Effective Single Photodynamic Treatment of ex Vivo Onychomycosis Using a Multifunctional Porphyrin Photosensitizer and Green Light
Abstract
:1. Introduction
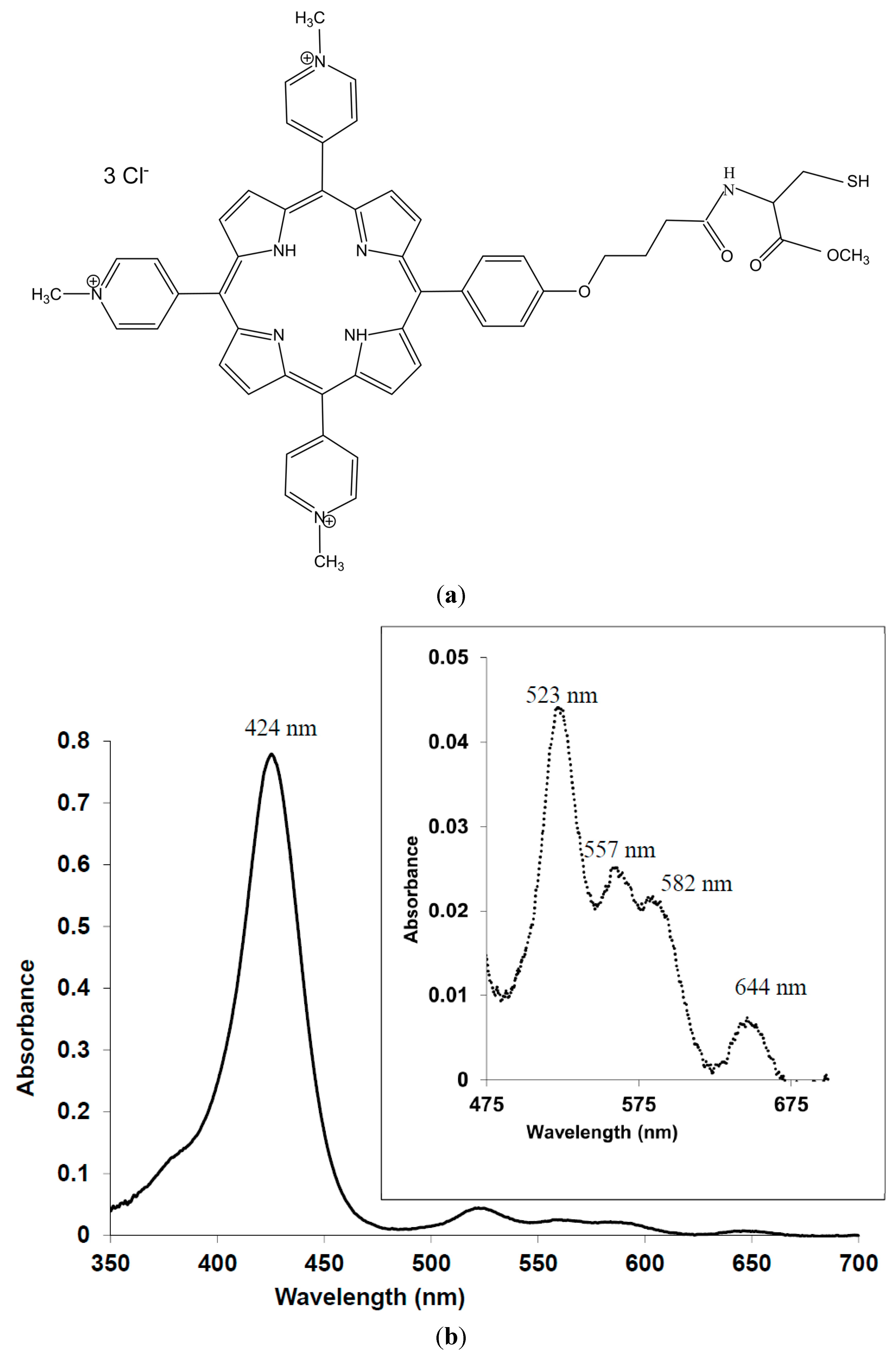
2. Experimental Section
2.1. Chemicals
2.2. Strains
2.3. Nails
2.4. Microconidia Preparation
2.5. Ex Vivo Onychomycosis Model
2.6. Light Sources
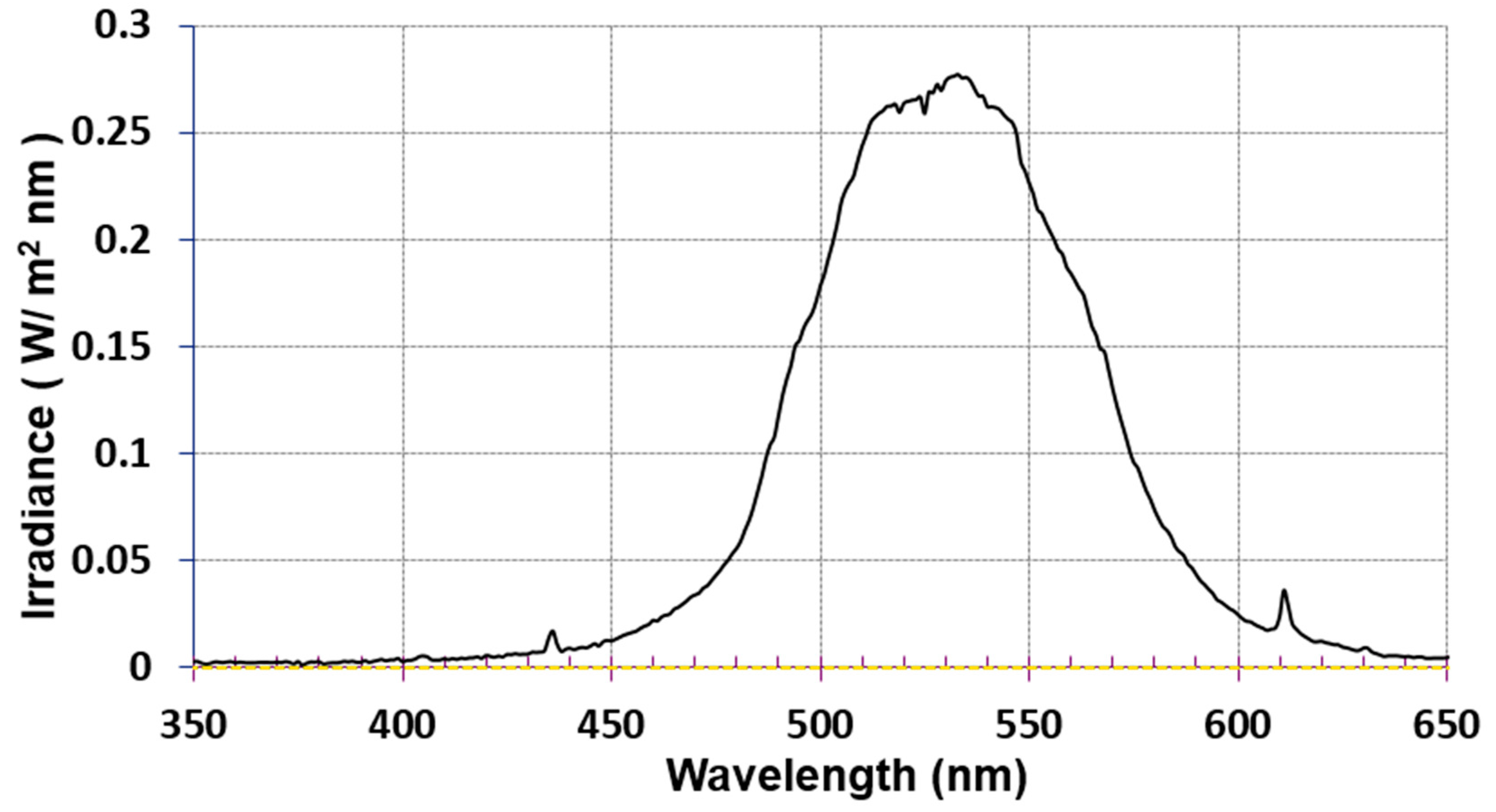
2.7. PDTs
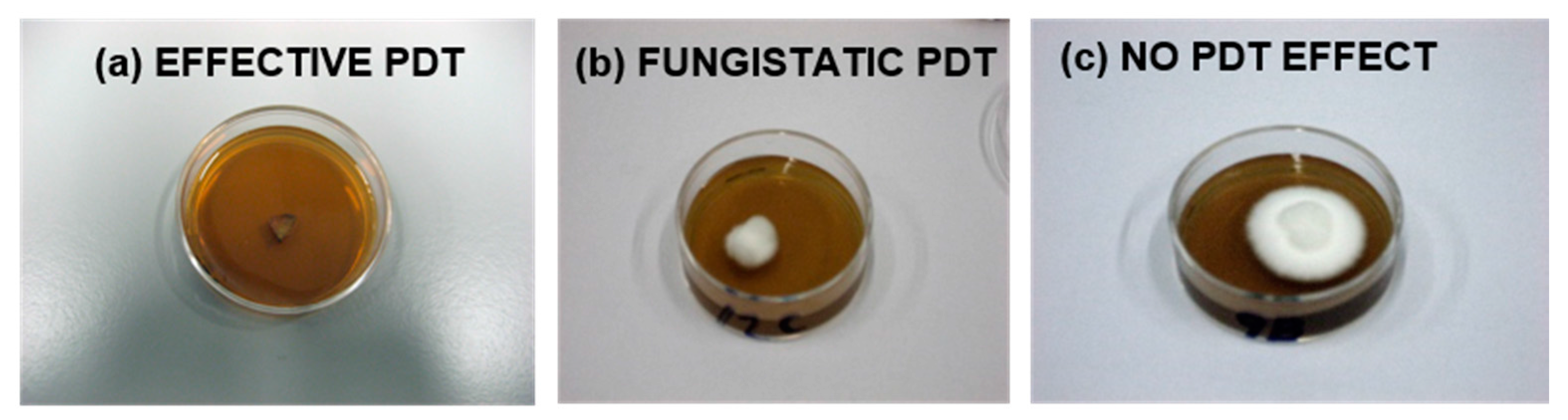
2.8. Statistical Analysis
3. Results
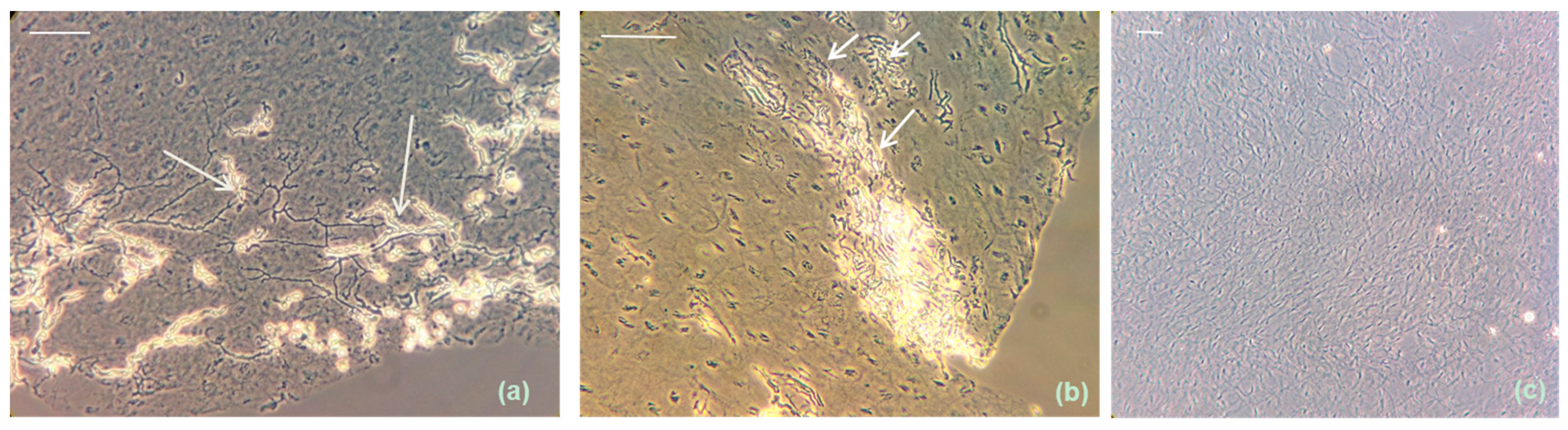
3.1. Extensive ex Vivo Dermatophytic Nail Invasion at 37 °C and 6.4% CO2
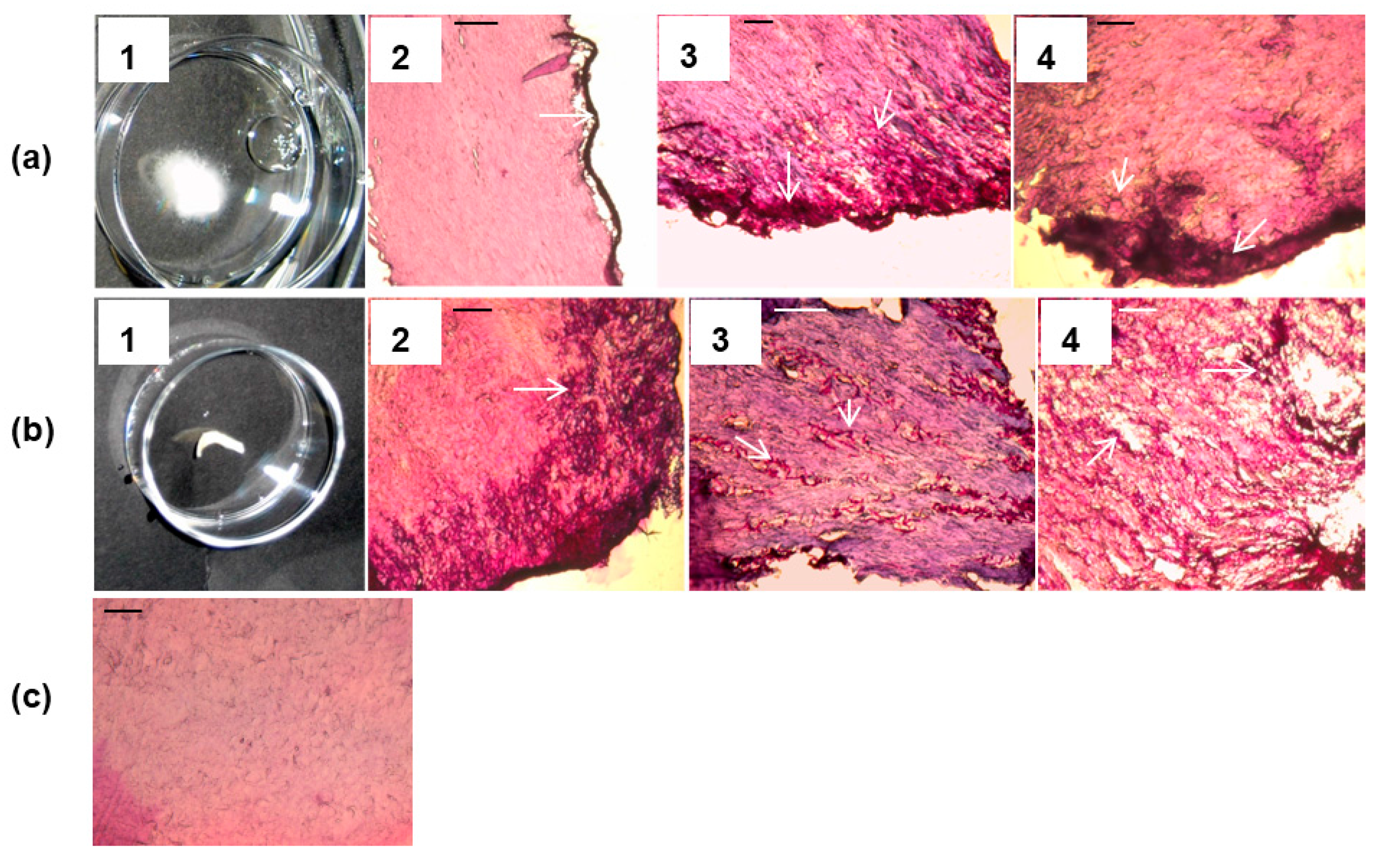
3.2. Arthroconidia Production in ex Vivo Human Nail at 37 °C and 6.4% CO2
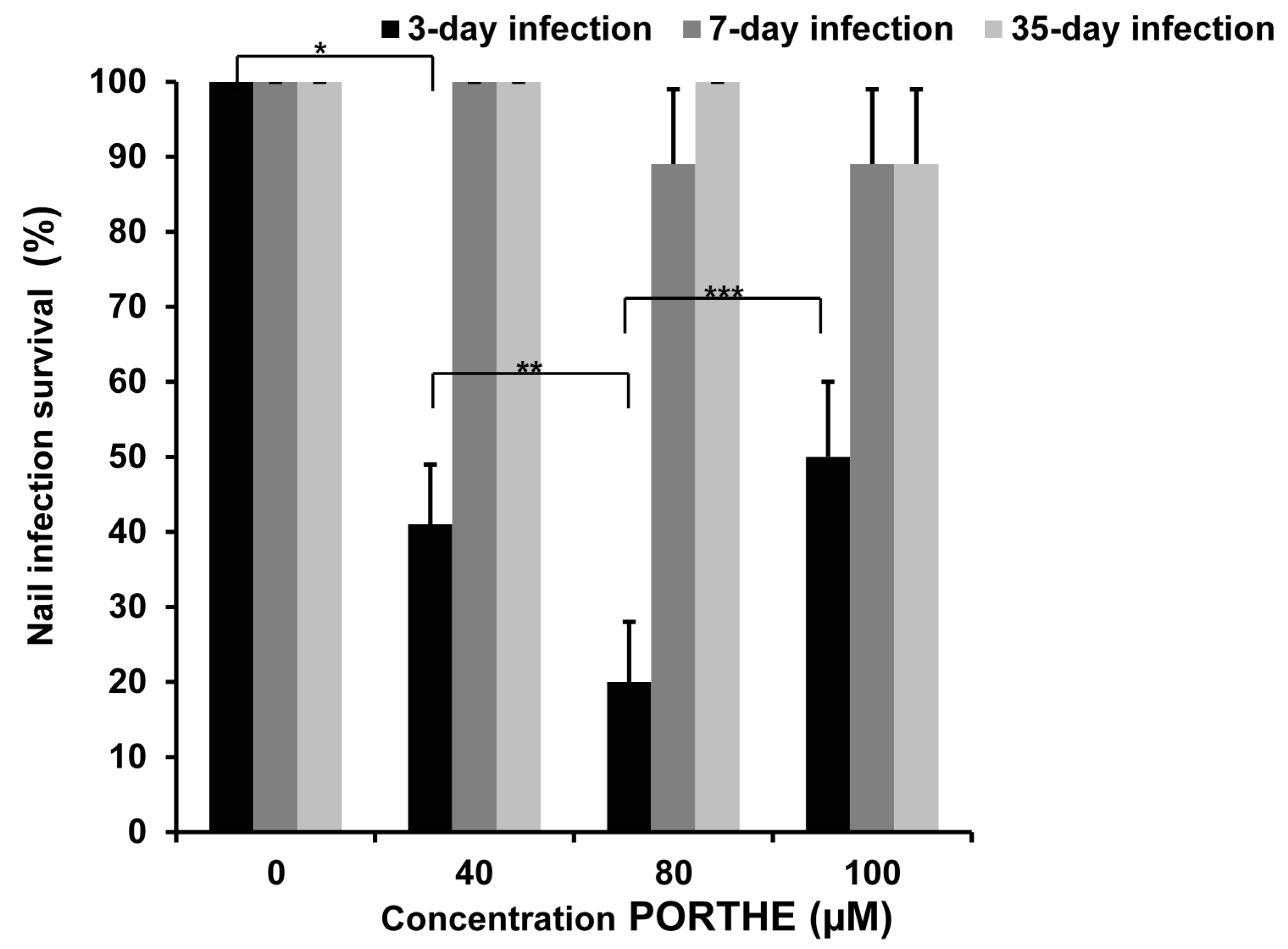
3.3. One-Time PORTHE Laser PDT (27 J/cm2) at pH 5 Only Effective for Non-Invasive 3-Day Onychomycoses
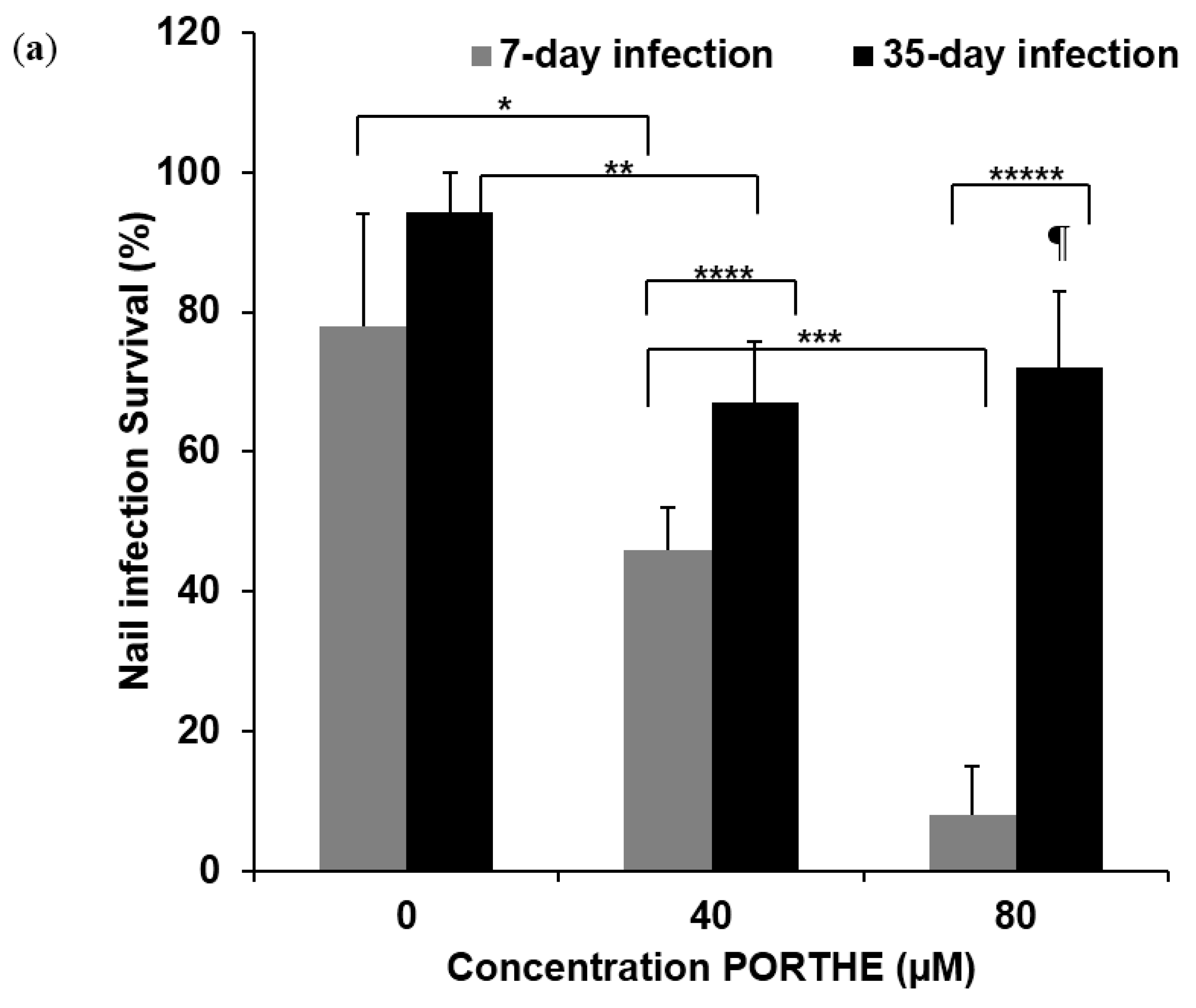
