Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review
Abstract
:1. Introduction
2. Physiopathology
3. Mycology
4. Different Forms of Dermatophytosis
- Localised to single or multiple perifollicular (hair follicle) sites e.g., Majocchi granuloma, nodular perifolliculitis.
- Deep dermatophytosis, not confined to the perifollicular area e.g., in the presence of immunosuppression or CARD9 deficiency, with or without dissemination to extra-cutaneous sites.
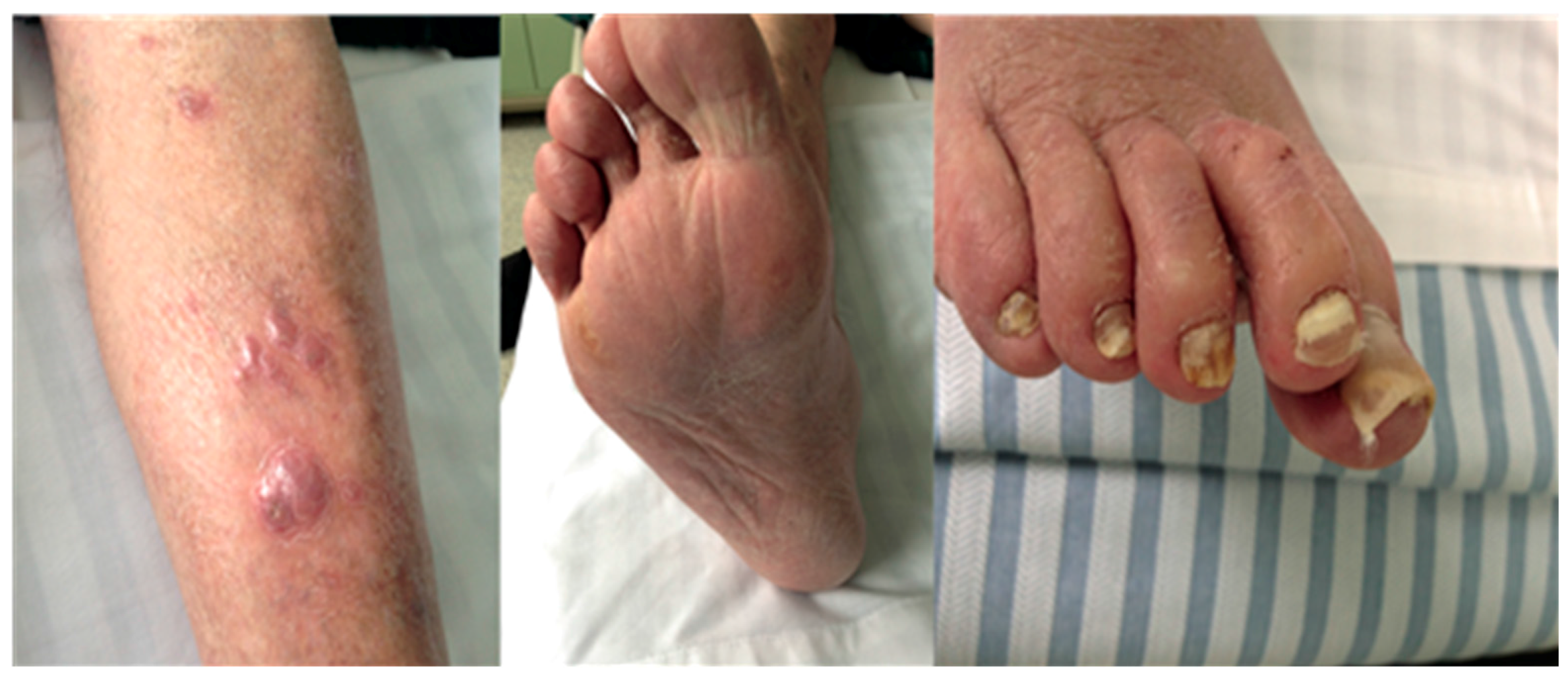
5. Diagnosis of Severe Dermatophytosis

6. Underlying Conditions Responsible for Severe Dermatophytsosis
| Underlying Conditions Associated with Severe Dermatophytosis | Number of Cases | Clinical Involvement | References |
|---|---|---|---|
| Solid organ transplant | 28 | Skin | [22,26,27,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] |
| HIV infection | 9 | Skin | [38,54,55,56,57,58,59,60] |
| Systemic corticosteroid treatment | 7 | Skin | [21,31,61,62,63,64,65] |
| Others immunosuppressive treatments ± steroid | 6 | Skin/nodes | [19,25,66,67,68,69] |
| Hematological malignancy | 7 | Skin | [70,71,72,73,74] |
| Liver disease | 3 | Skin | [17,75,76] |
| Topical steroid only | 1 | Skin/nodes | [77] |
| Cushing disease, congenital adrenal hyperplasia | 2 | Skin | [78,79] |
| Atopy | 1 | Skin | [80] |
| Diabetes mellitus | 1 | Skin | [64] |
| CARD9 deficiency | 19 | Skin/nodes/organs | [11,23,81,82,83,84,85,86,87] |
6.1. Solid Organ Transplant
6.2. HIV Infection
6.3. Other Secondary Immunodeficiencies
6.4. Primary Immunodeficiency
7. Treatment of Severe Dermatophytosis
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Ameen, M. Epidemiology of superficial fungal infections. Clin. Dermatol. 2010, 28, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Havlickova, B.; Czaika, V.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.; Johns, N.; Williams, H.; Bolliger, I.; Dellavalle, R.; Margolis, D.; Marks, R.; Naldi, L.; Weinstock, M.; Wulf, S.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Invest. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, C.; Bouchara, J.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, R.J. Dermatophytosis and other superficial mycoses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 2985–2994. [Google Scholar]
- Zurita, J.; Hay, R. Adherence of dermatophyte microconidia and arthroconidia to human keratinocytes in vitro. J. Invest. Dermatol. 1987, 89, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Baldo, A.; Monod, M.; Mathy, A.; Cambier, L.; Bagut, E.; Defaweux, V.; Symoens, F.; Antoine, N.; Mignon, B. Mechanisms of skin adherence and invasion by dermatophytes. Mycoses 2012, 55, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Venturini, J.; Alvares, A.; Camargo, M.; Marchetti, C.; Fraga-Silva, T.; Luchini, A.; Arruda, M. Dermatophyte-host relationship of a murine model of experimental invasive dermatophytosis. Microbes Infect. 2012, 14, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; McLoone, N.; Hay, R. Could proximal white subungual onychomycosis be a complication of systemic spread? The lessons to be learned from Maladie Dermatophytique and other deep infections. Br. J. Dermatol. 2005, 153, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Baran, R. Deep dermatophytosis: Rare infections or common, but unrecognised, complications of lymphatic spread? Curr. Opin. Infect Dis. 2004, 17, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Pathan, S.; Vincent, Q.; Liu, L.; Cypowyj, S.; Prando, C.; Migaud, M.; Taibi, L.; Ammar-Khodja, A.; Boudghene Stambouli, O.; et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 2013, 369, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Sohnle, P. Cutaneous defenses against dermatophytes and yeasts. Clin. Microbiol. Rev. 1995, 8, 317–335. [Google Scholar] [PubMed]
