barone, A. Bacterial plaque colonization around dental implant surfaces. Implant Dent. 2006, 15, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Gadalla, H.; Lundgren, T. Bacterial colonization of the implant-abutment interface (IAI) of dental implants with a sloped marginal design: An in-vitro study. Clin. Implant. Dent. Relat. Res. 2016, 18, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Mesia, R.; Calderon, N.; Wong, F.; Wallet, S. The effect of dynamic loading on bacterial colonization of the dental implant fixture-abutment interface: An in vitro study. J. Oral Implantol. 2014, 40, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Ata-Ali, J.; Candel-Marti, M.E.; Flichy-Fernandez, A.J.; Penarrocha-Oltra, D.; Balaguer-Martinez, J.F.; Penarrocha Diago, M. Peri-implantitis: Associated microbiota and treatment. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e937–e943. [Google Scholar] [CrossRef] [PubMed]
- Miani, P.K.; do Nascimento, C.; Sato, S.; Filho, A.V.; da Fonseca, M.J.; Pedrazzi, V. In vivo evaluation of a metronidazole-containing gel for the adjuvant treatment of chronic periodontitis: Preliminary results. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Bergstrom, C.; Lekholm, U. Microbiologic diagnostics at titanium implants. Clin. Implant Dent. Relat. Res. 2003, 5, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Pye, A.D.; Lockhart, D.E.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Al Groosh, D.; Roudsari, G.B.; Moles, D.R.; Ready, D.; Noar, J.H.; Pratten, J. The prevalence of opportunistic pathogens associated with intraoral implants. Lett. Appl. Microbiol. 2011, 52, 501–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; Lopez-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bower, R.C.; Radny, N.R.; Wall, C.D.; Henry, P.J. Clinical and microscopic findings in edentulous patients 3 years after incorporation of osseointegrated implant-supported bridgework. J. Clin. Periodontol. 1989, 16, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, G.; Belibasakis, G.N. Microbiome of peri-implant infections: Lessons from conventional, molecular and metagenomic analyses. Virulence 2015, 6, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kawamura, Y.; Yamaki, K.; Ishida, N.; Tian, L.; Takeuchi, Y.; Hashimoto, K.; Abiko, Y.; Mayanagi, G.; Washio, J.; et al. Oral microbiota in crevices around dental implants: Profiling of oral biofilm. In Interface Oral Health Science 2014; Keiichi, S., Osamu, S., Nobuhiro, T., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Alcoforado, G.A.; Rams, T.E.; Feik, D.; Slots, J. Microbial aspects of failing osseointegrated dental implants in humans. J. Parodontol. 1991, 10, 11–18. [Google Scholar] [PubMed]
- Hultin, M.; Gustafsson, A.; Hallstrom, H.; Johansson, L.A.; Ekfeldt, A.; Klinge, B. Microbiological findings and host response in patients with peri-implantitis. Clin. Oral Implants Res. 2002, 13, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Renvert, S.; Dahlen, G. Microbial findings at failing implants. Clin. Oral Implants Res. 1999, 10, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Melo, L.; Ferrari, D.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin. Oral Implants Res. 2008, 19, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, L.; Wang, Z.; Li, L.; Zhang, J.; Zhang, Q.; Chen, T.; Lin, J.; Chen, F. Subgingival microbiome in patients with healthy and ailing dental implants. Sci. Rep. 2015, 5, 10948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, A.; Maiden, M.F.; Lee, K.; Shulman, L.B.; Weber, H.P. Dental implant infections. Clin. Infect. Dis. 1997, 25 (Suppl. 2), S213–S217. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, C.; Pita, M.S.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. In vivo evaluation of Candida spp. Adhesion on titanium or zirconia abutment surfaces. Arch. Oral Biol. 2013, 58, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hirota, K.; Goto, T.; Yumoto, H.; Miyake, Y.; Ichikawa, T. Biofilm formation of Candida albicans on implant overdenture materials and its removal. J. Dent. 2012, 40, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; An, Y.; Liang, H.; Tong, Y.; Guo, T.; Ma, C. The effect of different titanium nitride coatings on the adhesion of Candida albicans to titanium. Arch. Oral Biol. 2013, 58, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rivero, M.E.; Del Pozo, J.L.; Valentin, A.; Fornes, V.; Molina de Diego, A.; Peman, J.; Canton, E. Activity of Amphotericin B and Anidulafungin, Alone and Combined, Against Candida tropicalis Biofilms Developed on Teflon® and Titanium. Rev. Iberoam. Micol. 2017, 34, 175–179. [Google Scholar] [PubMed]
- Fernandez-Rivero, M.E.; Del Pozo, J.L.; Ramirez, P.; Valentin, E.; Ruiz-Gaitan, A.; Peman, J.; Canton, E. Time-kill assays of amphotericin b plus anidulafungin against Candida tropicalis biofilms formed on two different biomaterials. Int. J. Artif. Organs 2017, 41, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An in vitro model for oral mixed biofilms of Candida albicans and Streptococcus gordonii in synthetic saliva. Front. Microbiol. 2016, 7, 686. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Sissons, C. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch. Oral Biol. 2001, 46, 477–486. [Google Scholar] [CrossRef]
- Wang, S.K.; Reid, B.M.; Dugan, S.L.; Roggenbuck, J.A.; Read, L.; Aref, P.; Taheri, A.P.; Yeganeh, M.Z.; Simmer, J.P.; Hu, J.C. FAM20A mutations associated with enamel renal syndrome. J. Dent. Res. 2014, 93, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tummala, S.; Lopez-Ribot, J.L. A 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J. Vis. Exp. 2010, pii: 2287. [Google Scholar] [CrossRef]
- Dental Implants Facts and Figures. Available online: http://www.aaid.com/about/press_room/dental_implants_faq.html (accessed on 31 January 2017).
