Onychomycosis: A Review
Abstract
:1. Introduction
2. Clinical Features
2.1. Distal and Lateral Subungual Onychomycosis
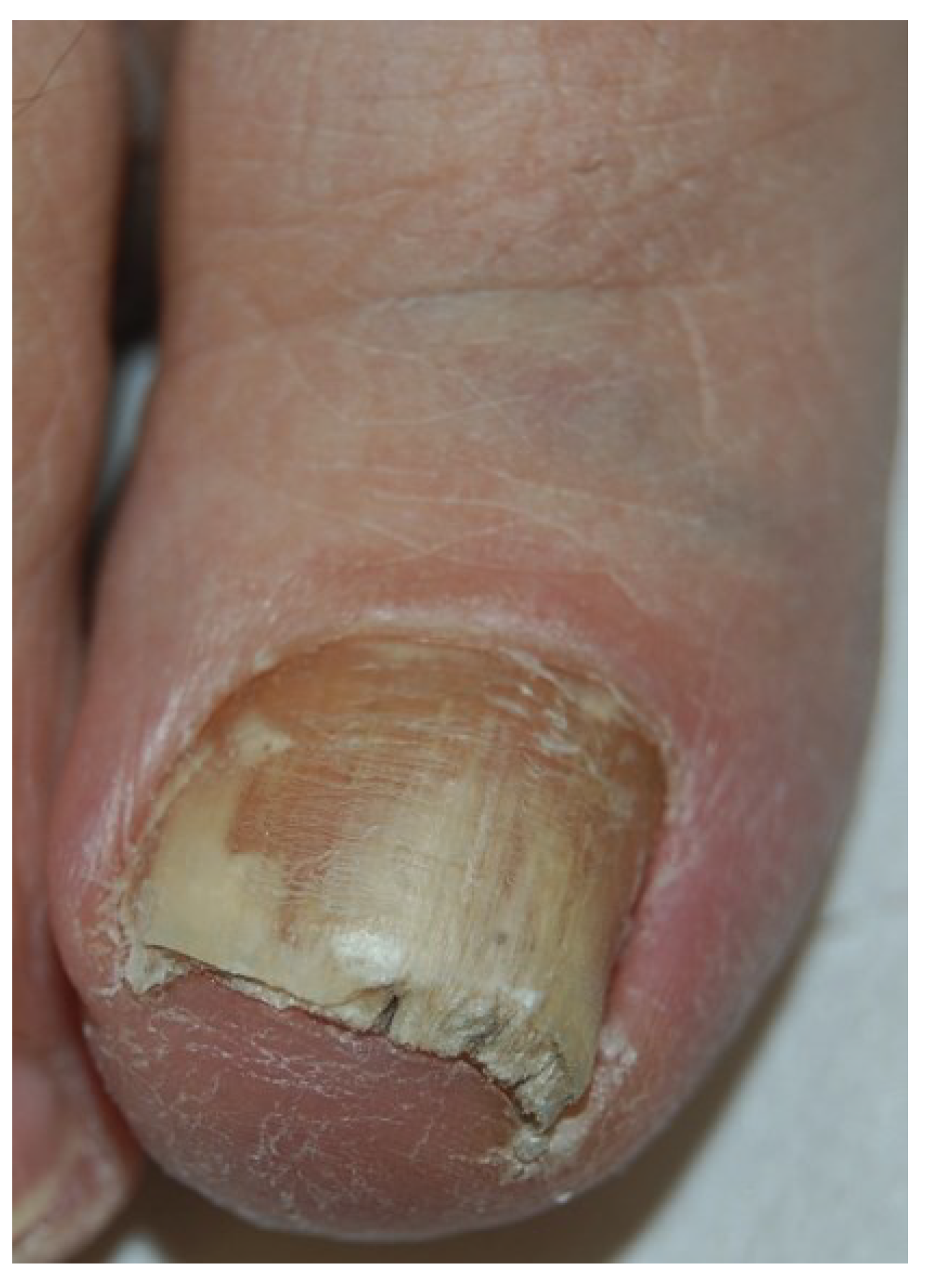
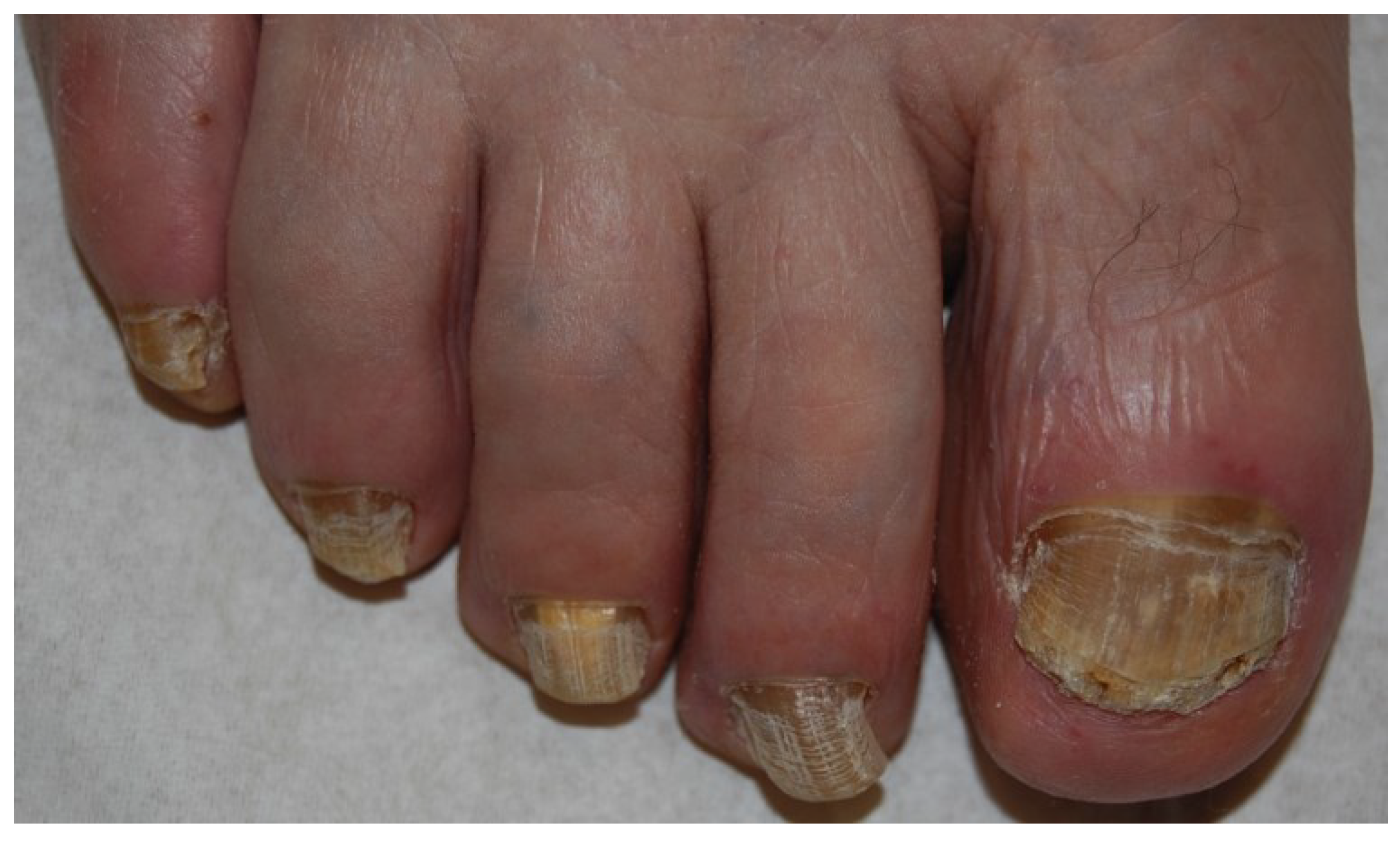
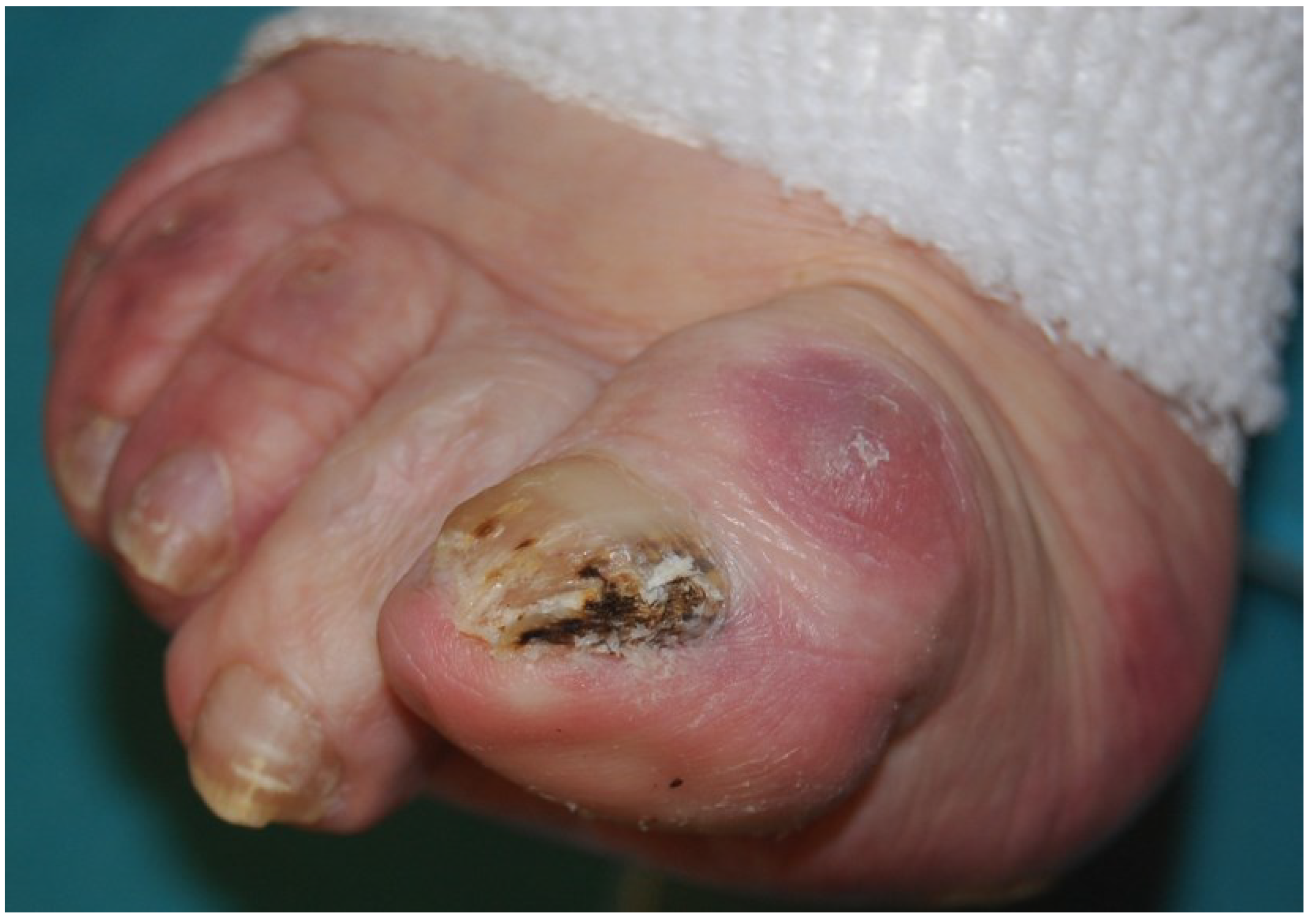

2.2. White Superficial Onychomycosis

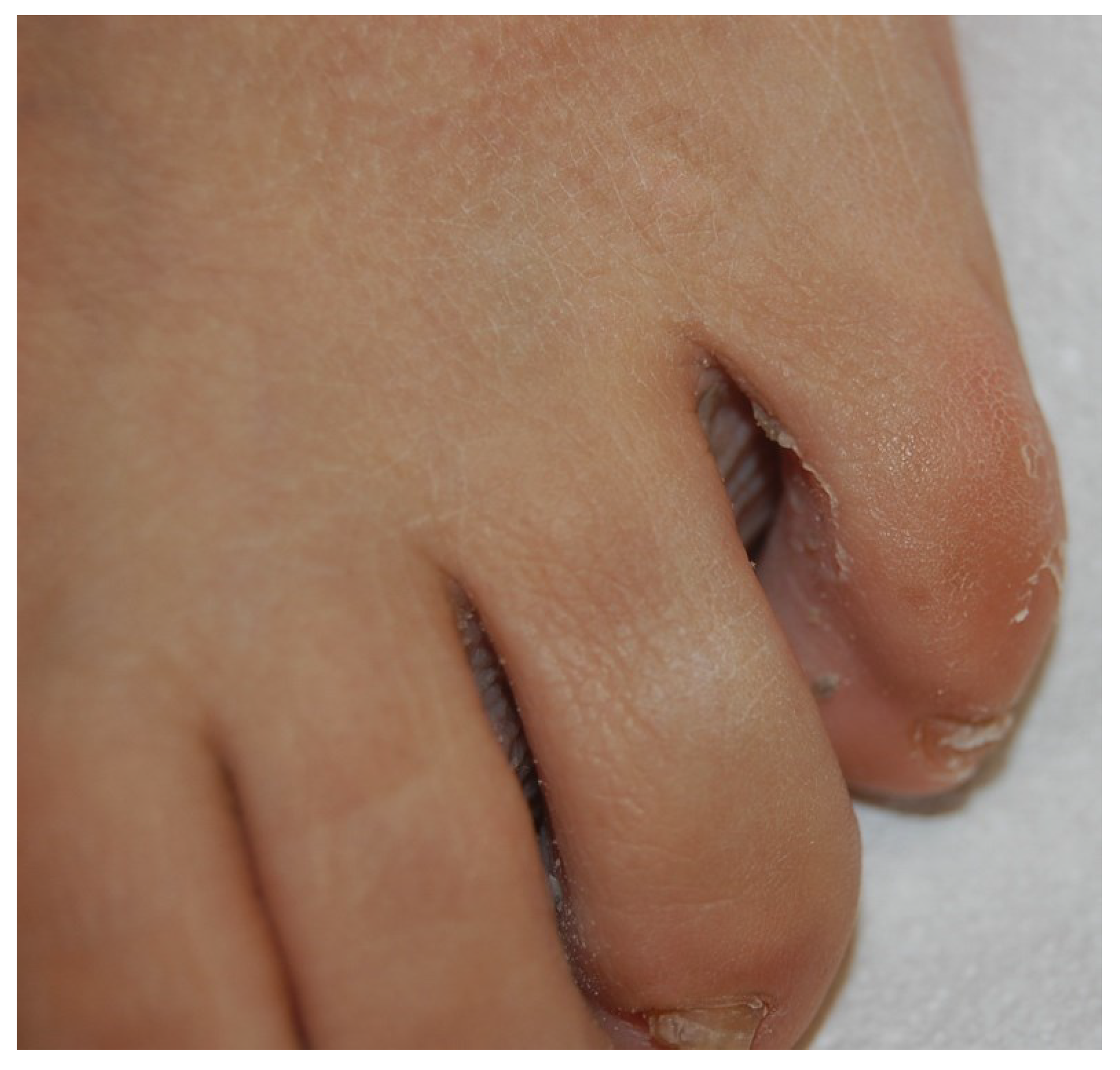
2.3. Proximal Subungual Onychomycosis
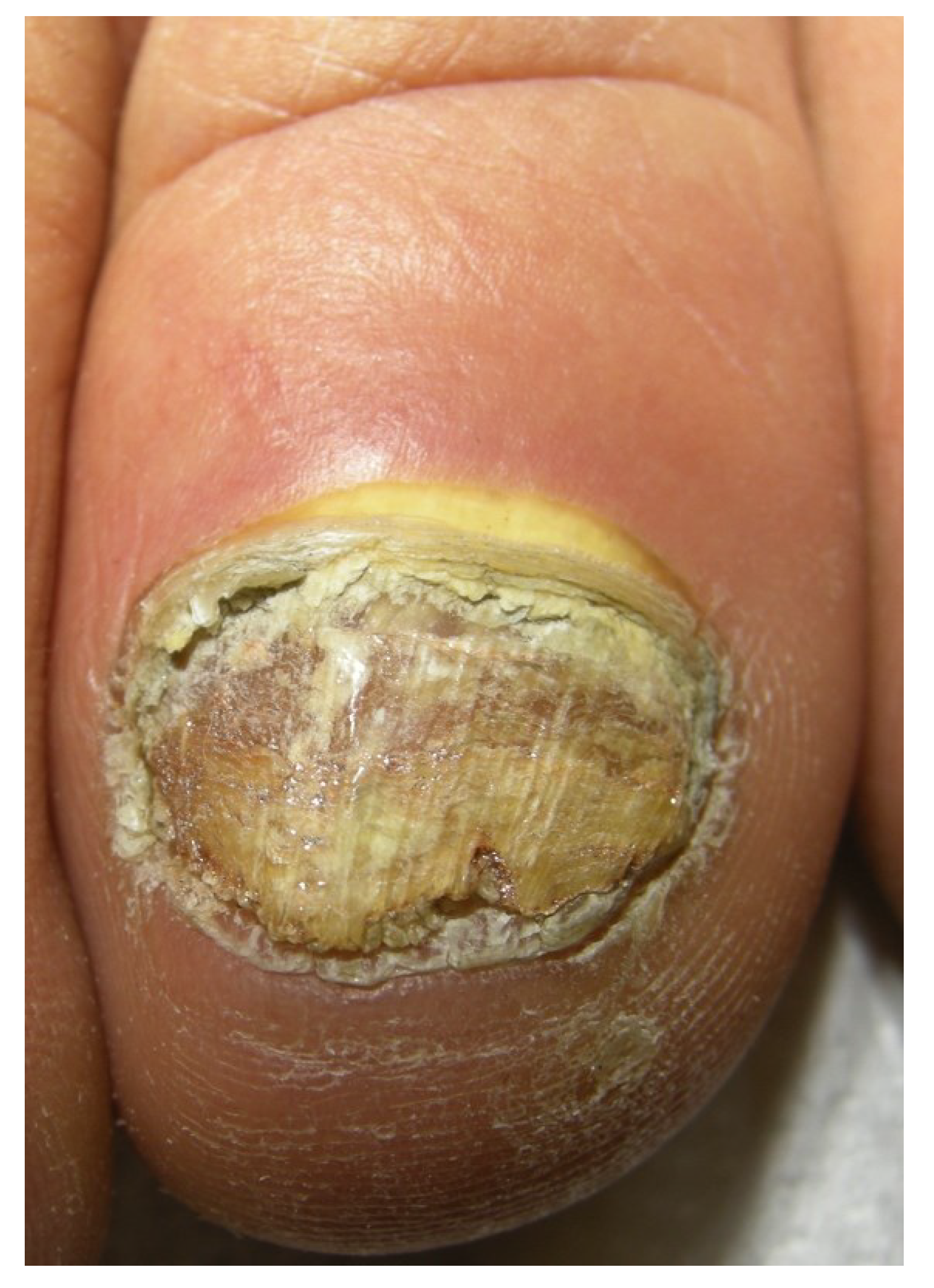
2.4. Endonyx Onychomycosis
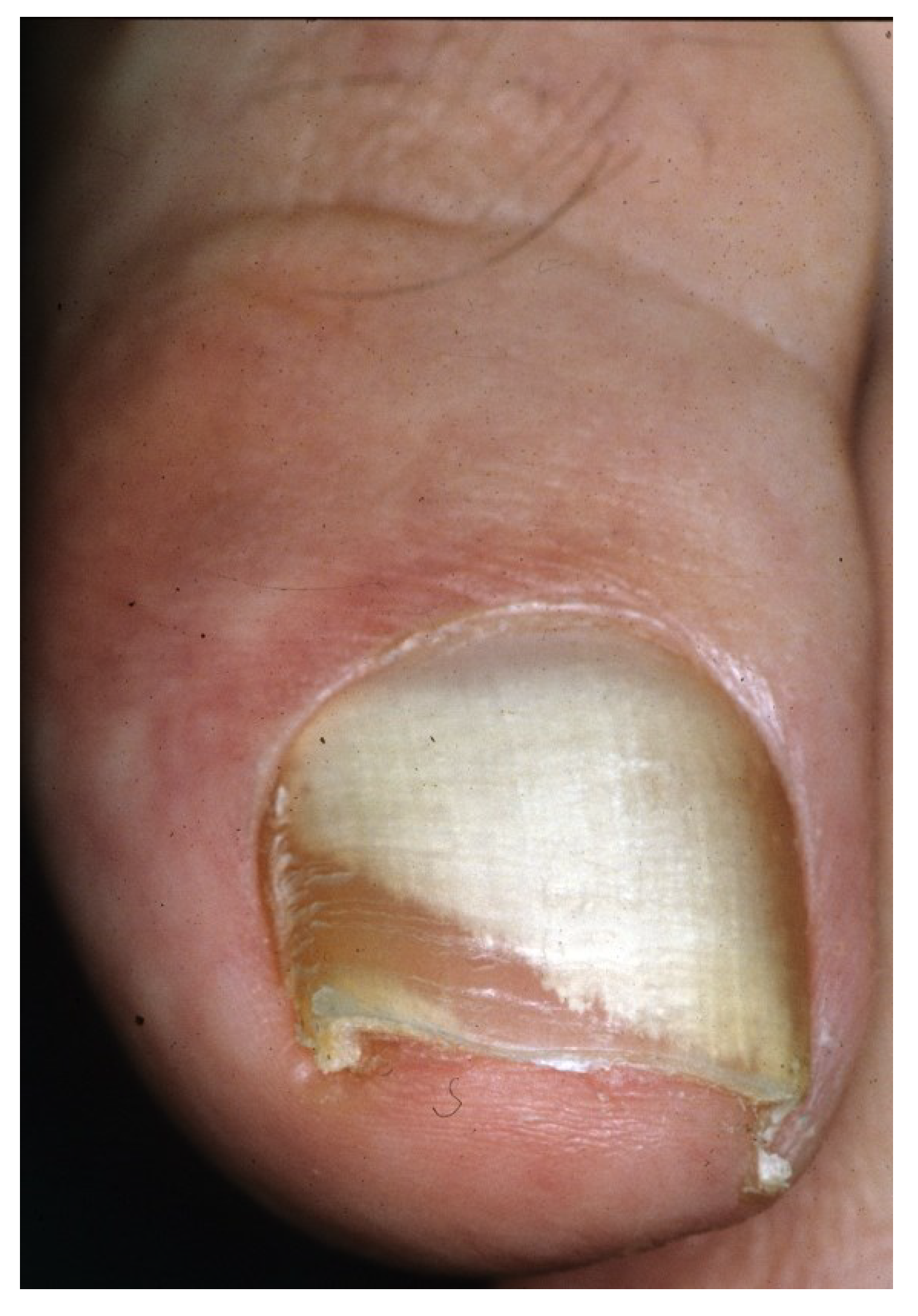
2.5. Total Dystrophic Onychomycosis
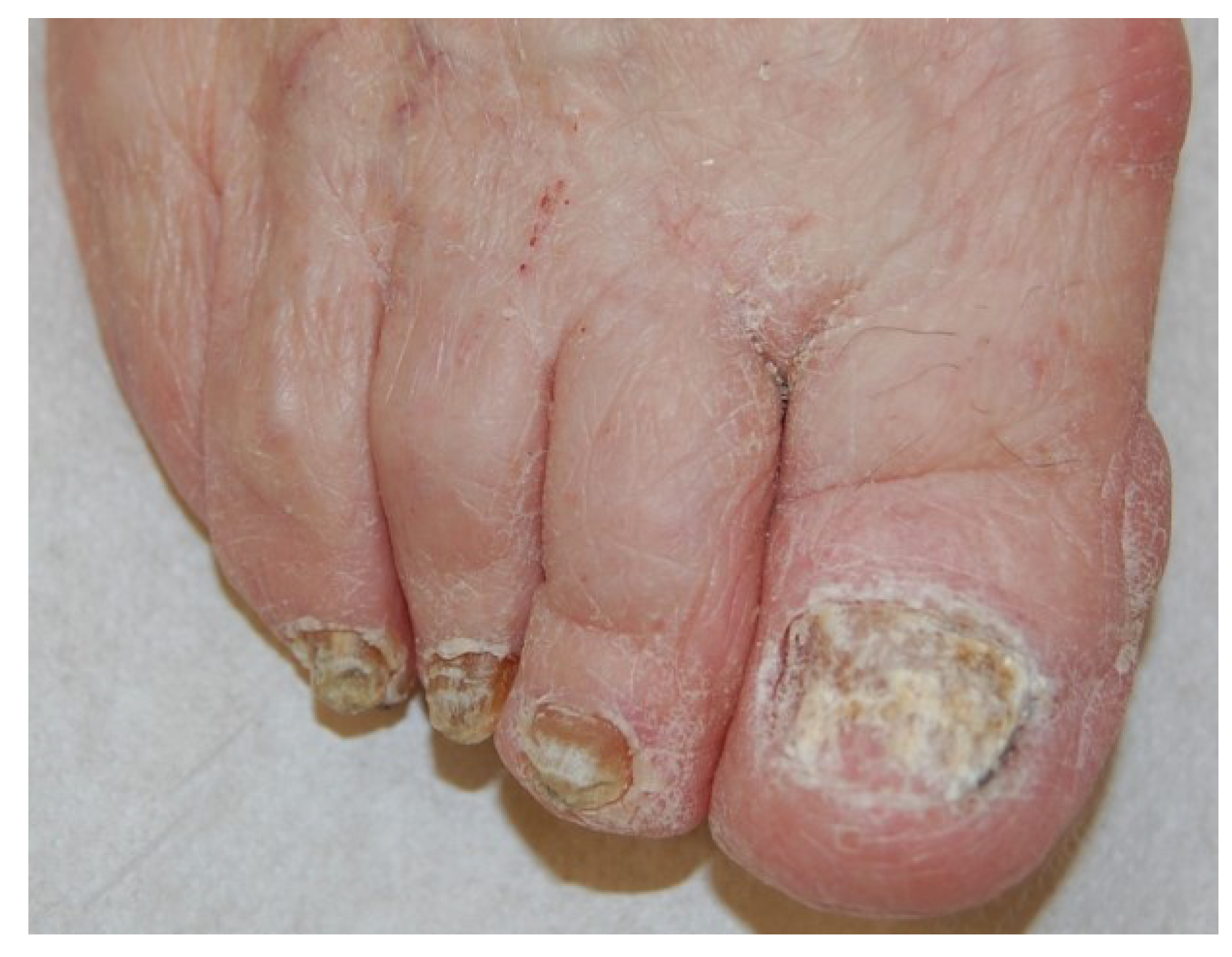
3. Diagnosis of Onychomycosis

4. Treatment
4.1. Topical Treatment
4.2. Systemic Treatment
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Heikkla, H.; Stubb, S. The prevalence of onychomycosis in Finland. Br. J. Dermatol. 1995, 133, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Roseeuw, D. Achilles foot screening project: Preliminary results of patients screened by dermatologists. J. Eur. Acad. Dermatol. Venereol. 1999, 12, S6–S9. [Google Scholar] [CrossRef] [PubMed]
- Scher, R.K.; Rich, P.; Pariser, D.; Elewski, B. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin. Cutan. Med. Surg. 2013, 32, S2–S4. [Google Scholar] [PubMed]
- Nouripour-Sisakht, S.; Mirhendi, H.; Shidfar, M.R.; Ahmadi, B.; Rezaei-Matehkolaei, A.; Geramishoar, M.; Zarei, F.; Jalalizand, N. Aspergillus species as emerging causative agents of onychomycosis. J. Mycol. Med. 2015. [Google Scholar] [CrossRef]