- Nakamura, T.; Nishibu, A.; Yasoshima, M.; Tanoue, C.; Yoshida, N.; Hatta, J.; Miyamoto, T.; Nishii, M.; Yanagibashi, T.; Nagai, Y.; et al. Analysis of Trichophyton antigen-induced contact hypersensitivity in mouse. J. Dermatol. Sci. 2012, 66, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yang, X.; Yudate, T.; Chung, J.; Wu, J.; Luby-Phelps, K.; Kimberly, R.; Underhill, D.; Cruz, P.J.; Ariizumi, K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J. Biol. Chem. 2006, 281, 38854–38866. [Google Scholar] [CrossRef] [PubMed]
- Calderon, R.; Hay, R. Cell-mediated immunity in experimental murine dermatophytosis. II. Adoptive transfer of immunity to dermatophyte infection by lymphoid cells from donors with acute or chronic infections. Immunology 1984, 53, 465–472. [Google Scholar] [PubMed]
- Campos, M.; Russo, M.; Gomes, E.; Almeida, S. Stimulation, inhibition and death of macrophages infected with Trichophyton rubrum. Microbes Infect. 2006, 8, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.; Kradin, R.; Marty, F.; Hospenthal, D.; Kotton, C. Disseminated dermatophytosis in a patient with hereditary hemochromatosis and hepatic cirrhosis: Case report and review of the literature. Med. Mycol. 2010, 48, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Inaoki, M.; Nishijima, C.; Miyake, M.; Asaka, T.; Hasegawa, Y.; Anzawa, K.; Mochizuki, T. Case of dermatophyte abscess caused by Trichophyton rubrum: A case report and review of the literature. Mycoses 2015, 58, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Seddon, M.; Thomas, M. Invasive disease due to Epidermophyton floccosum in an immunocompromised patient with Behçet’s syndrome. Clin. Infect. Dis. 1997, 25, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Majocchi, D. A new trichophytic granuloma: Clinical and mycological studies. Bull. R. Acad. Med. Roma 1883, 9, 220–223. [Google Scholar]
- Das, S.; Saha, R.; Bhattacharya, S. Disseminated nodular granulomatous perifolliculitis. Indian J. Med. Microbiol. 2007, 25, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Chastain, M.A.; Reed, R.J.; Pankey, G.A. Deep dermatophytosis: Report of 2 cases and review of the literature. Cutis 2001, 67, 457–462. [Google Scholar] [PubMed]
- Boudghène-Stambouli, O.; Mérad-Boudia, A.; Allal, M. Cerebral injury in dermatophytic disease. J. Mycol. Med. 1992, 2, 106–108. [Google Scholar]
- Cheikhrouhou, F.; Makni, F.; Masmoudi, A.; Sellami, A.; Turki, H.; Ayadi, A. A fatal case of dermatomycoses with retropharyngeal abscess. Ann. Dermatol. Venereol. 2010, 137, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Nir-Paz, R.; Elinav, H.; Pierard, G.; Walker, D.; Maly, A.; Shapiro, M.; Barton, R.; Polacheck, I. Deep Infection by Trichophyton rubrum in an Immunocompromised Patient. J. Clin. Microbiol. 2003, 41, 5298–5301. [Google Scholar] [CrossRef] [PubMed]
