Development of an Expression Vector to Overexpress or Downregulate Genes in Curvularia protuberata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Culture
2.2. Vector Construction
2.3. Overexpression and RNAi Constructs
2.4. Protoplast Isolation
2.5. Transformation and Screening
2.6. Semi-Quantitative Reverse Transcription-PCR
2.7. Melanin Extraction and Quantification
2.8. Trehalose Assay
2.9. Catalase Assay
3. Results
3.1. Vector Construction
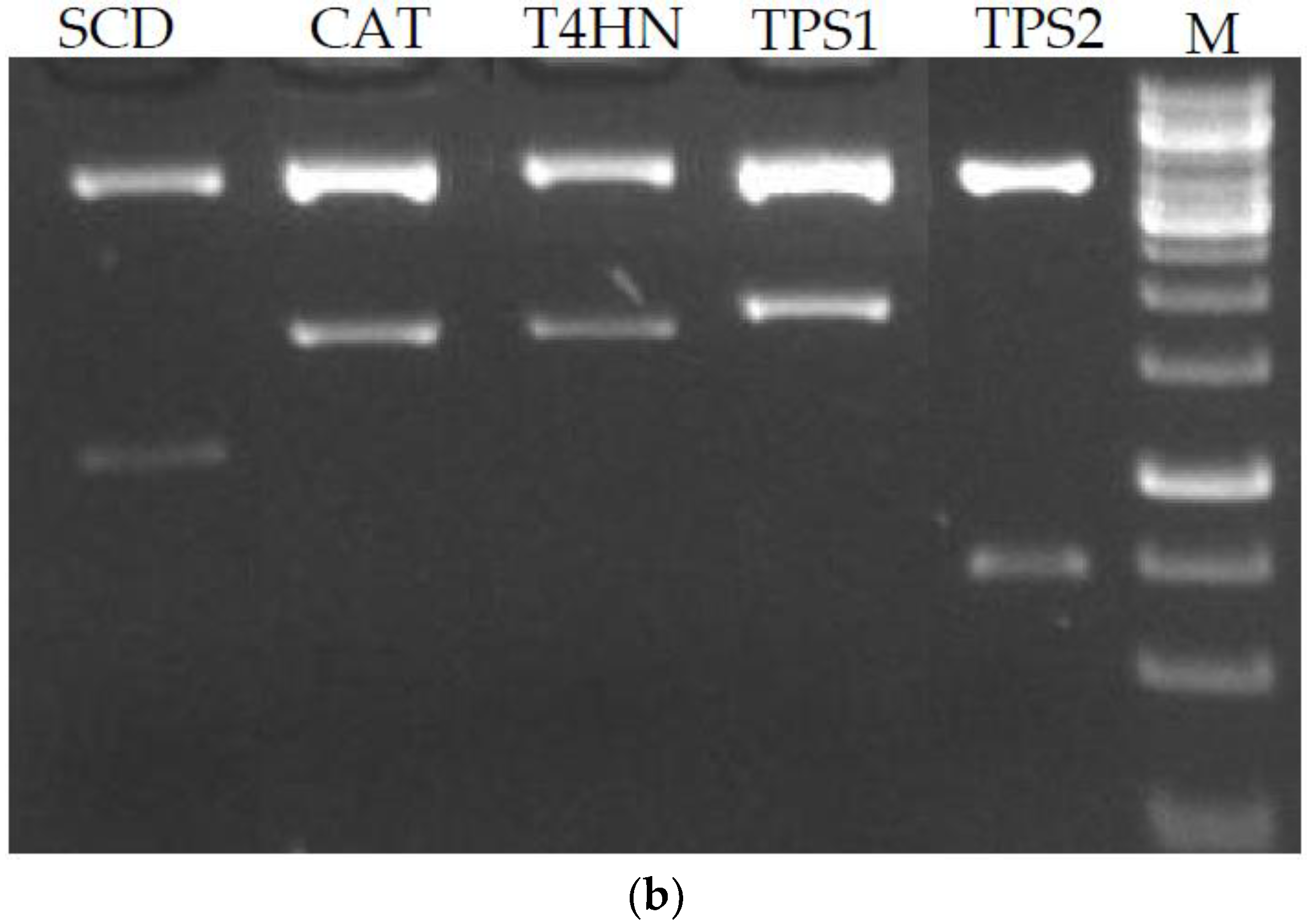
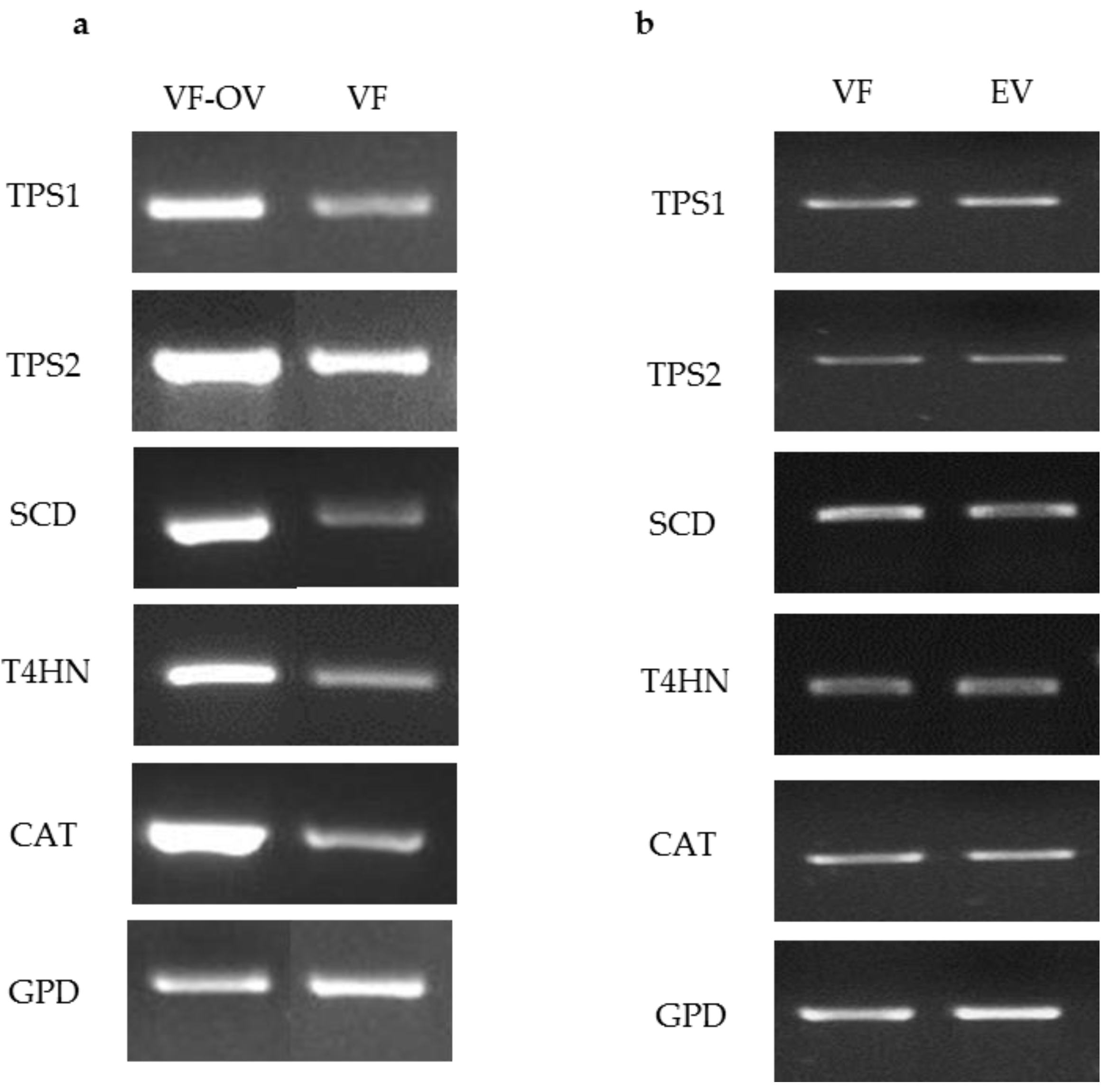
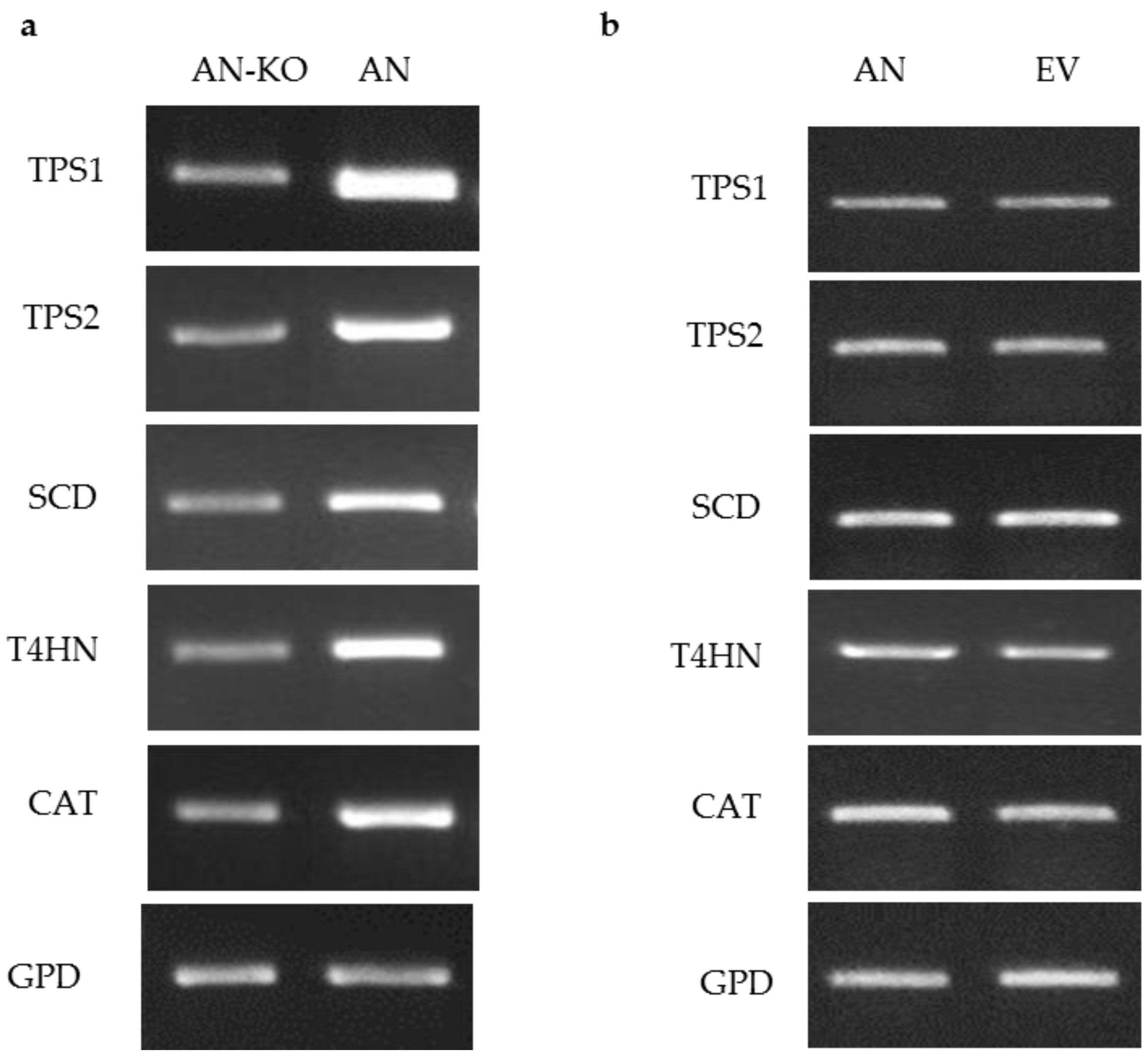
3.2. Molecular Analysis of Transformants
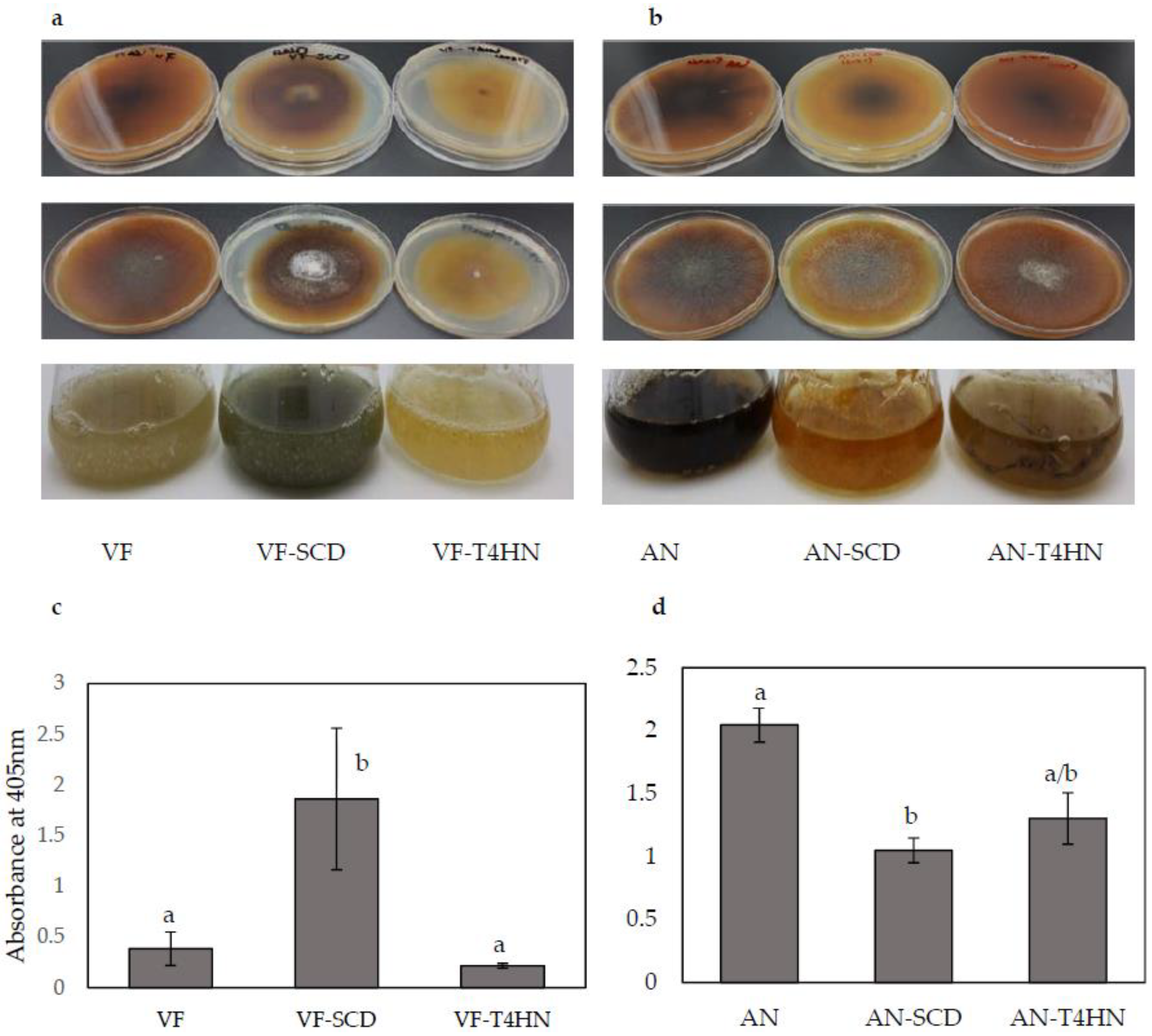
3.3. Melanin Analysis
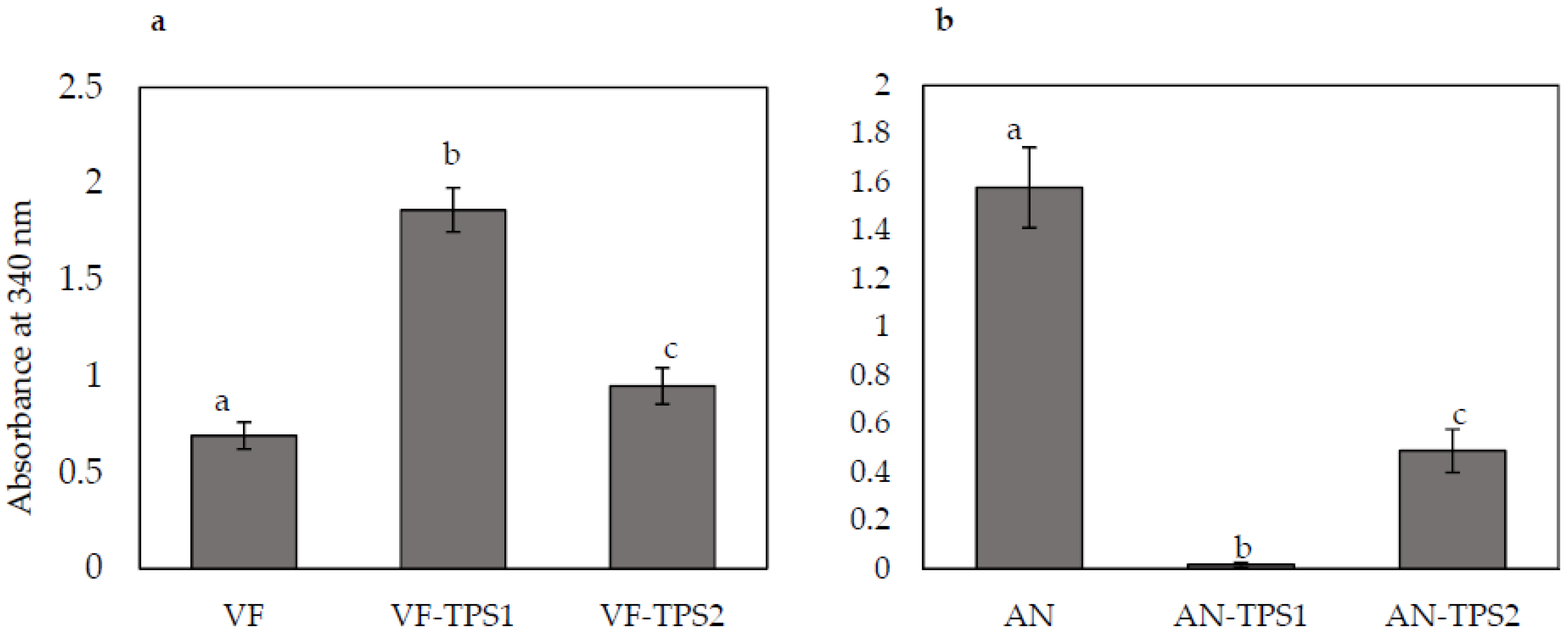
3.4. Trehalose Assay
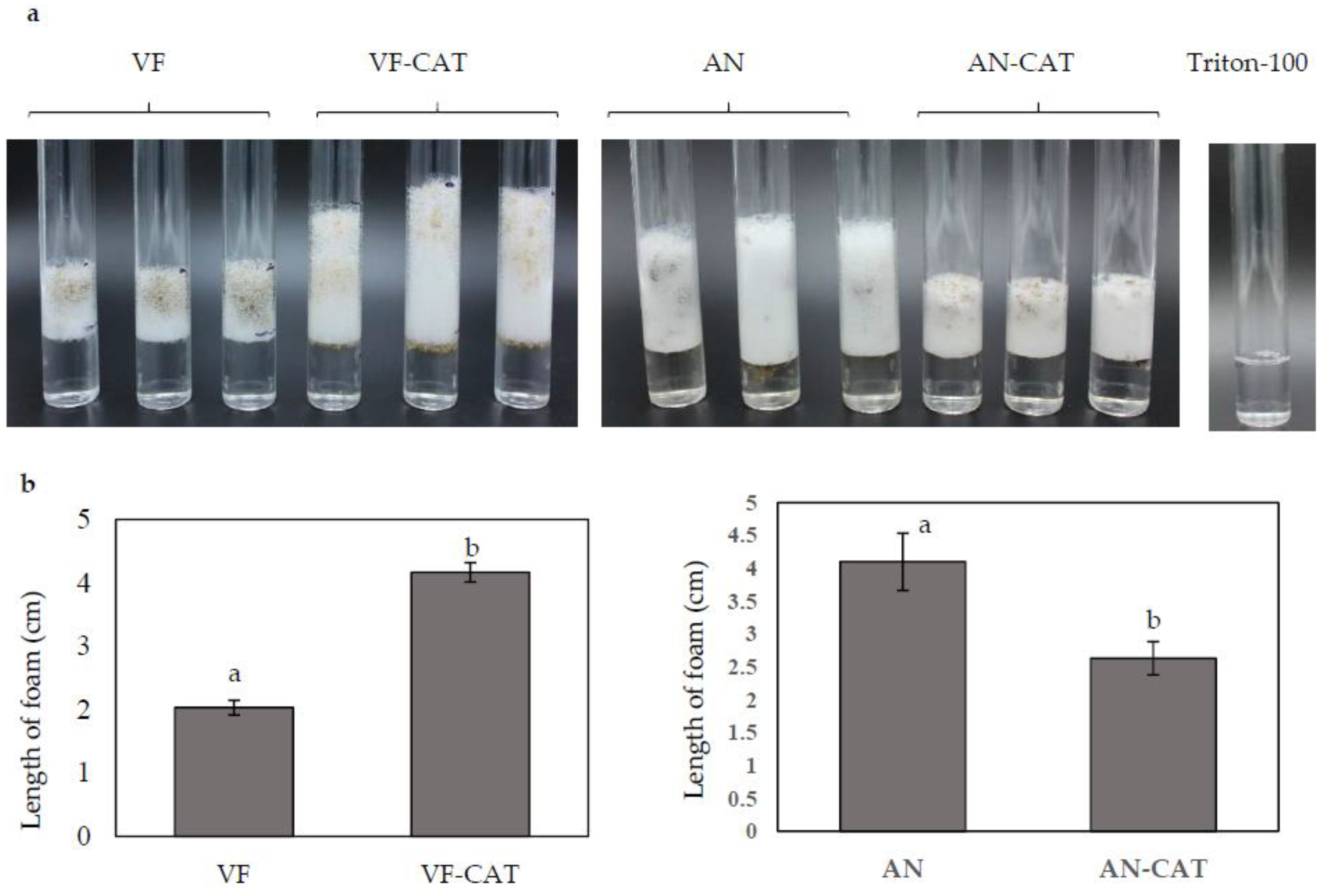
3.5. Catalase Assay
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.R.; Oswald, J.; He, J.; Tang, Y.; Roossinck, M.J. Teasing apart a three-way symbiosis: Transcriptome analyses of Curvularia protuberata in response to viral infection and heat stress. Biochem. Biophys. Res. Commun. 2010, 401, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Andrie, R.M.; Martinez, J.P.; Ciuffetti, L.M. Development of ToxA and ToxB promoter-driven fluorescent protein expression vectors for use in filamentous ascomycetes. Mycologia 2005, 97, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Lorang, J.M.; Tuori, R.P.; Martinez, J.P.; Sawyer, T.L.; Redman, R.S.; Rollins, J.A.; Wolpert, T.J.; Johnson, K.B.; Rodriguez, R.J.; Dickman, M.B.; et al. Green fluorescent protein is lighting up fungal biology. Appl. Environ. Microbiol. 2001, 67, 1987–1994. [Google Scholar] [PubMed]