- Gupta, A.K.; Drummond-Main, C.; Cooper, E.A.; Brintnell, W.; Piraccini, B.M.; Tosti, A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Dermatol. 2012, 66, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, J.A.; Tilakatatne, W.M.; Panagoda, G.J. Candidal onychomycosis: A mini-review. Mycopathologia 2009, 168, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Pichardo-Geisinger, R.; Mora, D.C.; Newman, J.C.; Arcury, T.A.; Feldman, S.R.; Quandt, S.A. Comorbidity of tinea pedis and onychomycosis and evaluation of risk factors in Latino immigrant poultry processing and other manual laborers. South. Med. J. 2014, 107, 374–349. [Google Scholar] [CrossRef] [PubMed]
- Wulkan, A.J.; Tosti, A. Pediatric nail conditions. Clin. Dermatol. 2013, 31, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Mukherjee, P.K.; Warshaw, E.M.; Evans, S.; Korman, N.J.; Tavakkol, A. Molecular analysis of dermatophytes suggests spread of infection among household members. Cutis 2013, 91, 237–245. [Google Scholar] [PubMed]
- Piraccini, B.M.; Starace, M.; Bruni, F. Onychomycosis in children. Expert Rev. Dermatol. 2012, 7, 569–578. [Google Scholar] [CrossRef]
- Finch, J.; Arenas, R.; Baran, R. Fungal melanonychia. J. Am. Acad. Dermatol. 2012, 66, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Tosti, A. White superficial onychomycosis: Epidemiological, clinical, and pathological study of 79 patients. Arch. Dermatol. 