- Budihardja, D.; Freund, V.; Mayser, P. Widespread erosive tinea corporis by Arthroderma benhamiae in a renal transplant recipient: Case report. Mycoses 2010, 53, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Smith, A. ‘‘Tinea pseudoimbricata’’: Tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin. Exp. Dermatol. 2003, 28, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, P.; Passeron, T.; Gari-Toussaint, M. Epidermophytie rapidement extensive à Trichopyton mentagrophytes chez un sujet immunocompétent. Med. Mycol. 2011, 21, 277–280. (in France). [Google Scholar] [CrossRef]
- Mansouri, P.; Farshi, S.; Khosravi, A.; Naraghi, Z.; Chalangari, R. Trichophyton Schoenleinii-induced widespread tinea corporis mimicking parapsoriasis. J. Mycol. Med. 2012, 22, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Mansouri, P.; Nikaein, D.; Sharifzadeh, A.; Erfanmanesh, A.; Chalangari, R.; Safaei-Naraghi, Z.; Safar, F. Severe tinea corporis due to Trichophyton verrucosum mimicking discoid lupus erythematosus. J. Mycol. Med. 2012, 22, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Neafie, R.; Skelton, H.G., 3rd; Barrett, T.; Graham, J.; Lupton, G. Majocchi’s granuloma. J. Cutan. Pathol. 1991, 18, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Pappert, A.; Garzon, M.; Silvers, D. Invasive Trichophyton rubrum infection in the immunocompromised host: Report of three cases. J. Am. Acad. Dermatol. 1995, 33, 315–318. [Google Scholar] [CrossRef]
- Lillis, J.; Dawson, E.; Chang, R.; White, C.J. Disseminated dermal Trichophyton rubrum infection—An expression of dermatophyte dimorphism? J. Cutan. Pathol. 2009, 37, 1168–1169. [Google Scholar] [CrossRef] [PubMed]
- Azib, S.; Ingen-Housz-Oro, S.; Foulet, F.; Ortonne, N.; Penso-Assathiany, D.; Lang, P.; Wolkenstein, P.; Chosidow, O. Nodules on the legs in a renal transplant recipient. JAMA Dermatol. 2013, 149, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Voisard, J.; Weill, F.; Beylot-Barry, M.; Vergier, B.; Dromer, C.; Beylot, C. Dermatophytic granuloma caused by Microsporum canis in a heart-lung recipient. Dermatolgy 1999, 198, 317–319. [Google Scholar] [CrossRef]
- Novick, N.; Tapia, L.; Bottone, E. Invasive Trichophyton rubrum infection in an immunocompromised host: Case report and review of the literature. Am. J. Med. 1987, 82, 321–325. [Google Scholar] [CrossRef]
- Solá, M.; Vázquez Doval, F.; Serna, M.; Quintanilla, E. Papulo-nodular lesions in a heart transplant patient: Invasive infection by Trichophyton rubrum. Med. Clin. 1992, 98, 437–438. [Google Scholar]
- King, D.; Cheever, L.; Hood, A.; Horn, T.; Rinaldi, M.; Merz, W. Primary invasive cutaneous Microsporum canis infections in immunocompromised patients. J. Clin. Microbiol. 1996, 34, 460–462. [Google Scholar] [PubMed]
- Squeo, R.; Beer, R.; Silvers, D.; Weitzman, I.; Grossman, M. Invasive Trichophyton rubrum resembling blastomycosis infection in the immunocompromised host. J. Am. Acad. Dermatol. 1998, 39, 379–380. [Google Scholar] [CrossRef]
- Franco, R. Deep dermatophytosis in a post transplant recipient. Int. J. Dermatol. 2001, 40, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chu, S.; Hsiao, G.; Chou, N.; Wang, S.; Chiu, H. Majocchi’s granuloma caused by Trichophyton tonsurans in a cardiac transplant recipient. Br. J. Dermatol. 1999, 140, 1194–1196. [Google Scholar] [PubMed]
- Tse, K.; Yeung, C.; Tang, S.; Chan, H.; Li, F.; Chan, T.; Lai, K. Majocchi’s Granuloma and posttransplant lymphoproliferative disease in a renal transplant recipient. Am. J. Kidney Dis. 2001, 38, E38. [Google Scholar] [CrossRef]