- De Silva, A.P.; Bolton, M.D.; Nelson, B.D. Transformation of Sclerotinia sclerotiorum with the green fluorescent protein gene and fluorescence of hyphae in four inoculated hosts. Plant Pathol. 2009, 58, 487–496. [Google Scholar] [CrossRef]
- Bennett, R.P.; Cox, C.A.; Hoeffler, J.P. Fusion of green fluorescent protein with the Zeocin™-resistance marker allows visual screening and drug selection of transfected eukaryotic Cells. BioTechniques 1998, 24, 478–482. [Google Scholar] [PubMed]
- Pfeifera, T.A.; Hegedusa, D.D.; Grigliattia, T.A.; Theilmannab, D.A. Baculovirus immediate-early promoter-mediated expression of the Zeocin™ resistance gene for use as a dominant selectable marker in Dipteran and Lepidopteran insect cell lines. Gene 1997, 188, 183–190. [Google Scholar] [CrossRef]
- Rasala, B.A.; Chao, S.; Pier, M.; Barrera, D.J.; Mayfield, S.P. Enhanced genetic tools for engineering multigene traits into green algae. PLoS ONE 2014, 9, e94028. [Google Scholar]
- Wilber, A.; Linehan, J.L.; Tian, X.; Woll, P.S.; Morris, J.K.; Belur, L.R.; McIvor, R.S.; Kaufman, D.S. Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells 2007, 25, 2919–2927. [Google Scholar] [PubMed]
- Alderton, A.J.; Burr, I.; Mühlschlegel, F.A.; Tuite, M.F. Zeocin resistance as a dominant selective marker for transformation and targeted gene deletions in Candida glabrata. Mycoses 2006, 49, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, J.; Kozarich, J.W. Mechanisms of bleomycin-induced DNA degradation. Chem. Rev. 1987, 87, 1107–1136. [Google Scholar] [CrossRef]
- Van Peer, A.F.; de Bekker, C.; Vinck, A.; Wosten, H.A.; Lugones, L.G. Phleomycin increases transformation efficiency and promotes single integrations in Schizophyllum commune. Appl. Environ. Microbiol. 2009, 75, 1243–1247. [Google Scholar] [PubMed]