3.4. PG Required for Successful One-Time PORTHE Laser PDT of 7-Day and 35-Day Onychomycoses
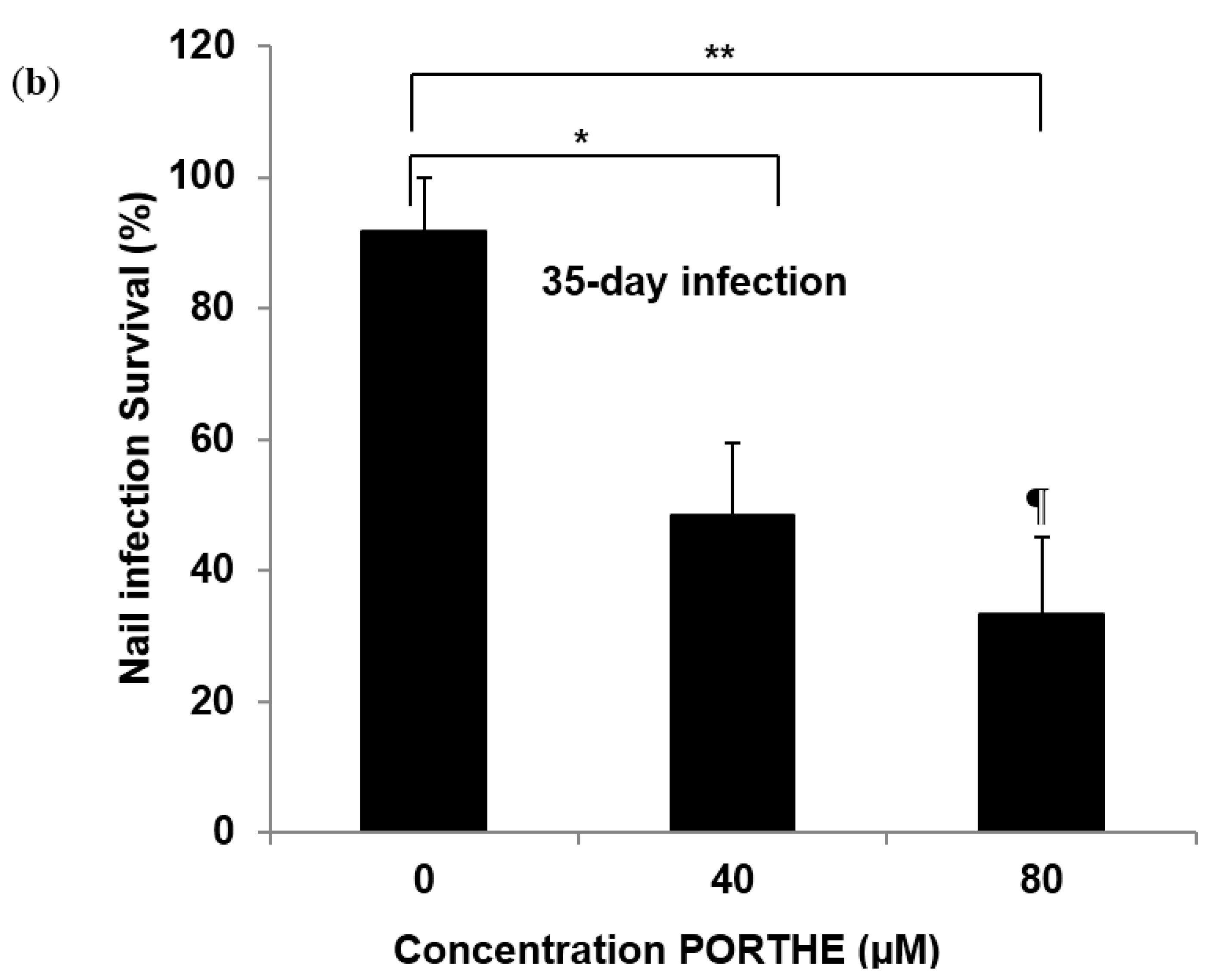
3.5. Equally Effective One-Time LED (28 J/cm2) PDT of 35-Day T. Mentagrophytes, T. Rubrum and T. Tonsurans Onychomycoses with pH 5 PORTHE/PG Formulation
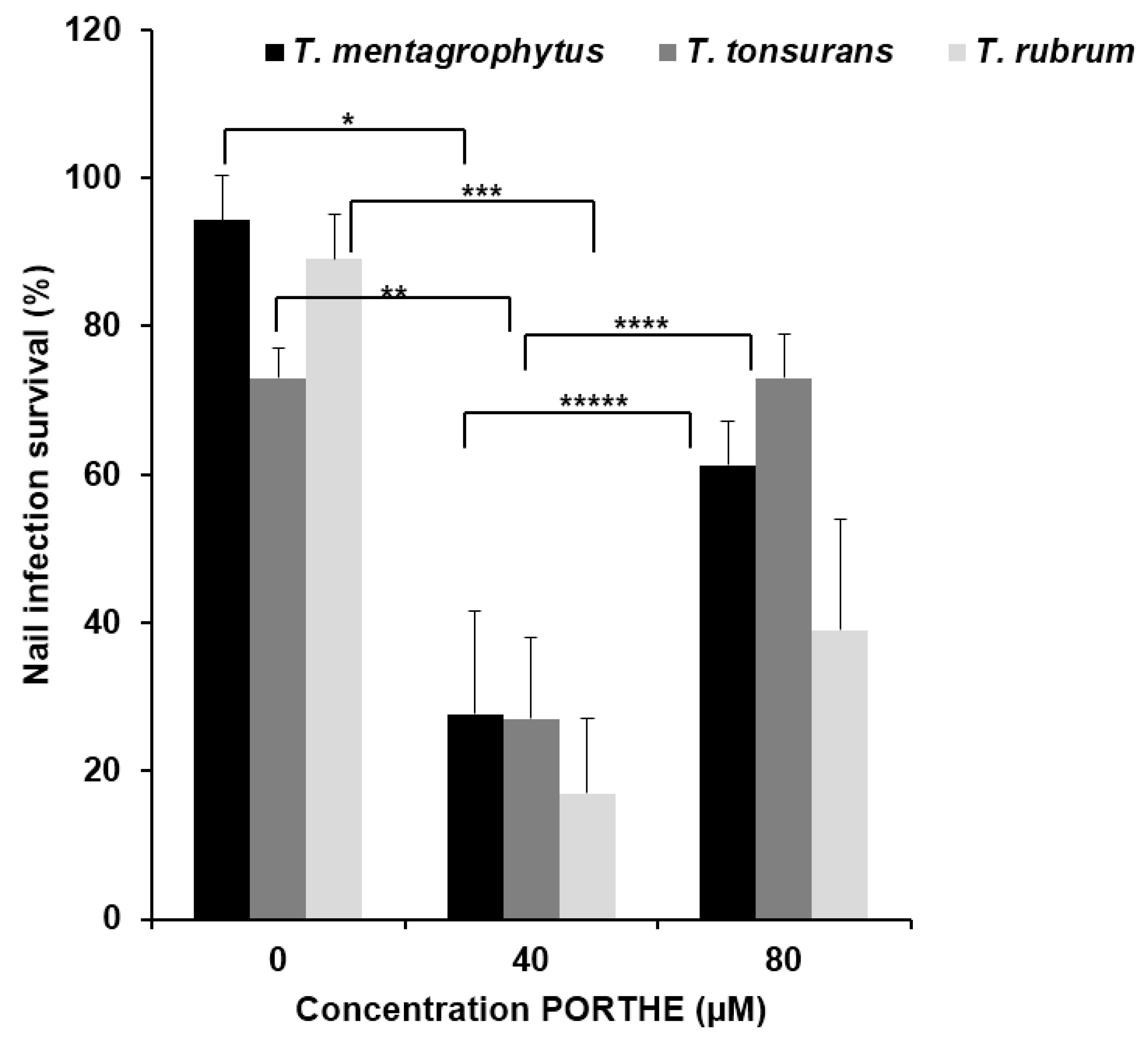
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grover, C.; Khurana, A. Onychomycosis: Newer insights in pathogenesis and diagnosis. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ramos-E-Silva, M.; Lima, C.M.; Schechtman, R.C.; Trope, B.M.; Carneiro, S. Superficial mycoses in immunodepressed patients (AIDS). Clin. Dermatol. 2010, 28, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Humke, S. The prevalence and management of onychomycosis in diabetic patients. Eur. J Dermatol. 2000, 10, 379–384. [Google Scholar] [PubMed]