2004, 140, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Lorenzi, S.; Tosti, A. ‘Deep’ white superficial onychomycosis due to molds. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Baran, R.; Piraccini, B.M.; Fanti, P.A. “Endonyx” onychomycosis: A new modality of nail invasion by dermatophytes. Acta Derm. Venereol. 1999, 79, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Balestri, R.; Starace, M.; Rech, G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Fouilloux, B.; Perrot, J.L.; Labeille, B.; Douchet, C.; Cambazard, F. Confocal microcopy for healthy and pathological nail. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Arrese, J.E.; Quatresooz, P.; Pierard-Franchimont, C.; Pierard, G.E. Nail histomycology. Protean aspects of a human fungal bed. Ann. Dermatol. Venereol. 2003, 130, 1254–1259. [Google Scholar] [PubMed]
- Tsunemi, Y.; Takehara, K.; Miura, Y.; Nakagami, G.; Sanada, H.; Kawashima, M. Screening for tinea unguium by Dermatophyte Test Strip. Br. J. Dermatol. 2014, 170, 328–331. [Google Scholar] [CrossRef]
- Idriss, M.H.; Khali, A.; Elston, D. The diagnostic value of fungal fluorescence in onychomycosis. J. Cutan. Pathol. 2013, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Jachtenberg, J.W.; Pavel, S.; Bakker-Schut, T.C.; Willemse-Erix, D.; de Haas, E.R.; Sterenborg, H. Detection and differentiation of causative organisms of onychomycosis in an ex vivo nail model by means of Raman spectroscopy. J. Eur. Acad. Dermatol .Venereol. 2013, 28, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J. Clin. Aesthet. Dermatol. 2014, 7, 10–18. [Google Scholar] [PubMed]