- Lanza, A.M.; Kim, D.S.; Alper, H.S. Evaluating the influence of selection markers on obtaining selected pools and stable cell lines in human cells. Biotechnol. J. 2013, 8, 811–821. [Google Scholar] [PubMed]
- Fernandez, C.W.; Koide, R.T. The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecol. 2013, 6, 479–486. [Google Scholar] [CrossRef]
- Gómez, B.L.; Nosanchuk, J.D. Melanin and fungi. Curr. Opin. Infect. Dis. 2003, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006, 50, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Singaravelan, N.; Grishkan, I.; Beharav, A.; Wakamatsu, K.; Ito, S.; Nevo, E. Adaptive melanin response of the soil fungus Aspergillus niger to UV radiation stress at ‘‘Evolution Canyon’’, Mount Carmel, Israel. PLoS ONE 2008, 3, e2993. [Google Scholar]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [PubMed]
- Tseng, M.N.; Chung, P.C.; Tzean, S.S. Enhancing the stress tolerance and virulence of an entomopathogen by metabolic engineering of dihydroxynaphthalene melanin biosynthesis genes. Appl. Environ. Microbiol. 2011, 77, 4508–4519. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef]
- Thompson, J.E.; Fahnestock, S.; Farrall, L.; Liao, D.I.; Valent, B.; Jordan, D.B. The second naphthol reductase of fungal melanin biosynthesis in Magnaporthe grisea: Tetrahydroxynaphthalene reductase. J. Biol. Chem. 2000, 275, 34867–34872. [Google Scholar] [PubMed]
- Tanaka, N.; Haruki, Y.; Ueno, M.; Arase, S.; Kihara, J. Expression of T4HR1, a 1,3,6,8-tetrahydroxynaphthalene reductase gene involved in melanin biosynthesis, is enhanced by near-ultraviolet irradiation in Bipolaris oryzae. Adv. Microbiol. 2015, 5, 166–176. [Google Scholar]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose a multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [PubMed]