- Nazar, J.R.; Gerosa, P.E.; Diaz, O.A. Onychomycoses: Epidemiology, causative agents and assessment of diagnostic laboratory methods. Rev. Argent Microbiol. 2012, 44, 21–25. [Google Scholar] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.; Rossi, A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia 2008, 166, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Leidich, S.D.; Isham, N.; Leitner, I.; Ryder, N.S.; Ghannoum, M.A. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob. Agents Chemother. 2003, 47, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. The susceptibility of dermatophytes to photodynamic treatment with special focus on Trichophyton rubrum. Photochem. Photobiol. 2011, 87, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Koshi, E.; Philip, K.; Mohan, A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011, 15, 323–327. [Google Scholar] [PubMed]
- Smijs, T.; Dame, Z.; de Haas, E.R.; Aans, J.B.; Pavel, S.; Sterenborg, H. Photodynamic and Nail Penetration Enhancing Effects of Novel Multifunctional Photosensitizers Designed for The Treatment of Onychomycosis. Photochem. Photobiol. 2013, 90, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Cronin, L.; Moffitt, M.; Mawad, D.; Morton, O.C.; Lauto, A.; Stack, C. An in vitro study of the photodynamic effect of rose bengal on trichophyton rubrum. J. Biophotonics. 2012, 11, 1–9. [Google Scholar]
- Ghavam, S.A.; Aref, S.; Mohajerani, E.; Shidfar, M.R.; Moravvej, H. Laser irradiation on growth of trichophyton rubrum: An in vitro study. J. Lasers Med. Sci. 2015, 6, 10–16. [Google Scholar] [PubMed]
- Zurita, J.; Hay, R.J. Adherence of dermatophyte microconidia and arthroconidia to human keratinocytes in vitro. J. Invest Dermatol. 