- Tietz, H.J.; Hay, R.; Querner, S.; Delcker, A.; Kurka, P.; Merk, H.F. Efficacy of 4 weeks topical bifonazole treatment for onychomycosis after nail ablation with 40% urea: A double-blind, randomized, placebo-controlled multicenter study. Mycoses 2013, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Baran, R. Onychomycosis: A proposed revision of the clinical classification. J. Am. Acad. Dermatol. 2011, 65, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Daigle, D.; Foley, K.A. Topical therapy for toenail onychomycosis: An evidence-based review. Am. J. Clin. Dermatol. 2014, 15, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Ryder, J.E.; Baran, R. The use of topical therapies to treat onychomycosis. Dermatol. Clin. 2003, 21, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Paquet, M.; Simpson, F.C. Therapies for the treatment of onychomycosis. Clin. Dermatol. 2013, 31, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A. Efinaconazole solution 10%: Topical antifungal therapy for toenail onychomycosis. Cutis 2013, 92, 203–208. [Google Scholar] [PubMed]
- Rich, P. Efinaconazole topical solution, 10%: The benefits of treating onychomycosis early. J. Drugs Dermatol. 2015, 14, 58–62. [Google Scholar] [PubMed]
- Elewski, B.E.; Tosti, A. Tavaborole for the treatment of onychomycosis. Expert Opin. Pharmacother. 2014, 15, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Tavaborole: First global approval. Drugs 2014, 74, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Bahena, M.E.; Bucko, A.; Ocampo-Candiani, J.; Herz-Ruelas, M.E.; Jones, T.M.; Jarratt, M.T.; Pollak, R.A.; Zane, L.T. The efficacy and safety of tavaborole, a novel, boron-based pharmaceutical agent: Phase 2 studies conducted for the topical treatment of toenail onychomycosis. J. Drugs Dermatol. 2014, 13, 1124–1132. [Google Scholar] [PubMed]
- Elewski, B.E.; Ghannoum, M.A.; Mayser, P.; Gupta, A.K.; Korting, H.C.; Shouey, R.J.; Baker, D.R.; Rich, P.A.; Ling, M.; Hugot, S.; et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild to moderate toenail onychomycosis: Results from three randomized studies using double-blind vehicle controlled and open-label active controlled designs. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Dominicus, R.; Weidner, C.; Tate, H.; Kroon, H.A. Open-label study of the efficacy and safety of topical treatment with TDT 067 (terbinafine in Transfersome®) in patients with onychomycosis. Br. J. Dermatol. 2012, 166, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Gianni, C. Update on the management of onychomycosis. G. Ital. Dermatol. Venereol. 2013, 148, 633–638. [Google Scholar] [PubMed]