- Iordachescu, M.; Imai, R. Trehalose biosynthesis in response to abiotic stresses. J. Integr. Plant Biol. 2008, 50, 1223–1229. [Google Scholar] [PubMed]
- Argüelles, J.C. Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch. Microbiol. 2000, 174, 217–224. [Google Scholar] [PubMed]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [PubMed]
- Nunes, C.; Schluepmann, H.; Delatte, T.L.; Wingler, A.; Silva, A.B.; Fevereiro, P.S.; Jansen, M.; Fiorani, F.; Wiese-Klinkenberg, A.; Paul, M. Regulation of growth by the trehalose pathway: Relationship to temperature and sucrose. Plant Signal. Behav. 2013, 8, e26626. [Google Scholar] [PubMed]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Berndt, P.; Hahn, M. Trehalose metabolism is important for heat stress tolerance and spore germination of Botrytis cinerea. Microbiology 2006, 152, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. TIBS 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Banerjee, M.; Bihani, S.C.; Ballal, A. A salt-Inducible Mn-catalase (KatB) protects cyanobacterium from oxidative stress. Plant Physiol. 2016, 170, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Morris, V.B.; Labhasetwar, V.; Ghorpade, A. Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress. Cell Death Dis. 2013, 4, e903. [Google Scholar] [PubMed]
- Vighi, I.L.; Benitez, L.C.; do Amaral, M.N.; Auler, P.A.; Moraes, G.P.; Rodrigues, G.S.; da Maia, L.C.; Pinto, L.S.; Braga, E.J. Changes in gene expression and catalase activity in Oryza sativa L. under abiotic stress. Genet. Mol. Res. 2016, 15, 1–15. [Google Scholar]
