Invasive Candidiasis in Various Patient Populations: Incorporating Non-Culture Diagnostic Tests into Rational Management Strategies
Abstract
:1. Introduction
2. Invasive Candidiasis in Various Populations
| Risk of IC * | Patient Characteristics | Type of IC ** | Incidence *** | References |
|---|---|---|---|---|
| Low |
| Candidemia | <1% | [11,12,20] |
| Low-to-moderate |
| Intra-abdominal candidiasis | ~3%–6% | [21] |
| Candidemia | ~3%–7% | [13,14,16] | |
| Moderate |
| Candidemia | ~10%–15% | [13,16] |
| High |
| Intra-abdominal candidiasis | ~20%–40% | [7,8,19] |
3. Non-Culture Diagnostic Test Performance
4. Interpreting Non-Culture Tests in Various Populations
| Type of IC | Risk Category | Pre-Test Probability of IC * | Post-Test Probability of IC | |||||
|---|---|---|---|---|---|---|---|---|
| β-d-Glucan ** | PCR *** | Ideal Assay **** | ||||||
| Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | |||
| Candidemia | Low | 1% | 4% | <1% | 8% | <1% | 48% | <1% |
| Low-to-moderate | 5% | 17% | 1% | 32% | <1% | 83% | <1% | |
| Moderate | 10% | 31% | 3% | 50% | 1% | 91% | 1% | |
| Intra-abdominal candidiasis | Low-to-moderate | 5% | 11% | 3% | 12% | 1% | 83% | <1% |
| Moderate | 10% | 21% | 6% | 23% | 3% | 91% | 1% | |
| High | 30% | 51% | 22% | 53% | 11% | 97% | 4% | |
5. Integrating Non-Culture Tests into Patient Management Strategies
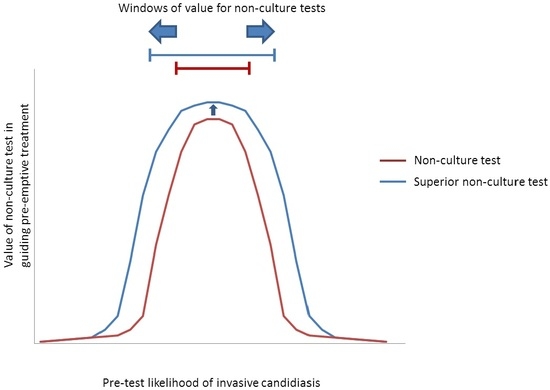
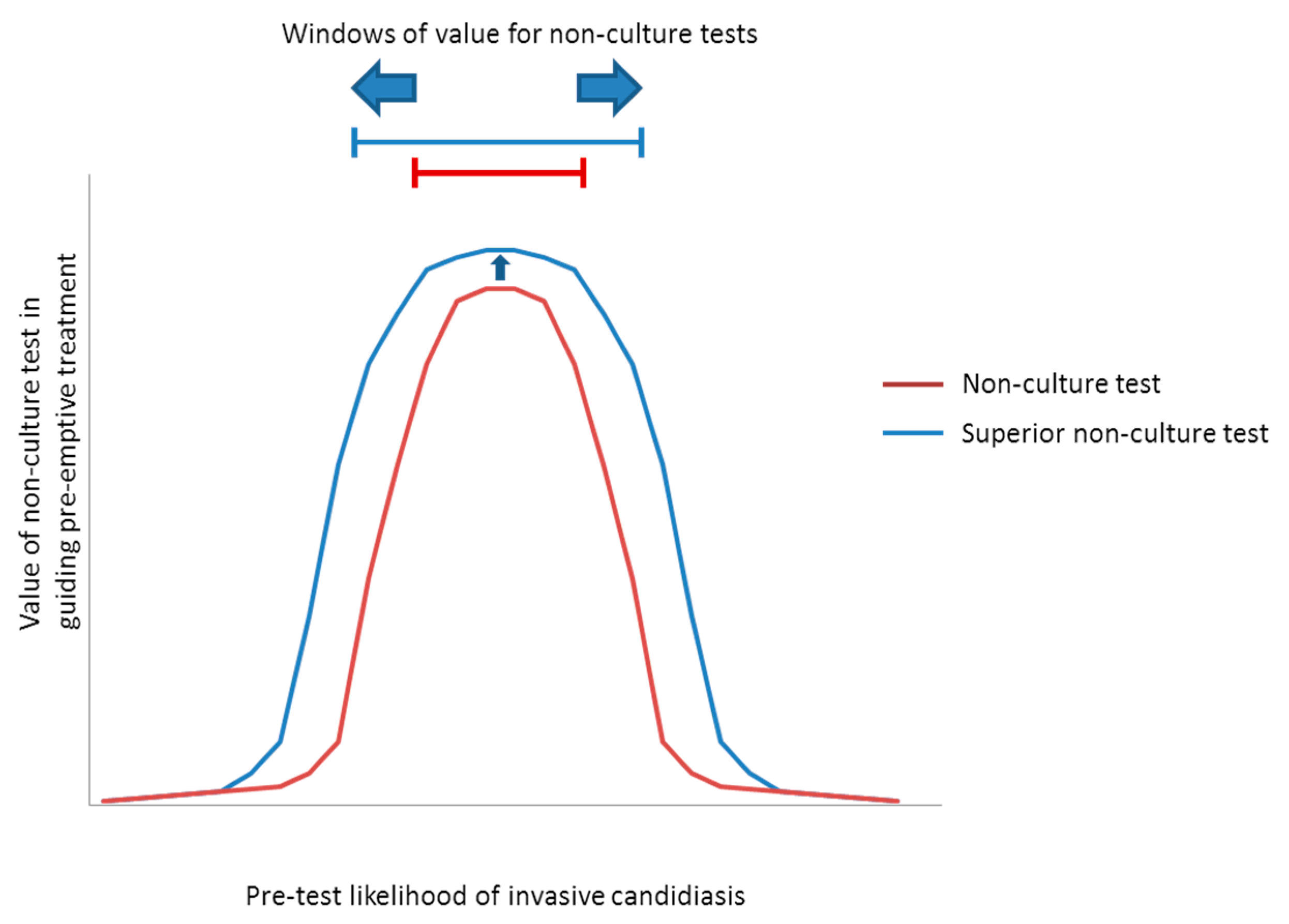
5.1. Screening for Antifungal Treatment
| Predominant Type of IC | Test | Windows † | Comments | |
|---|---|---|---|---|
| Pre-test Likelihoods | Corresponding Populations | |||
| Candidemia (±DSC) | β-DG 1 | ~5% to 40% * | Low-to-moderate- to high-risk ICU patients | NPVs are ≥~85% at pre-test likelihoods as high as the upper limits of these ranges, suggesting that an antifungal strategy would remain viable. Compared to β-d-glucan, a hypothetical PCR or other ideal assay would broaden the window for antifungal treatment to include lower-risk patients, including those in septic shock. Based on the particulars of a case, clinicians may decide to stop antifungal treatment if blood cultures are negative despite positive non-culture test results. With negative blood cultures, β-d-glucan or PCR PPVs are <15% in all settings with pre-test likelihood <~15%. |
| PCR 2 | ~2% to 60% * | Low- to high-risk ICU patients, and patients in septic shock | ||
| Ideal assay 3 | ~1% to 60% * | |||