- Rajpara, V.; Frankel, S.; Rogers, C.; Nouri, K. Trichophyton tonsurans associated tinea corporis infection with the development of Mojocchi’s granuloma in a renal transplant patient. J. Drugs Dermatol. 2005, 4, 767–769. [Google Scholar] [PubMed]
- Seçkin, D.; Arikan, S.; Haberal, M. Deep dermatophytosis caused by Trichophyton rubrum with concomitant disseminated nocardiosis in a renal transplant recipient. J. Am. Acad. Dermatol. 2004, 51, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Hamacher, K.; Roberts, G. Pseudomycetoma caused by Microsporum canis in an immunosuppressed patient: A case report and review of the literature. J. Cutan. Pathol. 2007, 34, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Brod, C.; Benedix, F.; Röcken, M.; Schaller, M. Trichophytic Majocchi granuloma mimicking Kaposi sarcoma. J. Dtsch. Dermatol. Ges. 2007, 5, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.; Jaekel, D.; Kiss, E.; Kliem, V. Majocchi’s granuloma after kidney transplantation. Exp. Clin. Transplant. 2006, 4, 518–520. [Google Scholar] [PubMed]
- Gega, A.; Ketsela, G.; Glavin, F.; Soldevilla-Pico, C.; Schain, D. Majocchi’s granuloma after antithymocyte globulin therapy in a liver transplant patient. Transpl. Infect. Dis. 2010, 12, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, R.; Yu, J.; Wang, C.; Wan, Z.; Wang, X. Majocchi’s granuloma in a liver transplant recipient caused by a Trichophyton spp., phenotypically consistent with Trichophyton rubrum var. raubitschekii. Med. Mycol. 2009, 47, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Deziel, P.; Razonable, R. Majocchi’s granuloma in solid organ transplant recipients. Transpl. Infect. Dis. 2011, 13, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gönül, M.; Saraçlı, M.; Demiriz, M.; Gül, U. Deep Trichophyton rubrum infection presenting with umbilicated papulonodules in a cardiac transplant recipient. Mycoses 2013, 56, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Won, C.; Chang, S.; Lee, M.; Choi, J.; Moon, K. Majocchi’s granuloma mimicking Kaposi sarcoma in a heart transplant patient. J. Dermatol. 2011, 38, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Steiner, U.; Trüeb, R.; Schad, K.; Kamarashev, J.; Koch, S.; French, L.; Hofbauer, G. Trichophyton rubrum-induced Majocchi’s Granuloma in a heart transplant recipient. A therapeutic challenge. J. Dermatol. Case Rep. 2012, 6, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Del Palacio, A.; Pereiro-Miguens, M.; Gimeno, C.; Cuétara, M.; Rubio, R.; Costa, R.; Romero, G. Widespread dermatophytosis due to Microsporum (Trichophyton) gallinae in a patient with AIDS a case report from Spain. Clin. Exp. Dermatol. 1992, 17, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Hadacek, B.; Nassif, A.; Roux, A.; Desplaces, N.; Huerre, M.; de Bievre, C.; Aerts, J.; Raguin, G. Trichophyton tonsurans dermatophyte granuloma in an HIV-1 infected patient. Br. J. Dermatol. 1999, 140, 762–763. [Google Scholar] [PubMed]