- Jones, T.; Tavakkol, A. Safety and Tolerability of Luliconazole Solution 10-Percent in Patients with Moderate to Severe Distal Subungual Onychomycosis. Antimicrob. Agents Chemother. 2013, 57, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Scher, R.K.; Nakamura, N.; Tavakkol, A. Luliconazole: A review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses 2014, 57, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.E.; Avram, M.M.; Wanner, M.A. A review of lasers and light for the treatment of onychomycosis. Lasers Surg. Med. 2014, 46, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ledon, J.A.; Savas, J.; Franca, K.; Chacon, A.; Nouri, K. Laser and light therapy for onychomycosis: A systematic review. Lasers Med. Sci. 2014, 29, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Souza, L.W.; Souza, S.V.; Botelho, A.C. Randomized controlled trial comparing photodynamic therapy based on methylene blue dye and fluconazole for toenail onychomycosis. Dermatol. Ther. 2014, 27, 43–47. [Google Scholar]
- Simmons, B.J.; Griffith, R.D.; Falto-Aizpurua, L.A.; Nouri, K. An update on photodynamic therapies in the treatment of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2015. [Google Scholar] [CrossRef]
- Zhang, R.N.; Wang, D.K.; Zhuo, F.L.; Duan, X.H.; Zhang, X.Y.; Zhao, J.Y. Long-pulse Nd:YAG 1064-nm laser treatment for onychomycosis. Chin. Med. J. (Engl.) 2012, 125, 3288–3291. [Google Scholar]