| IAC | β-DG 4 | ~7% to 20% | Patients with severe pancreatitiis | Compared to β-d-glucan, a hypothetical PCR assay would broaden the window for antifungal treatment to include high-risk surgery patients with GI leaks, or high-risk liver transplant recipients with bile leaks. An ideal assay for intra-abdominal candidiasis would further broaden the window to include lower-risk patients. Using negative blood culture results to justify withholding pre-emptive treatment or discontinuing prophylaxis among patients with negative β-d-glucan results may broaden the window of pre-test likelihoods to ~7%–30%. Otherwise, negative blood cultures do not significantly impact antifungal strategies. |
| PCR 5 | ~7% to 40% | Moderate- to high-risk GI surgery patients, and patients with severe pancreatitis | ||
| Ideal assay 3 | ~1% to 60% | Peritoneal dialysis patients with peritonitis, in addition to groups above | ||
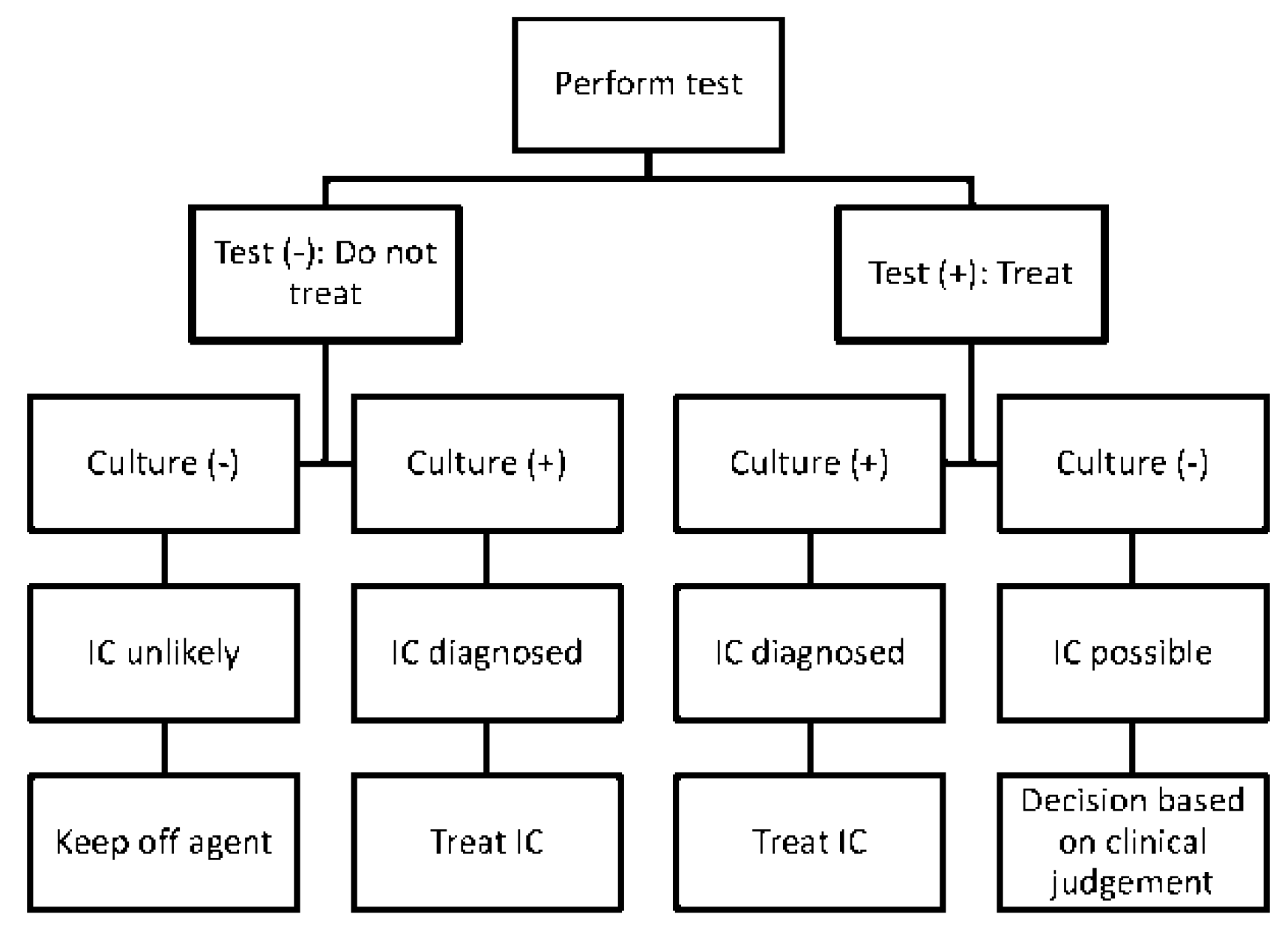
5.2. Treating Individual Patients
6. Conclusions
Author Contributions
Conflicts of Interests
References
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.; Fraser, V.J.; Kollef, M.H. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: A potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005, 49, 3640–3645. [Google Scholar] [CrossRef] [PubMed]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Undiagnosed invasive candidiasis: Incorporating non-culture diagnostics into rational prophylactic and preemptive antifungal strategies. Expert Rev. Anti Infect. Ther. 2014, 12, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Wissel, M.C.; Shields, R.K.; Salomoni, M.A.; Hao, B.; Press, E.G.; Shields, R.M.; Cheng, S.; Mitsani, D.; Vadnerkar, A.; et al. Performance of Candida real-time polymerase chain reaction, β-d-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. 2012, 54, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Tissot, F.; Lamoth, F.; Hauser, P.M.; Orasch, C.; Fluckiger, U.; Siegemund, M.; Zimmerli, S.; Calandra, T.; Bille, J.; Eggimann, P.; et al. β-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am. J. Respir. Crit. Care Med. 2013, 188, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Bille, J.; Schneider, R.; Mosimann, F.; Francioli, P. Clinical significance of Candida isolated from peritoneum in surgical patients. Lancet 1989, 2, 1437–1440. [Google Scholar] [CrossRef]
- Leroy, O.; Gangneux, J.P.; Montravers, P.; Mira, J.P.; Gouin, F.; Sollet, J.P.; Carlet, J.; Reynes, J.; Rosenheim, M.; Regnier, B.; et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in france (2005–2006). Crit. Care Med. 2009, 37, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.S.; Geroulanos, S.; Mentzelopoulos, S.D. Determinants of candidemia and candidemia-related death in cardiothoracic icu patients. Chest 2003, 124, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Kett, D.H.; Azoulay, E.; Echeverria, P.M.; Vincent, J.L. Candida bloodstream infections in intensive care units: Analysis of the extended prevalence of