- Kwon, K.; Jang, H.; Son, H.; Oh, C.; Kwon, Y.; Kim, K.; Suh, S. Widespread and invasive Trichophyton rubrum infection mimicking Kaposi’s sarcoma in a patient with AIDS. J. Dermatol. 2004, 31, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Lowinger-Seoane, M.; Torres-Rodríguez, J.; Madrenys-Brunet, N.; Aregall-Fusté, S.; Saballs, P. Extensive dermatophytoses caused by Trichophyton mentagrophytes and Microsporum canis in a patient with AIDS. Mycopathologia 1992, 120, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pèrez, M.A.; Rodriquez-Pichardo, A.; Camacho, F.; Rios, J.J. Extensive and deep dermatophytosis caused by Trichophyton mentagrophytes var. Interdigitalis in an HIV-1 positive patient. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Porro, A.; Yoshioka, M.; Kaminski, S.; Palmeira Mdo, C.; Fischman, O.; Alchorne, M. Disseminated dermatophytosis caused by Microsporum gypseum in two patients with the acquired immunodeficiency syndrome. Mycopathologia 1997, 137, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Tsang, P.; Hopkins, T.; Jimenez-Lucho, V. Deep dermatophytosis caused by Trichophyton rubrum in a patient with AIDS. J. Am. Acad. Dermatol. 1996, 34, 1090–1091. [Google Scholar] [CrossRef]
- Akiba, H.; Motoki, Y.; Satoh, M.; Iwatsuki, K.; Kaneko, F. Recalcitrant trichophytic granuloma associated with NK cell deficiency in a SLE patient treated with corticosteroid. Eur. J. Dermatol. 2001, 11, 58–62. [Google Scholar] [PubMed]
- Barson, W. Granuloma and pseudogranuloma of the skin due to Microsporum canis. Successful management with local injections of miconazole. Arch. Dermatol. 1985, 121, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Demidovich, C.; Kornfeld, B.; Gentry, R.; Fitzpatrick, J. Deep dermatophyte infection with chronic draining nodules in an immunocompromised patient. Cutis 1995, 55, 237–240. [Google Scholar] [PubMed]
- Erbağci, Z. Deep dermatophytoses in association with atopy and diabetes mellitus: Majocchi’s granuloma tricophyticum or dermatophytic pseudomycetoma? Mycopathologia 2002, 154, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tirado-González, M.; Ball, E.; Ruiz, A.; Rodriguez, Y.; Goudet, C.; Finkel, O.; Golan, H.; de Morentin, H.; Sprecher, H.; Sprecher, E.; et al. Disseminated dermatophytic pseudomycetoma caused by Microsporum species. Int. J. Dermatol. 2012, 51, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Lowther, A.; Somani, A.; Camouse, M.; Florentino, F.; Somach, S. Invasive Trichophyton rubrum infection occurring with infliximab and long-term prednisone treatment. J. Cutan. Med. Surg. 2007, 11, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Ota, K.; Sato, K.; Nara, S.; Yagushi, T.; Nakano, H.; Sawamura, D. Deep pseudocystic dermatophytosis caused by Trichophyton rubrum in a patient with myasthenia gravis. Acta Derm. Venereol. 2013, 93, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ishida, E.; Yasuda, H.; Yamamoto, O.; Tokura, Y. Tinea profunda cysticum caused by Trichophyton rubrum. J. Am. Acad. Dermatol. 2006, 54, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Petrov, I.; Kempf, W.; Stoilova, D.; Broshtilova, V.; Mateev, G.; Balabanova, M. Disseminated dermatophytic pseudomycetomas arising in an immunocompromised patient. Br. J. Dermatol. 2006, 155, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Faergemann, J.; Gisslén, H.; Dahlberg, E.; Westin, J.; Roupe, G. Trichophyton rubrum abscesses in immunocompromised patients. A case report. Acta Derm. Venereol. 1989, 69, 244–247. [Google Scholar] [PubMed]
- Baker, R.; Para, M. Successful use of ketoconazole for invasive cutaneous Trichophyton rubrum infection. Arch. Intern. Med. 1984, 144, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Welsh, M.; Skelton, H. Trichophyton rubrum showing deep dermal invasion directly from the epidermis in immunosuppressed patients. Br. J. Dermatol. 2001, 145, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.; Sullivan, J. Dermatophytes as opportunistic pathogens. J. Am. Acad. Dermatol. 1994, 30, 1021–1022. [Google Scholar] [CrossRef]
