Of Tracts, Rings, Nodes, Cusps, Sinuses, and Arrhythmias—A Comment on Szili-Torok et al.’s Paper Entitled “The ‘Dead-End Tract’ and Its Role in Arrhythmogenesis”. J. Cardiovasc. Dev. Dis. 2016, 3, 11
1. Introduction
2. How Do We Define the “Conduction System”?
3. What, then, Is the “Dead-End Tract”?
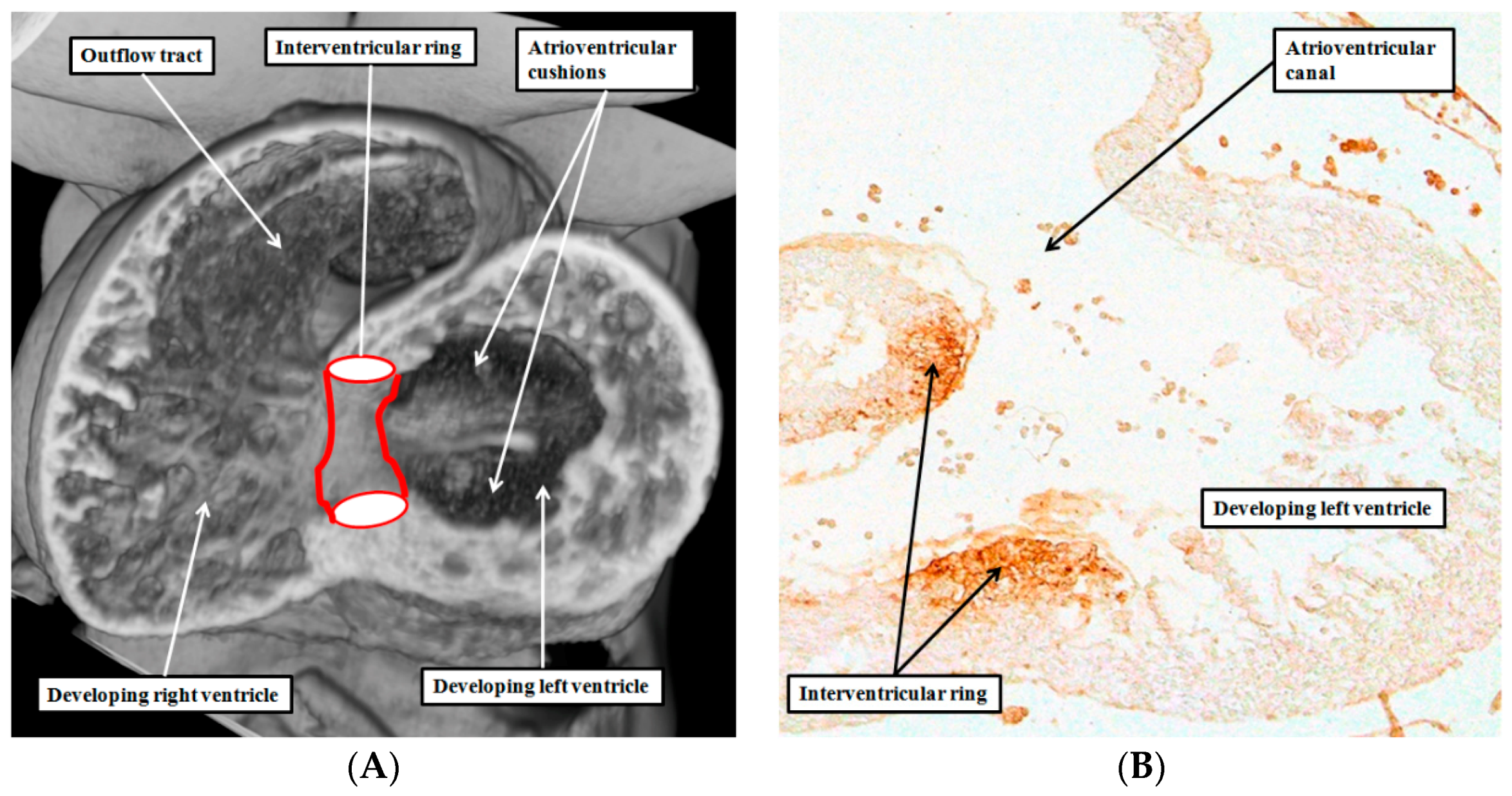
4. Are There Other Rings of “Conducting Tissues”?
5. Is There a Difference between “Tracts” and “Nodes”?
6. Are There Differences between the Developmental Structures and Their Postnatal Remnants?
7. Are the Remnants the Substrates for Idiopathic Ventricular Tachycardias?
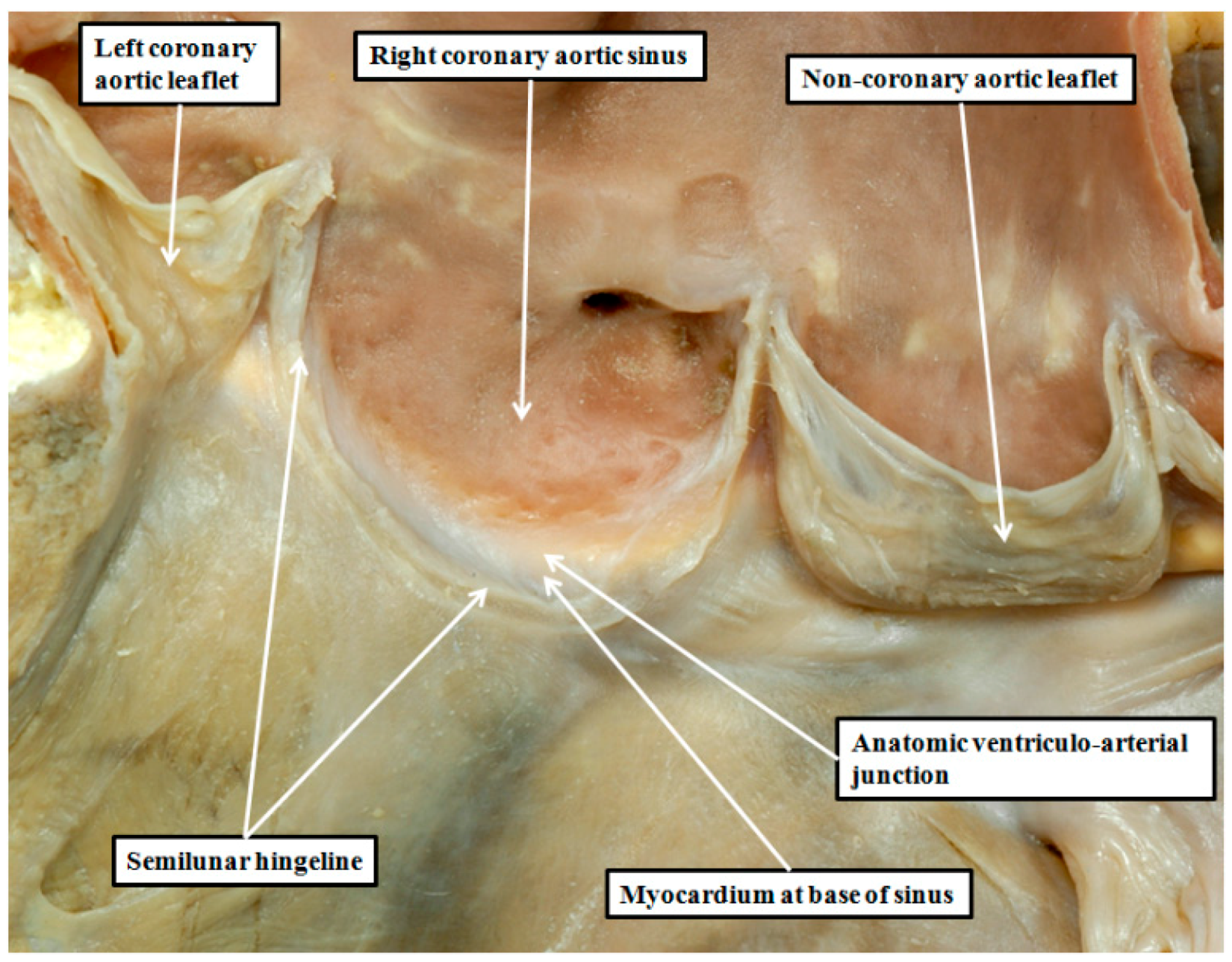
8. Are There Differences in the Anatomy of the Arterial Roots?
9. Can We Demonstrate the Specific Anatomy during Life?
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Vries, L.; Hendriks, A.; Szili-Torok, T. The “Dead-End Tract” and Its Role in Arrhythmogenesis. J. Cardiovasc. Dev. Dis. 2016, 3, 11. [Google Scholar] [CrossRef]
- Tawara, S. The Conduction System of the Mammalian Heart. An Anatomico-Histological Study of the Atrioventricular Bundle and the Purkinje Fibers; Imperial Collage Press: London, UK, 2000. [Google Scholar]
- Aschoff, L. Referat uber die Herzstorungen in ihren Beziehungen zu den Spezifischen Muskelsystem des Herzens. Verh. Dtsch. Ges. Pathol. 1910, 14, 3–35. [Google Scholar]
- Mönckeberg, J.G. Beitrage zur normalen und pathologischen Anatomie des Herzens. Verh. Dtsch. Ges. Pathol. 1910, 14, 64–71. [Google Scholar]
- Thorel, C. Vorlaufige mitteilung uber eine besondere muskelverbindung zwischen der cava superior und den Hischen bundel. Munch. Med. Wochenschr. 1909, 36, 2159–2164. [Google Scholar]