- Di Fiore, P.M.; Tam, L.; Thai, H.T.; Hittelman, E.; Norman, R.G. Retention of teeth versus extraction and implant placement: Treatment preferences of dental faculty and dental students. J. Dent. Educ. 2008, 72, 352–358. [Google Scholar] [PubMed]
- Setzer, F.C.; Kim, S. Comparison of long-term survival of implants and endodontically treated teeth. J. Dent. Res. 2014, 93, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Zarifian, A.; Ghoddusi, J. Choice of treatment plan based on root canal therapy versus extraction and implant placement: A mini review. Iran. Endod. J. 2015, 10, 152–155. [Google Scholar] [PubMed]
- Bamford, C.V.; d’Mello, A.; Nobbs, A.H.; Dutton, L.C.; Vickerman, M.M.; Jenkinson, H.F. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 2009, 77, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Bamford, C.V.; Nobbs, A.H.; Barbour, M.E.; Lamont, R.J.; Jenkinson, H.F. Functional regions of Candida albicans hyphal cell wall protein Als3 that determine interaction with the oral bacterium Streptococcus gordonii. Microbiology 2015, 161, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.F.; Douglas, L.J. Interactions between Candida species and bacteria in mixed infections. In Polymicrobial Diseases; Brogden, K.A., Guthmiller, J.M., Eds.; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Diaz, P.I.; Xie, Z.; Sobue, T.; Thompson, A.; Biyikoglu, B.; Ricker, A.; Ikonomou, L.; Dongari-Bagtzoglou, A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 2012, 80, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jenkinson, H.F.; Dongari-Bagtzoglou, A. Innocent until proven guilty: Mechanisms and roles of Streptococcus-Candida interactions in oral health and disease. Mol. Oral Microbiol. 2014, 29, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.J.; Nobbs, A.H.; Vickerman, M.M.; Barbour, M.E.; Jenkinson, H.F. Interaction of Candida albicans cell wall als3 protein with Streptococcus gordonii sspb adhesin promotes development of mixed-species communities. Infect. Immun. 2010, 78, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Howlin, R.P.; Fabbri, S.; Offin, D.G.; Symonds, N.; Kiang, K.S.; Knee, R.J.; Yoganantham, D.C.; Webb, J.S.; Birkin, P.R.; Leighton, T.G.; et al. Removal of dental biofilms with an ultrasonically activated water stream. J. Dent. Res. 2015, 94, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An In Vitro Model for Candida albicans–Streptococcus gordonii Biofilms on Titanium Surfaces. J. Fungi 2018, 4, 66. https://doi.org/10.3390/jof4020066
Montelongo-Jauregui D, Srinivasan A, Ramasubramanian AK, Lopez-Ribot JL. An In Vitro Model for Candida albicans–Streptococcus gordonii Biofilms on Titanium Surfaces. Journal of Fungi. 2018; 4(2):66. https://doi.org/10.3390/jof4020066
Chicago/Turabian StyleMontelongo-Jauregui, Daniel, Anand Srinivasan, Anand K. Ramasubramanian, and Jose L. Lopez-Ribot. 2018. "An In Vitro Model for Candida albicans–Streptococcus gordonii Biofilms on Titanium Surfaces" Journal of Fungi 4, no. 2: 66. https://doi.org/10.3390/jof4020066