- Lestringant, G.; Lindley, S.; Hillsdon-Smith, J.; Bouix, G. Deep dermatophytosis to Trichophyton rubrum and T. verrucosum in an immunosuppressed patient. Int. J. Dermatol. 1988, 27, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Sun, P.L.; Chang, Y.T. Extensive deep dermatophytosis cause by Trichophyton rubrum in a patient with liver cirrhosis and chronic renal failure. Mycopathologia 2013, 176, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, B.; Radotra, B.; Rai, R. Majocchi’s granuloma trichophyticum in an immunocompromised patient. Int. J. Dermatol. 2000, 39, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Swart, E.; Smit, F. Trichophyton violaceum abscesses. Br. J. Dermatol. 1979, 101, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, I.; Maquine, G.; Francesconi, V.; Francesconi, F. Dermatophytosis caused by Tricophyton rubrum as an opportunistic infection in patients with Cushing disease. An. Bras. Dermatol. 2010, 85, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Vezon, G.; Desbois, N.; Boisseau-Garsaud, A.; Helenon, R.; Jouannelle, A.; Saint-Cyr, I.; Cales-Quist, D. Microsporum canis mycetoma of the scalp. Ann. Dermatol. Venereol. 2000, 127, 729–731. [Google Scholar] [PubMed]
- Tateishi, Y.; Sato, H.; Akiyama, M.; Abe, M.; Kobayashi, H.; Umehara, S.; Yamaguchi, J.; Shibaki, H.; Shimizu, H. Severe generalized deep dermatophytosis due to Trichophyton rubrum. Arch. Dermatol. 2004, 140, 624–625. [Google Scholar] [PubMed]
- Jachiet, M.; Lanternier, F.; Rybojad, M.; Bagot, M.; Ibrahim, L.; Casanova, J.; Puel, A.; Bouaziz, J. Extensive skin and nail dermatophytosis due to autosomal recessive CARD9 deficiency in an Egyptian patient cured with posaconazole. J. Am. Acad. Dermatol. 2015, 151, 192–194. [Google Scholar]
- Boudghène-Stambouli, O.; Mérad-Boudia, A. Dermatophytic disease in Algeria: A new case and review of the literature. Ann. Dermatol. Venereol. 1991, 118, 17–21. [Google Scholar] [PubMed]
- Boudghène-Stambouli, O.; Mérad-Boudia, A. Antifungal agents in dermato-phytic disease: Failure of griseofulvin, ketoconazole and itraconazole. Bull. Soc. Pathol. Exot. 1990, 83, 170–176. [Google Scholar] [PubMed]
- Boudghène-Stambouli, O.; Mérad-Boudia, A. Trichophyton rubrum dermatophytic disease: A new case. Ann. Dermatol. Venereol. 1989, 116, 725–727. [Google Scholar] [PubMed]
- Pruszkowski, A.; Bourgault Villada, I.; Cremer, G.; Ammar-Khodja, A.; Emilie, D.; Revuz, J. Dermatophytic disease: Role of type TC2 CD8 lymphocytes. Ann. Dermatol. Venereol. 1995, 122, 1–55. [Google Scholar]
- Boudghène-Stambouli, O.; Mérad-Boudia, A. Dermatophytic disease: Exuberant hyperkeratosis with cutaneous horns. Ann. Dermatol. Venereol. 1998, 125, 705–707. [Google Scholar] [PubMed]
- Grumach, A.; de Queiroz-Telles, F.; Migaud, M.; Lanternier, F.; Filho, N.R.; Palma, S.; Constantino-Silva, R.; Casanova, J.; Puel, A. A homozygous CARD9 mutation in a Brazilian patient with deep dermatophytosis. J. Clin. Immunol. 2015, 35, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Cypowyj, S.; Picard, C.; Bustamante, J.; Lortholary, O.; Casanova, J.; Puel, A. Primary immunodeficiencies underlying fungal infections. Curr. Opin. Pediatr. 2013, 25, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Hadida, E.; Schousboe, A. Aspects de la maladie dermatophytique. Algerie Med. 1959, 63, 139–141. (In France) [Google Scholar]
