On the Morphology of the Drosophila Heart
Abstract
:1. The Circulatory System of Flies
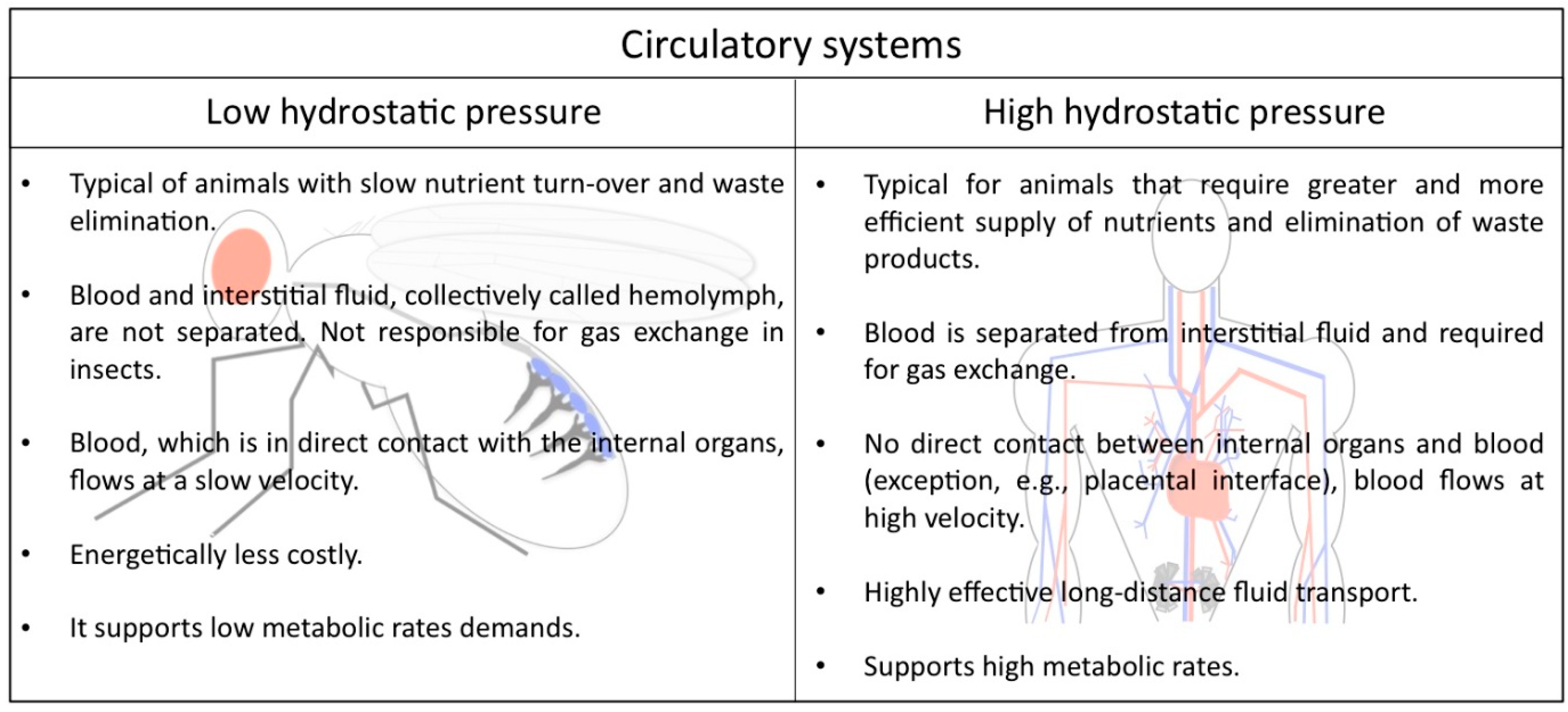
2. Low and High Hydrostatic Pressure Circulatory Systems: The Main Difference between Insects and Mammals
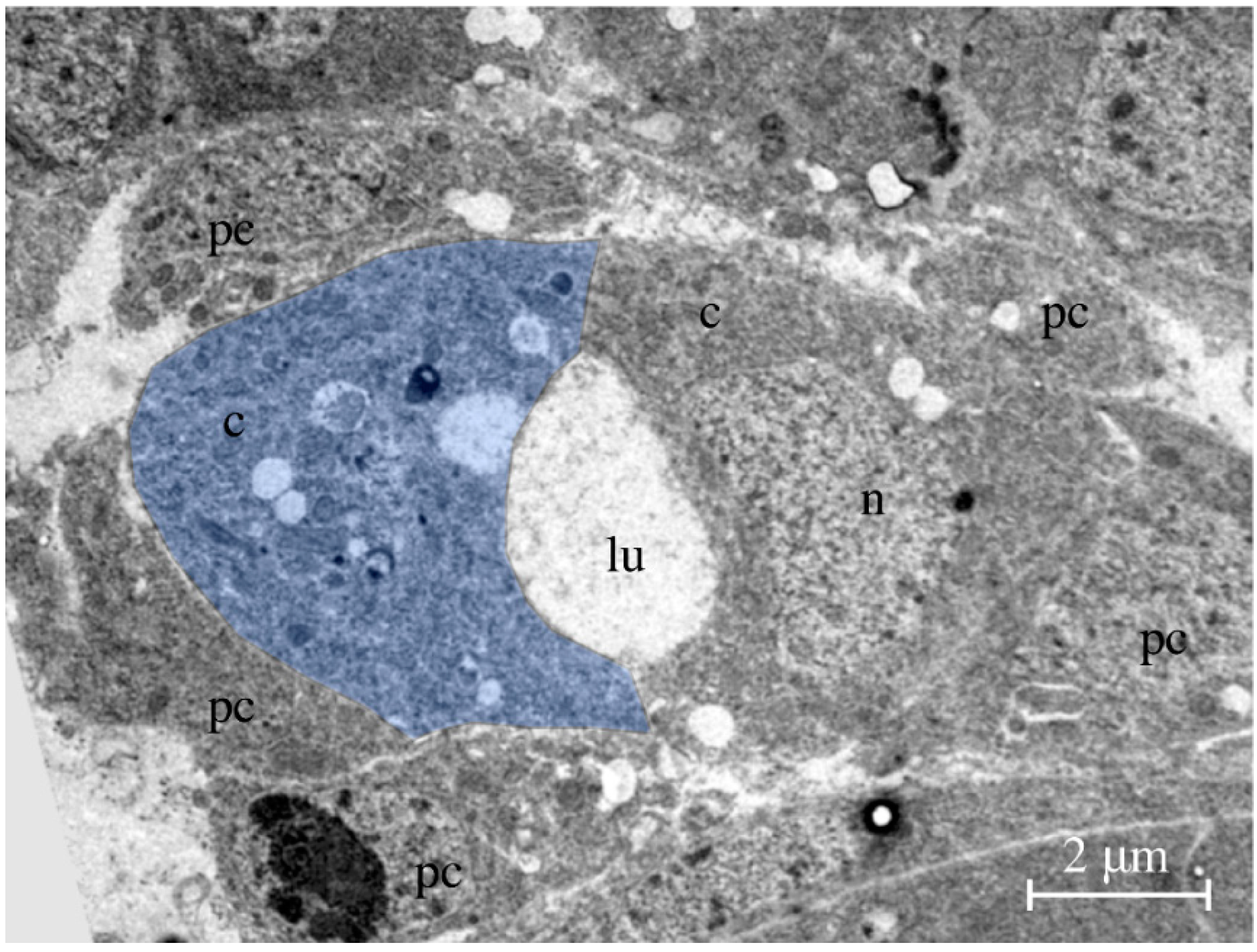
3. Histology of the Fly Heart
4. Cell Types that Constitute the Fly Heart
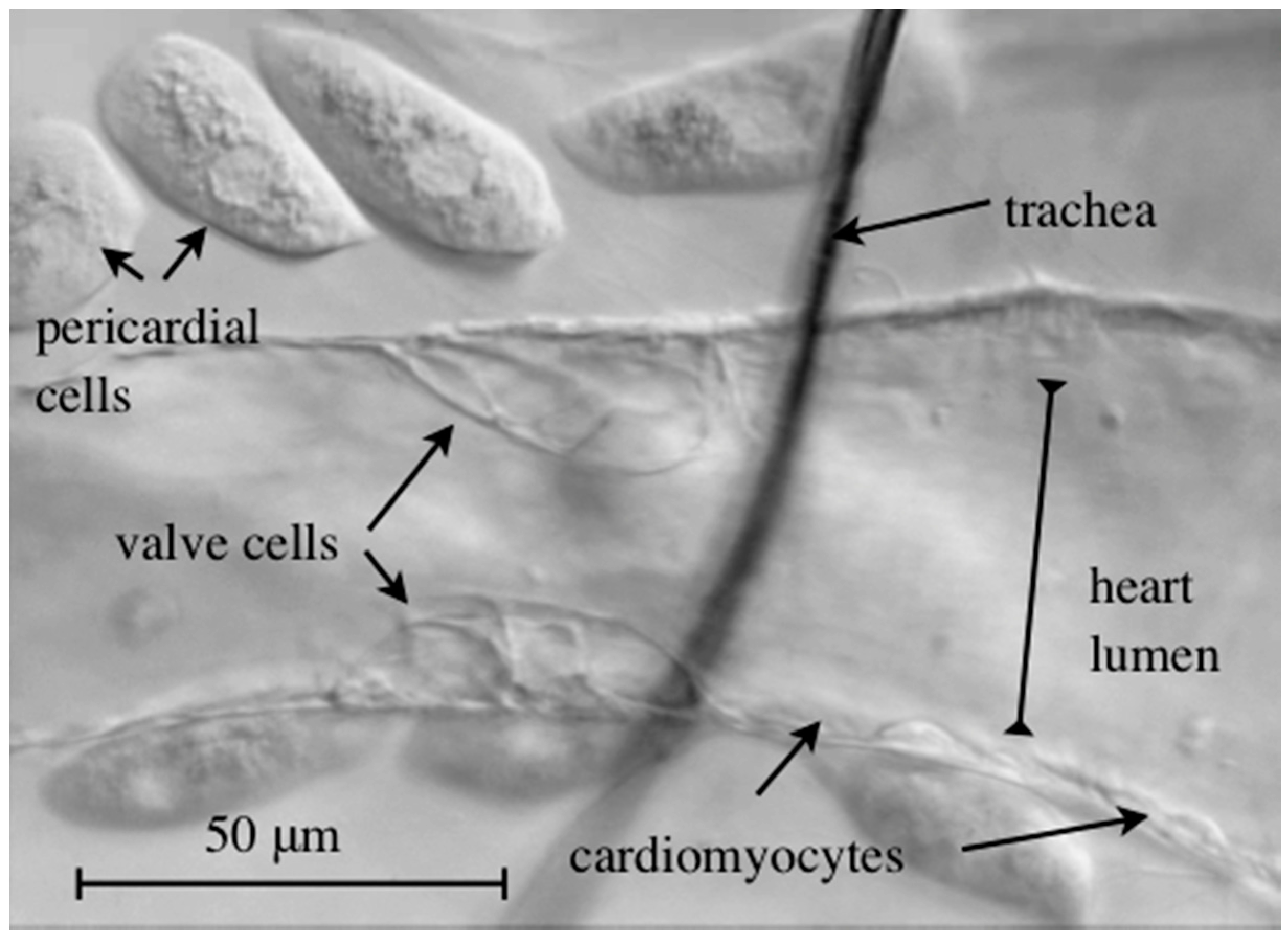
4.1. Cardiomyocytes
4.2. Ostial Cells
4.3. Intracardiac Valves
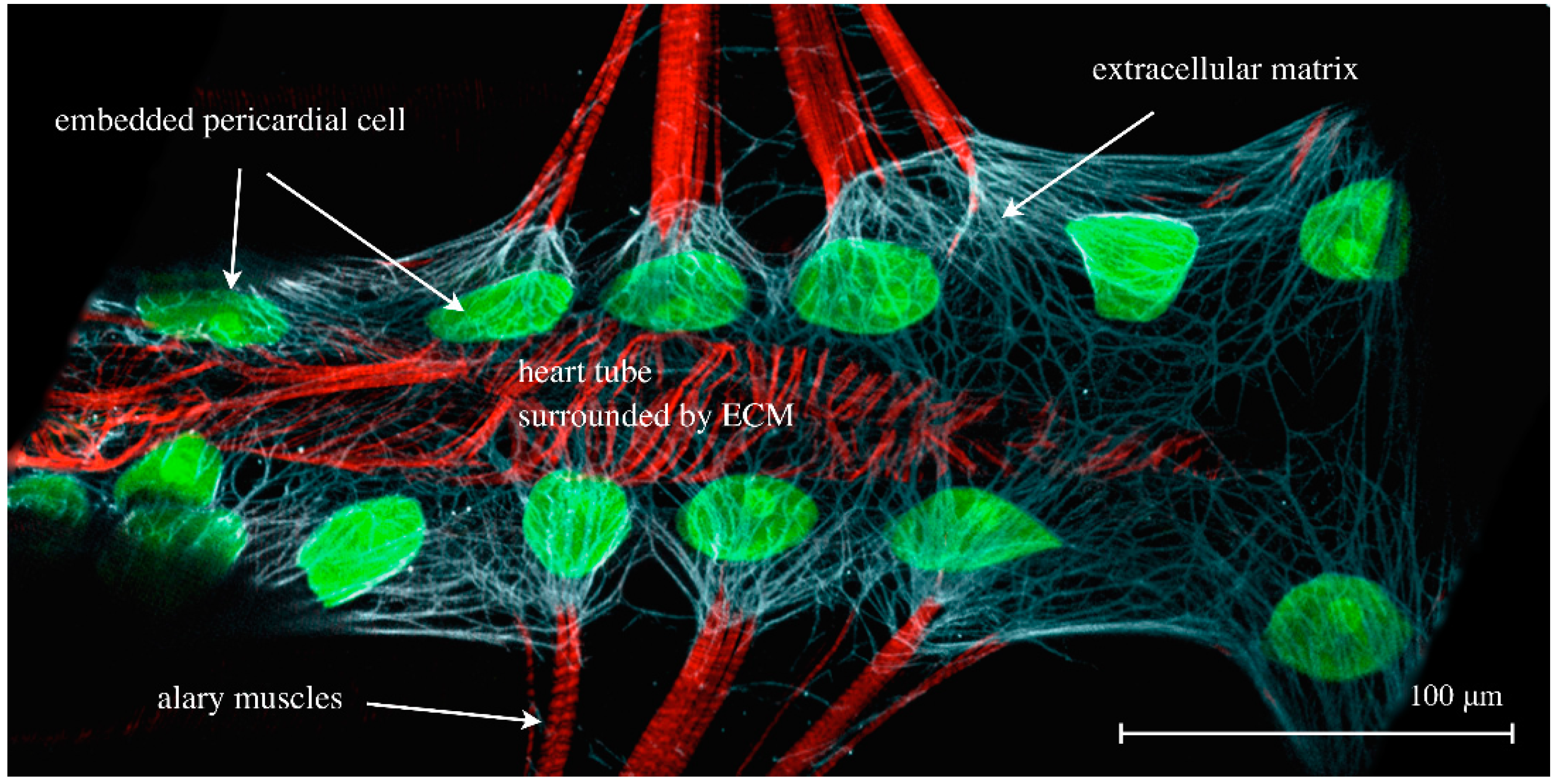
4.4. Alary Muscles
4.5. Pericardial Cells
4.6. Ventral Longitudinal Muscles
4.7. Connective Tissue—ECM
5. Accessory Hearts
6. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, A. The internal anatomy and histology of the imago. In Biology of Drosophila; Demerec, M., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1950; pp. 420–534. [Google Scholar]
- Rizki, T.M. The circulatory system and associated cells and tissues. In The Genetics and Biology of Drosophila; Ashburner, M., Wright, T.R.F., Eds.; Academic Press: New York, NY, USA, 1978; Volume 2b, pp. 397–452. [Google Scholar]
- Bunt, S.; Hooley, C.; Hu, N.; Scahill, C.; Weavers, H.; Skaer, H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell 2010, 19, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.; Jacinto, A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat. Rev. Mol. Cell Biol. 2007, 8, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Zaffran, S.; Astier, M.; Gratecos, D.; Guillen, A.; Sémériva, M. Cellular interactions during heart morphogenesis in the Drosophila embryo. Biol. Cell 1995, 84, 13–24. [Google Scholar] [CrossRef]
- Sellin, J.; Albrecht, S.; Kölsch, V.; Paululat, A. Dynamics of heart differentiation, visualized utilizing heart enhancer elements of the Drosophila melanogaster bHLH transcription factor Hand. Gene Expr. Patterns 2006, 6, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Aradhya, R.; Ashoka, D.; Inamdar, M. Post-embryonic pericardial cells of Drosophila are required for overcoming toxic stress but not for cardiac function or adult development. Cell Tissue Res. 2007, 331, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Lehmacher, C.; Abeln, B.; Paululat, A. The ultrastructure of Drosophila heart cells. Arthropod Struct. Dev. 2012, 41, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Sellin, J.; Drechsler, M.; Nguyen, H.T.; Paululat, A. Antagonistic function of Lmd and Zfh1 fine tunes cell fate decisions in the Twi and Tin positive mesoderm of Drosophila melanogaster. Dev. Biol. 2009, 326, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Monier, B.; Astier, M.; Sémériva, M.; Perrin, L. Steroid-dependent modification of Hox function drives myocyte reprogramming in the Drosophila heart. Development 2005, 132, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.R.; Cripps, R.M. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 2001, 109, 51–59. [Google Scholar] [CrossRef]