infection in intensive care unit study. Crit. Care Med. 2011, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Sable, C.; Sobel, J.; Alexander, B.D.; Donowitz, G.; Kan, V.; Kauffman, C.A.; Kett, D.; Larsen, R.A.; Morrison, V.; et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hadley, S.; Lee, W.W.; Ruthazer, R.; Nasraway, S.A., Jr. Candidemia as a cause of septic shock and multiple organ failure in nonimmunocompromised patients. Crit. Care Med. 2002, 30, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Shoham, S.; Vazquez, J.; Reboli, A.; Betts, R.; Barron, M.A.; Schuster, M.; Judson, M.A.; Revankar, S.G.; Caeiro, J.P.; et al. Msg-01: A randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin. Infect. Dis. 2014, 58, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Paphitou, N.I.; Ostrosky-Zeichner, L.; Rex, J.H. Rules for identifying patients at increased risk for Candidal infections in the surgical intensive care unit: Approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med. Mycol. 2005, 43, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Eschenauer, G.A.; Kwak, E.J.; Humar, A.; Potoski, B.A.; Clarke, L.G.; Shields, R.K.; Abdel-Massih, R.; Silveira, F.P.; Vergidis, P.; Clancy, C.J.; et al. Targeted versus universal antifungal prophylaxis among liver transplant recipients. Am. J. Transplant. 2014, 15, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Matuszkiewicz-Rowinska, J. Update on fungal peritonitis and its treatment. Perit Dial. Int. 2009, 29, S161–S165. [Google Scholar] [PubMed]
- Hall, A.M.; Poole, L.A.; Renton, B.; Wozniak, A.; Fisher, M.; Neal, T.; Halloran, C.M.; Cox, T.; Hampshire, P.A. Prediction of invasive Candidal infection in critically ill patients with severe acute pancreatitis. Crit. Care 2013, 17, R49. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Clancy, C.J.; Ostrosky-Zeichner, L.; Garey, K.W.; Alangaden, G.J.; Vazquez, J.A.; Groeger, S.J.; Judson, M.A.; Vinagre, Y.M.; Heard, S.O.; et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: A clinical trial. Clin. Infect. Dis. 2015, 60, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Karageorgopoulos, D.E.; Vouloumanou, E.K.; Ntziora, F.; Michalopoulos, A.; Rafailidis, P.I.; Falagas, M.E. β-d-glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin. Infect. Dis. 2011, 52, 750–770. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hang, J.P.; Zhang, L.; Wang, F.; Zhang, D.C.; Gong, F.H. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-d-glucan for invasive fungal infection: Focus on cutoff levels. J. Microbiol. Immunol. Infect. 2015, 48, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Neely, L.A.; Audeh, M.; Phung, N.A.; Min, M.; Suchocki, A.; Plourde, D.; Blanco, M.; Demas, V.; Skewis, L.R.; Anagnostou, T.; et al. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci. Transl. Med. 2013, 5, 182ra154. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Leibovici, L.; Paul, M. PCR diagnosis of invasive candidiasis: Systematic review and meta-analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Sugawara, Y.; Kaneko, J.; Tamura, S.; Makuuchi, M. Preemptive treatment of fungal infection based on plasma (1→3)β-d-glucan levels after liver transplantation. Infection 2007, 35, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Smith, P.B.; Davis, R.D.; Perfect, J.R.; Reller, L.B. The (1,3)β-d-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J. Clin. Microbiol. 2010, 48, 4083–4088. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Winston, D.J.; Limaye, A.P.; Pelletier, S.; Safdar, N.; Morris, M.I.; Meneses, K.; Busuttil, R.W.; Wagener, M.M.; Wheat, L.J. Performance characteristics of galactomannan and β-d-glucan in high-risk liver transplant recipients. Transplantation 2015, 99, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.D.; Samsa, G.P.; Schell, W.A.; Reller, L.B.; Perfect, J.R.; Alexander, B.D. Quantitation of Candida CFU in initial positive blood cultures. J. Clin. Microbiol. 2011, 49, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Telenti, A.; Steckelberg, J.M.; Stockman, L.; Edson, R.S.; Roberts, G.D. Quantitative blood cultures in candidemia. Mayo Clin. Proc. 1991, 66, 1120–1123. [Google Scholar] [CrossRef]
- Kiehn, T.E.; Wong, B.; Edwards, F.F.; Armstrong, D. Comparative recovery of bacteria and yeasts from lysis-centrifugation and a conventional blood culture system. J. Clin. Microbiol. 1983, 18, 300–304. [Google Scholar] [PubMed]
- Brannon, P.; Kiehn, T.E. Large-scale clinical comparison of the lysis-centrifugation and radiometric systems for blood culture. J. Clin. Microbiol. 1985, 22, 951–954. [Google Scholar] [PubMed]
- Muldoon, E.G.; Denning, D.W. Editorial commentary: Prophylactic echinocandin: Is there a subgroup of intensive care unit patients who benefit? Clin. Infect. Dis. 2014, 58, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Knitsch, W.; Vincent, J.L.; Utzolino, S.; Francois, B.; Dinya, T.; Dimopoulos, G.; Ozgunes, I.; Valia, J.C.; Eggimann, P.; Leon, C.; et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin. Infect. Dis. 2015, 61, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Webster, A.C.; Sorrell, T.C.; Craig, J.C. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: Systematic review and meta-analysis of randomized clinical trials. J. Antimicrob Chemother. 