- Anderson, R.H.; Christoffels, V.M.; Moorman, A.F.M. Controversies concerning the anatomical definition of the conduction tissues. Anat. Rec. 2004, 280, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Vermeulen, J.L.; Verbeek, F.J.; Virágh, S.; Kálmán, F.; Lamers, W.H.; Moorman, A.F.M. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle. III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat. Rec. 1992, 232, 97–111. [Google Scholar] [PubMed]
- Lamers, W.H.; Wessels, A.; Verbeek, F.J.; Moorman, A.F.; Virágh, S.; Wenink, A.C.; Gittenberger-de Groot, A.C.; Anderson, R.H. New findings concerning ventricular septation in the human heart. Implication for maldevelopment. Circulation 1992, 86, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Davies, F. The conducting system of the bird’s heart. J. Anat. 1930, 64, 129. [Google Scholar] [PubMed]
- Vassall-Adams, P.R. The development of the atrioventricular bundle and its branches in the avian heart. J. Anat. 1982, 134, 169–183. [Google Scholar] [PubMed]
- Anderson, R.H. The disposition, morphology and innervation of cardiac specialized tissues in the guinea pig. J. Anat. 1972, 111, 453–468. [Google Scholar] [PubMed]
- Anderson, R.H. The disposition and innervation of atrioventricular ring specialized tissue in rats and rabbits. J. Anat. 1972, 113, 197–211. [Google Scholar] [PubMed]
- Anderson, R.H.; Taylor, I.M. Development of atrioventricular specialized tissue in human heart. Br. Heart J. 1972, 34, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Yanni, J.; Boyett, M.R.; Anderson, R.H.; Dobrzynski, H. The extent of the specialized atrioventricular ring tissues. Heart Rhythm. 2009, 6, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Davies, M.J.; Becker, A.E. Atrioventricular ring specialized tissue in the normal heart. Eur. J. Cardiol. 1974, 2, 219–230. [Google Scholar]
- Anderson, R.H.; Ho, S.Y.; Gillette, P.C.; Becker, A.E. Mahaim, Kent and abnormal atrioventricular conduction. Cardiovasc. Res. 1996, 31, 480–491. [Google Scholar] [CrossRef]