- Huang, Z.; Ali, S.; Ren, S. Catalase production influences germination, stress tolerance and virulence of Lecanicillium muscarium conidia. Biocontrol Sci. Technol. 2012, 22, 249–260. [Google Scholar]
- Hernandez, C.E.M.; Guerrero, I.E.P.; Hernandez, G.A.G. Catalase overexpression reduces the germination time and increases the pathogenicity of the fungus Metarhizium anisopliae. Appl. Microbiol. Biotechnol. 2010, 87, 1033–1044. [Google Scholar]
- Young, C.; Itoh, Y.; Johnson, R.; Garthwaite, I.; Miles, C.O.; Munday-Finch, S.C.; Scott, B. Paxilline-negative mutants of Penicillium paxilli generated by heterologous and homologous plasmid integration. Curr. Genet. 1998, 33, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Johnson, R.; Scott, B. Integrative transformation of the mycotoxin-producing fungus, Penicillium paxilli. Curr. Genet. 1994, 25, 508–513. [Google Scholar] [PubMed]
- Fernandes, C.; Prados-Rosales, R.; Silva, B.M.; Nakouzi-Naranjo, A.; Zuzarte, M.; Chatterjee, S.; Stark, R.E.; Casadevall, A.; Goncalves, T. Activation of melanin synthesis in Alternaria infectoria by antifungal drugs. Antimicrob. Agents Chemother. 2016, 60, 1646–1655. [Google Scholar]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [PubMed]
- Iwase, T.; Tajima, A.; Sugimoto, S.; Okuda, K.; Hironaka, I.; Kamata, Y.; Takada, K.; Mizunoe, Y. A simple assay for measuring catalase activity: A visual approach. Sci. Rep. 2013, 3, 3081. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Shen, X.; Chen, B. Development of an efficient vector system for gene knock-out and near in-cis gene complementation in the sugarcane smut fungus. Sci. Rep. 2017, 7, 3113. [Google Scholar] [PubMed]