2006, 57, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Chung, K.; Jackson, W.L.; Waterman, P.E.; Kollef, M.H. Fluconazole prophylaxis in critically ill surgical patients: A meta-analysis. Crit. Care Med. 2005, 33, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Francioli, P.; Bille, J.; Schneider, R.; Wu, M.M.; Chapuis, G.; Chiolero, R.; Pannatier, A.; Schilling, J.; Geroulanos, S.; et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit. Care Med. 1999, 27, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Garbino, J.; Lew, D.P.; Romand, J.A.; Hugonnet, S.; Auckenthaler, R.; Pittet, D. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: A randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 2002, 28, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Pelz, R.K.; Hendrix, C.W.; Swoboda, S.M.; Diener-West, M.; Merz, W.G.; Hammond, J.; Lipsett, P.A. Double-blind placebo-controlled trial of fluconazole to prevent Candidal infections in critically ill surgical patients. Ann. Surg. 2001, 233, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.L.; Winston, D.J.; Greenfield, R.A.; Chandrasekar, P.H.; Fox, B.; Kaizer, H.; Shadduck, R.K.; Shea, T.C.; Stiff, P.; Friedman, D.J.; et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N. Engl. J. Med. 1992, 326, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.A.; Osborne, B.; Adams, R.; Levenstein, M.J.; Schoch, H.G.; Feldman, A.R.; Meyers, J.D.; Bowden, R.A. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—A prospective, randomized, double-blind study. J. Infect. Dis. 1995, 171, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.J.; Sabin, L.; Schmid, C.H. Why clinicians are natural Bayesians. BMJ 2005, 330, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Pauker, S.G.; Kopelman, R.I. Interpreting hoofbeats: Can bayes help clear the haze? N. Engl. J. Med. 1992, 327, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Kwa, A.L.; Cheng, S.; Du, C.; Clancy, C.J. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob. Agents Chemother. 2012, 56, 4862–4869. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clancy, C.J.; Shields, R.K.; Nguyen, M.H. Invasive Candidiasis in Various Patient Populations: Incorporating Non-Culture Diagnostic Tests into Rational Management Strategies. J. Fungi 2016, 2, 10. https://doi.org/10.3390/jof2010010
Clancy CJ, Shields RK, Nguyen MH. Invasive Candidiasis in Various Patient Populations: Incorporating Non-Culture Diagnostic Tests into Rational Management Strategies. Journal of Fungi. 2016; 2(1):10. https://doi.org/10.3390/jof2010010
Chicago/Turabian StyleClancy, Cornelius J., Ryan K. Shields, and M. Hong Nguyen. 2016. "Invasive Candidiasis in Various Patient Populations: Incorporating Non-Culture Diagnostic Tests into Rational Management Strategies" Journal of Fungi 2, no. 1: 10. https://doi.org/10.3390/jof2010010