- Van de Veerdonk, F.L.; Plantinga, T.S.; Hoischen, A.; Smeekens, S.P.; Joosten, L.A.; Gilissen, C.; Arts, P.; Rosentul, D.C.; Carmichael, A.J.; Smits-van der Graaf, C.A.; et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 2011, 365, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Okada, S.; Kong, X.F.; Kreins, A.Y.; Cypowyj, S.; Abhyankar, A.; Toubiana, J.; Itan, Y.; Audry, M.; Nitschke, P.; et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 2011, 208, 1635–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machan, M.; Kestenbaum, T.; Fraga, G. Diffuse hyperkeratosis in a deaf and blind 48-year-old woman—Quiz case. Diagnosis: Keratitis-ichthyosis-deafness (KID) syndrome with secondary dermatophytosis. Arch. Dermatol. 2012, 149, 1199–1200. [Google Scholar] [CrossRef] [PubMed]
- Lourdes, L.; Mitchell, C.; Glavin, F.; Schain, D.; Kaye, F. Recurrent dermatophytosis (Majocchi granuloma) associated eith chemotherapy-induced neutropenia. J. Clin. Oncol. 2014, 32, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nishioka, K. Deep Dermatophytosis during topical tacrolimus therapy for psoriasis. Acta Derm. Venereol. 2003, 83, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Araviysky, A.; Araviysky, R.; Eschkov, G. Deep generalized trichophytosis. (Endothrix in tissues of different origin). Mycopathologia 1975, 56, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liu, X.; Xu, H.; Zeng, X.; Chen, W.; Li, X. Deep dermatophytosis caused by Trichophyton rubrum: Report of two cases. Mycoses 2007, 50, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Tejasvi, T.; Sharma, V.K.; Sethuraman, G.; Singh, M.K.; Xess, I. Invasive dermatophytosis with lymph node involvement in an immunocompetent patient. Clin. Exp. Dermatol. 2005, 30, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Head, E. Subcutaneous abscess due to Trichophyton mentagrophytes. Int. J. Dermatol. 1982, 21, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Sentamilselvi, G.; Janaki, C.; Kamalam, A.; Thambiah, A. Deep dermatophytosis caused by Trichophyton rubrum—A case report. Mycopathologia 1998, 142, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Akman, A.; Savas, B.; Uguz, A.; Ozhak, B.; Okcu Heper, A.; Basaran, E.; Alpsoy, E. Invasive Trichophyton rubrum infection resembling blastomycosis in a patient with altered immune status during the course of chronic superficial Trichophyton rubrum infection. Eur. J. Dermatol. 2007, 17, 452–453. [Google Scholar] [PubMed]
- Ravaghi, M. Superficial and deep granulomatous lesions caused by Trichophyton violaceum. Cutis 1976, 17, 976–977. [Google Scholar] [PubMed]
- Mayou, S.; Calderon, R.; Goodfellow, A.; Hay, R. Deep (subcutaneous) dermatophyte infection presenting with unilateral lymphoedema. Clin. Exp. Dermatol. 1987, 12, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.; Sachdeva, B.; Gill, P.; Arora, B.; Deep, A.; Karan, J. Majocchi’s granuloma of the face in an immunocompetent patient. J. Dermatol. 2007, 34, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Barboza-Quintana, O.; Garza-Guajardo, R.; Assad-Morel, C.; Méndez-Olvera, N. Pseudomycetoma for Microsporum canis: Report of a case diagnosed by fine needle aspiration biopsy. Acta Cytol. 2007, 51, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Snyderman, R.; Meadows, L.; Pinnell, S. Generalized Microsporum audoninii infection and depressed cellular immunity associated with a missing plasma factor required for lymphocyte blastogenesis. Am. J. Med. 1977, 63, 991–1000. [Google Scholar] [CrossRef]