- Saitoh, K.-I.; Nishimura, M.; Kubo, Y.; Hayashi, N.; Minami, E.; Nishizawa, Y. Construction of a binary vector for knockout and expression analysis of Rice Blast Fungus genes. Biosci. Biotechnol. Biochem. 2008, 72, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Fetzner, R.; Seither, K.; Wenderoth, M.; Herr, A.; Fischer, R. Alternaria alternata transcription factor CmrA controls melanization and spore development. Microbiology 2014, 160, 1845–1854. [Google Scholar] [PubMed]
- Wheeler, M.H.; Abramczyk, D.; Puckhaber, L.S.; Naruse, M.; Ebizuka, Y.; Fujii, I.; Szaniszlo, P.J. New biosynthetic step in the melanin pathway of Wangiella (Exophiala) dermatitidis: Evidence for 2-acetyl-1,3,6,8-Tetrahydroxynaphthalene as a novel precursor. Eukaryot. Cell 2008, 7, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Eliahu, N.; Igbaria, A.; Rose, M.S.; Horwitz, B.A.; Lev, S. Melanin biosynthesis in the maize pathogen Cochliobolus heterostrophus depends on two mitogen-activated protein kinases, Chk1 and Mps1, and the transcription factor Cmr1. Eukaryot. Cell 2007, 6, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, C.; Flores, C. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004, 4, 351–359. [Google Scholar] [PubMed]
- Van Vaeck, C.; Wear, S.; Van Dijck, P.; Thevelein, M. Analysis and modification of trehalose 6-phosphate levels in the yeast Saccharomyces cerevisiae with the use of Bacillus subtilis phosphotrehalase. Biochem. J. 2001, 353, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.A.; Nagahisa, K.; Hirasawa, T.; Yoshikawa, K.; Ashitani, K.; Shimizu, H. Effect of trehalose accumulation on response to saline stress in Saccharomyces cerevisiae. Yeast 2009, 26, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Virgilio, C.; Bürckert, N.; Bell, W.; Jenö, P.; Boller, T.; Wiemken, A. Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur. J. Biochem. 1993, 212, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Ishii-Minami, N.; Saitoh, K.; Otake, Y.; Kaku, H.; Shibuya, N.; Nishizawa, Y.; Minami, E. The role of catalase-peroxidase secreted by Magnaporthe oryzae during early infection of rice Cells. Mol. Plant Microbe Interact. 2011, 24, 163–171. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers | Sequence 5′→3′ | Restriction Enzyme |
|---|---|---|---|
| Overexpression | |||
| TPS1 | Forward | TCGAATTCATGCCTGACGAACCCACAAGAC | EcoR1 |
| Reverse | GAGGATCCTCATTGGGCATTGGCAGGAGCAG | BamH1 | |
| TPS2 | Forward | GTGAATTCATGAGTGCCCCTACCGATGACAAG | EcoR1 |
| Reverse | TGCAGTCTAGACTATGGCACCGCCCGAGACTCAG | XbaI | |
| SCD | Forward | CAGAATTCATGTTTGAGAAGAACAAACTCC | EcoRI |
| Reverse | CACTGCAGTTACATGGCCAGCCCTGGCGCCTTC | PstI | |
| T4HN | Forward | TTGAATTCATGGTCATCAACGTTCCCAC | EcoRI |
| Reverse | TCGGATCCCTACTGGGATGATCCACCAGAG | BamHI | |
| CAT | Forward | CAGAATTCATGTCCAAAGGCGAGTGTCC | EcoRI |
| Reverse | CTGGATCCTCAAGTCGACTTGTTCTTGAC | BamHI | |
| Downregulation | |||
| TPS1 | Forward Sense | CAGCAAGCTTGAATTCGCTCCGAGATCTACCGAATC | EcoRI/HindIII |
| Reverse Sense | CAAACGGATCCGTGGAAGAAACAAGGCAGACG | BamHI | |
| Forward Anti-sense | TCCACGGATCCAAACTTACCATTGATGCGGCC | BamHI | |
| TPS2 | Forward Sense | CACCAAGCTTGAATTCACCTATCCCCGTTGATCCCA | EcoRI/HindIII |
| Reverse Sense | ACGTGGATCCACAATGTCGCCTGGCTTGTA | BamHI | |
| Forward Anti-sense | TTGTGGATCCTCCGTCGGCAGGCTCATTTTG | BamHI | |
| SCD | Forward Sense | CACCAAGCTTGAATTCAGCTACGACAGCAAGGACTG | EcoR1/HindIII |