- Kent, A.F.S. The structure of the cardiac tissues at the auricular-ventricular junction. J. Physiol. 1913, 47, xvii–xviii. [Google Scholar]
- Kanei, Y.; Friedman, M.; Ogawa, N.; Hanon, S.; Lam, P.; Schweitzer, P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann. Noninvasive Electrocardiol. 2008, 13, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Boyett, M.R.; Dobrzynski, H.; Moorman, A.F. The anatomy of the conduction system: Implications for the clinical cardiologist. J. Cardiovasc. Transl. Res. 2013, 6, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Merrick, A.F.; Yacoub, M.H.; Ho, S.Y.; Anderson, R.H. Anatomy of the muscular subpulmonary infundibulum with regard to the Ross procedure. Ann. Thorac. Surg. 2000, 69, 556–561. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, R.H.; Spicer, D.E.; Mori, S. Of Tracts, Rings, Nodes, Cusps, Sinuses, and Arrhythmias—A Comment on Szili-Torok et al.’s Paper Entitled “The ‘Dead-End Tract’ and Its Role in Arrhythmogenesis”. J. Cardiovasc. Dev. Dis. 2016, 3, 11. J. Cardiovasc. Dev. Dis. 2016, 3, 17. https://doi.org/10.3390/jcdd3020017
Anderson RH, Spicer DE, Mori S. Of Tracts, Rings, Nodes, Cusps, Sinuses, and Arrhythmias—A Comment on Szili-Torok et al.’s Paper Entitled “The ‘Dead-End Tract’ and Its Role in Arrhythmogenesis”. J. Cardiovasc. Dev. Dis. 2016, 3, 11. Journal of Cardiovascular Development and Disease. 2016; 3(2):17. https://doi.org/10.3390/jcdd3020017
Chicago/Turabian StyleAnderson, Robert H., Diane E. Spicer, and Shumpei Mori. 2016. "Of Tracts, Rings, Nodes, Cusps, Sinuses, and Arrhythmias—A Comment on Szili-Torok et al.’s Paper Entitled “The ‘Dead-End Tract’ and Its Role in Arrhythmogenesis”. J. Cardiovasc. Dev. Dis. 2016, 3, 11" Journal of Cardiovascular Development and Disease 3, no. 2: 17. https://doi.org/10.3390/jcdd3020017