- Hironaga, M.; Okazaki, N.; Saito, K.; Watanabe, S. Trichophyton mentagrophytes granulomas. Arch Dermatol. 1983, 119, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torres, B.; Mayayo, E.; Boronat, J.; Guarro, J. Subcutaneous infection by Microsporum gypseum. Br. J. Dermatol. 2002, 146, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Botterel, F.; Romand, S.; Cornet, M.; Recanati, G.; Dupont, B.; Bourée, P. Dermatophyte pseudomycetoma of the scalp: Case report and review. Br. J. Dermatol. 2001, 145, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.G.; Lamazor, E.A.; Roeser, E.H.; Wegner, C.J. Mycetoma or pseudomycetoma? A distinctive mycosis caused by dermatophytes. Mycopathologia 1983, 81, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Du, X.; Yang, R.; Huang, S.; Sang, H.; Liu, W. Chronically recurrent and widespread tinea corporis due to Trichophyton rubrum in an immunocompetent patient. Mycopathologia 2015, 179, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kohli, Y.; Batra, R. In vitro activities of posaconazole, ravuconazole, terbinafine, itraconazole and fluconazole against dermatophyte, yeast and non-dermatophyte species. Med. Mycol. 2005, 43, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Isham, N.; Sheehan, D. Voriconazole susceptibilities of dermatophyte isolates obtained from a worldwide tinea capitis clinical trial. J. Clin. Microbiol. 2006, 44, 2579–2580. [Google Scholar] [CrossRef] [PubMed]
- Badali, H.; Mohammadi, R.; Mashedi, O.; de Hoog, G.; Meis, J. In vitro susceptibility patterns of clinically important Trichophyton and Epidermophyton species against nine antifungal drugs. Mycoses 2015, 58, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Prussick, R.; Sibbald, R.; Knowles, S. Terbinafine in the treatment of Majocchi’s granuloma. Int. J. Dermatol. 1995, 34, 489. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Beresford, E.; Ma, L.; Vickery, D.; Martinho, M.; Yu, X.; Komjathy, S.; Tavakkol, A. Skin concentrations and pharmacokinetics of posaconazole after oral administration. Antimicrob. Agents Chemother. 2010, 54, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Balci, D.; Cetin, M. Widespread, chronic, and fluconazole-resistant Trichophyton rubrum infection in an immunocompetent patient. Mycoses 2008, 51, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kuo, J.; Chen, J.; Sun, C.; Huang, S. Dermatophyte pseudomycetoma: A case report. Br. J. Dermatol. 1993, 129, 729–732. [Google Scholar] [PubMed]
- Seki, Y.; Otsuka, F.; Ohara, K.; Takizawa, K.; Ishibashi, Y. Surgical treatment of a deep fungal infection of the skin by Trichosporon cutaneum. J. Dermatol. 1987, 14, 77–80. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. J. Fungi 2016, 2, 4. https://doi.org/10.3390/jof2010004
Rouzaud C, Hay R, Chosidow O, Dupin N, Puel A, Lortholary O, Lanternier F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. Journal of Fungi. 2016; 2(1):4. https://doi.org/10.3390/jof2010004
Chicago/Turabian StyleRouzaud, Claire, Roderick Hay, Olivier Chosidow, Nicolas Dupin, Anne Puel, Olivier Lortholary, and Fanny Lanternier. 2016. "Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review" Journal of Fungi 2, no. 1: 4. https://doi.org/10.3390/jof2010004