- Li, Y.; Yu, S.; Xu, J.; Zhang, R.; Zhao, J. Comparison of the Efficacy of Long-Pulsed Nd:YAG Laser Intervention for Treatment of Onychomycosis of Toenails or Fingernails. J. Drugs Dermatol. 2014, 13, 1258–1263. [Google Scholar] [PubMed]
- Landsman, A.S.; Robbins, A.H.; Angelini, P.F.; Wu, C.C.; Cook, J.; Oster, M.; Bornstein, E.S. Treatment of mild, moderate, and severe onychomycosis using 870- and 930-nm light exposure. J. Am. Podiatr. Med. Assoc. 2010, 100, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Renner, R.; Grüßer, K.; Sticherling, M. 1,064-nm Diode Laser Therapy of Onychomycosis: Results of a Prospective Open Treatment of 82 Toenails. Dermatology 2015, 230, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bristow, I.R. The effectiveness of lasers in the treatment of onychomycosis: A systematic review. J. Foot Ankle Res. 2014, 7. [Google Scholar] [CrossRef]
- De Sá, D.C.; Lamas, A.P.; Tosti, A. Oral therapy for onychomycosis: An evidence-based review. Am. J. Clin. Dermatol. 2014, 15, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Paquet, M.; Simpson, F.; Tavakkol, A. Terbinafine in the treatment of dermatophyte toenail onychomycosis: A meta-analysis of efficacy for continuous and intermittent regimens. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D.; Lynde, C.W. Pulse itraconazole vs. continuous terbinafine for the treatment of dermatophyte toenail onychomycosis in patients with diabetes mellitus. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Drummond-Main, C.; Paquet, M. Evidence-based optimal fluconazole dosing regimen for onychomycosis treatment. J. Dermatol. Treat. 2013, 24, 75–80. [Google Scholar] [CrossRef]
- Elewski, B.; Pollak, R.; Ashton, S.; Rich, P.; Schlessinger, J.; Tavakkol, A. A randomized, placebo and active controlled, parallel group, multicentre, investigator blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br. J. Dermatol. 2012, 166, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, B.; van Rossem, K.; Malahias, S.; Raterink, K. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J. Am. Acad. Dermatol. 2013, 69, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Lecha, M.; Effendy, I.; Feuilhade de Chauvin, M.; Di Chiacchio, N.; Baran, R. Treatment options—development of consensus guidelines. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 25–33. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Lcensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piraccini, B.M.; Alessandrini, A. Onychomycosis: A Review. J. Fungi 2015, 1, 30-43. https://doi.org/10.3390/jof1010030
Piraccini BM, Alessandrini A. Onychomycosis: A Review. Journal of Fungi. 2015; 1(1):30-43. https://doi.org/10.3390/jof1010030
Chicago/Turabian StylePiraccini, Bianca Maria, and Aurora Alessandrini. 2015. "Onychomycosis: A Review" Journal of Fungi 1, no. 1: 30-43. https://doi.org/10.3390/jof1010030