1987, 89, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Bouwstra, J.A.; Schuitmaker, H.J.; Talebi, M.; Pavel, S. A novel ex vivo skin model to study the susceptibility of the dermatophyte Trichophyton rubrum to photodynamic treatment in different growth phases. J. Antimicrob. Chemother. 2007, 59, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Bouwstra, J.A.; Talebi, M.; Pavel, S. Investigation of conditions involved in the susceptibility of the dermatophyte Trichophyton rubrum to photodynamic treatment. J. Antimicrob. Chemother. 2007, 60, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 1958, 74, 443–450. [Google Scholar] [CrossRef]
- Mellody, M.; Bigg, E. The fungicidal action of triethylene glycol. J. Infect. Dis. 1946, 79, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, T.; Koskela, M. Antibacterial and antifungal properties of propylene glycol, hexylene glycol, and 1,3-butylene glycol in vitro. Acta Derm. Venereol. 1991, 71, 148–150. [Google Scholar] [PubMed]
- Mehra, T.; Schaller, M.; Walker, B.; Braunsdorf, C.; Mailander-Sanchez, D.; Schynowski, F.; Hahn, R.; Rocken, M.; Koberle, M.; Borelli, C. Efficacy of antifungal PACT in an in vitro model of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Tauber, A.; Muller-Goymann, C.C. In vitro permeation and penetration of ciclopirox olamine from poloxamer 407-based formulations-comparison of isolated human stratum corneum, bovine hoof plates and keratin films. Int. J. Pharm. 2015, 489, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sleven, R.; Lanckacker, E.; Boulet, G.; Delputte, P.; Maes, L.; Cos, P. Development of a novel in vitro onychomycosis model for the evaluation of topical antifungal activity. J. Microbiol. Methods 2015, 112, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Ahmad, I.; Porretta, M.; Summerbell, R.C. Arthroconidial formation in Trichophyton raubitschekii. Mycoses 2003, 46, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; van der Haas, R.N.; Lugtenburg, J.; Liu, Y.; de Jong, R.L.; Schuitmaker, H.J. Photodynamic treatment of the dermatophyte Trichophyton rubrum and its microconidia with porphyrin photosensitizers. Photochem. Photobiol. 2004, 80, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ragas, X.; Dai, T.; Tegos, G.P.; Agut, M.; Nonell, S.; Hamblin, M.R. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: In vitro and in vivo studies. Lasers Surg. Med. 2010, 42, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lourens, N. Single Dose Local Tolerance Study with PORTHE in Göttingen Minipigs; Report No.: Project 507537, substance 206130/A; WIL Research: Den Bosch, The Netherlands, 2015. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollander, C.D.; Visser, J.; De Haas, E.; Incrocci, L.; Smijs, T. Effective Single Photodynamic Treatment of ex Vivo Onychomycosis Using a Multifunctional Porphyrin Photosensitizer and Green Light. J. Fungi 2015, 1, 138-153. https://doi.org/10.3390/jof1020138
Hollander CD, Visser J, De Haas E, Incrocci L, Smijs T. Effective Single Photodynamic Treatment of ex Vivo Onychomycosis Using a Multifunctional Porphyrin Photosensitizer and Green Light. Journal of Fungi. 2015; 1(2):138-153. https://doi.org/10.3390/jof1020138
Chicago/Turabian StyleHollander, Chelsea Den, Jasper Visser, Ellen De Haas, Luca Incrocci, and Threes Smijs. 2015. "Effective Single Photodynamic Treatment of ex Vivo Onychomycosis Using a Multifunctional Porphyrin Photosensitizer and Green Light" Journal of Fungi 1, no. 2: 138-153. https://doi.org/10.3390/jof1020138