| Reverse Sense | GCTACTGCAGTCCACTCGCCGTCAATCTTC | PstI | |
| Forward Anti-sense | GCACCTGCAGACGCATCCGTGTATCGCTG | PstI | |
| T4HN | Forward Sense | GACTAAGCTTGAATTCAGCCAACGAAGTGTGCGAC | EcoRI/HindIII |
| Reverse Sense | TCAAGGATCCTGGCTCGCCATAAGCGACTCG | BamHI | |
| Forward Anti-sense | AGCCAGGATCCTTGATGCCACCGGGGGCGAC | BamHI | |
| CAT | Forward Sense | CTTCTCTAGAGAATTCGCGCTTTGCTCCTCTCAATG | EcoRI/XbaI |
| Reverse Sense | GGAAAGGATCCTGGCAAGGTCCTCTGAGTTG | BamHI | |
| Forward Anti-sense | GCCAGGATCCTTTCCATATCGTTCATAGCC | BamHI | |
| Semi-quantitative RT-PCR | |||
| TPS1 | Forward | TGACGAACCCACAAGACTGG | |
| Reverse | CTCCTCCCGCAGCATAGAAG | ||
| TPS2 | Forward | GACATTGGCCTCATTACCAG | |
| Reverse | CTTCGTTTTGCCAGCTCAT | ||
| SCD | Forward | AACTCCAGCCTACCTTTGAGG | |
| Reverse | ACTCGTACCACCGAATGTCC | ||
| T4HN | Forward | CACCATGGTCATCAACGTTCCCA | |
| Reverse | TACTTCTCCTCGCTAATCTCC | ||
| CAT | Forward | GTGCCTGGTTCAAGCTTCTC | |
| Reverse | TGAACGTCAGTCTGCTCCTG | ||
| GPD | Forward | GCAACAACCTGACCGTCAAC | |
| Reverse | CCCACTCGTTGTCGTACCAA | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Cleckler, B.; Morsy, M. Development of an Expression Vector to Overexpress or Downregulate Genes in Curvularia protuberata. J. Fungi 2018, 4, 54. https://doi.org/10.3390/jof4020054
Liu C, Cleckler B, Morsy M. Development of an Expression Vector to Overexpress or Downregulate Genes in Curvularia protuberata. Journal of Fungi. 2018; 4(2):54. https://doi.org/10.3390/jof4020054
Chicago/Turabian StyleLiu, Chengke, Blake Cleckler, and Mustafa Morsy. 2018. "Development of an Expression Vector to Overexpress or Downregulate Genes in Curvularia protuberata" Journal of Fungi 4, no. 2: 54. https://doi.org/10.3390/jof4020054