- Wasserthal, L.T. Drosophila flies combine periodic heartbeat reversal with a circulation in the anterior body mediated by a newly discovered anterior pair of ostial valves and venous channels. J. Exp. Biol. 2007, 210, 3707–3719. [Google Scholar] [CrossRef] [PubMed]
- Schaub, C.; März, J.; Reim, I.; Frasch, M. Org-1-dependent lineage reprogramming generates the ventral longitudinal musculature of the Drosophila heart. Curr. Biol. 2015, 25, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.P.; Nongthomba, U.; Kelly Tanaka, K.K.; Denton, M.L.; Meadows, S.M.; Bancroft, N.; Molina, M.R.; Cripps, R.M. Cardiac remodeling in Drosophila arises from changes in actin gene expression and from a contribution of lymph gland-like cells to the heart musculature. Mech. Dev. 2011, 128, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Levine, R.B. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J. Comp. Neurol. 2003, 465, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Levine, R.B. Glutamatergic innervation of the heart initiates retrograde contractions in adult Drosophila melanogaster. J. Neurosci. 2005, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Morin, X.; Daneman, R.; Zavortink, M.; Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 15050–15055. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Frasch, M. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 2005, 132, 4911–4925. [Google Scholar] [CrossRef] [PubMed]
- Jagla, K.; Frasch, M.; Jagla, T.; Dretzen, G.; Bellard, F.; Bellard, M. ladybird, a new component of the cardiogenic pathway in Drosophila required for diversification of heart precursors. Development 1997, 124, 3471–3479. [Google Scholar] [PubMed]
- Zaffran, S.; Reim, I.; Qian, L.; Lo, P.C.; Bodmer, R.; Frasch, M. Cardioblast-intrinsic Tinman activity controls proper diversification and differentiation of myocardial cells in Drosophila. Development 2006, 133, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Wang, S.; Holz, A.; Bergter, A.; Paululat, A. The ADAM metalloprotease Kuzbanian is crucial for proper heart formation in Drosophila melanogaster. Mech. Dev. 2006, 123, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, A.L.; Cripps, R.M. Cardiac gene regulatory networks in Drosophila. Biochim. Biophys. Acta 2009, 1789, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.N.; Burnett, L.A.; Sellin, J.; Paululat, A.; Newfeld, S.J. Defective Dpp signaling results in heart overgrowth and reduced cardiac output in Drosophila. Genetics 2007, 176, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Monier, B.; Ponzielli, R.; Astier, M.; Sémériva, M. Drosophila cardiac tube organogenesis requires multiple phases of Hox activity. Dev. Biol. 2004, 272, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.M.; Hendren, J.D.; Helander, L.A.; Cripps, R.M. The NK homeodomain transcription factor Tinman is a direct activator of seven-up in the Drosophila dorsal vessel. Dev. Biol. 2006, 302, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Tauc, H.M.; Mann, T.; Werner, K.; Pandur, P. A role for Drosophila Wnt-4 in heart development. Genesis 2012, 50, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Tixier, V.; Bataille, L.; Jagla, K. Diversification of muscle types: Recent insights from Drosophila. Exp. Cell Res. 2010, 316, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.H.; Skeath, J.B.; Gajewski, K.; Schulz, R.A.; Frasch, M. Homeotic genes autonomously specify the anteroposterior subdivision of the Drosophila dorsal vessel into aorta and heart. Dev. Biol. 2002, 251, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Lovato, T.L.; Nguyen, T.P.; Molina, M.R.; Cripps, R.M. The Hox gene abdominal-A specifies heart cell fate in the Drosophila dorsal vessel. Development 2002, 129, 5019–5027. [Google Scholar] [PubMed]
- Ryan, K.M.; Hoshizaki, D.K.; Cripps, R.M. Homeotic selector genes control the patterning of seven-up expressing cells in the Drosophila dorsal vessel. Mech. Dev. 2005, 122, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Sénatore, S.; Salmand, P.A.; Lalevée, N.; Perrin, L.; Sémériva, M. The fabulous destiny of the Drosophila heart. Curr. Opin. Gene.t Dev. 2009, 19, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Moyer, K.; Yacoub, N.; Soldaat, C.; Komosa, M.; Vassilieva, K.; Wilk, R.; Hu, J.; Vasquez Pas, L.D.; Syed, Q.; et al. Syndecan contributes to heart cell specification and lumen formation during Drosophila cardiogenesis. Dev. Biol. 2011, 356, 279–290. [Google Scholar] [CrossRef] [PubMed]
- MacMullin, A.; Jacobs, J.R. Slit coordinates cardiac morphogenesis in Drosophila. Dev. Biol. 2006, 293, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Martínez, E.; Soplop, N.H.; Patel, R.; Kramer, S.G. Repulsion by Slit and Roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during Drosophila heart tube lumen formation. J. Cell Biol. 2008, 182, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Martinez, E.; Soplop, N.H.; Kramer, S.G. Lateral positioning at the dorsal midline: Slit and Roundabout receptors guide Drosophila heart cell migration. Proc. Natl. Acad. Sci. USA 2006, 103, 12441–12446. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, N.; Ordan, E.; Ocorr, K.; Bodmer, R.; Volk, T. Multiplexin promotes heart but not aorta morphogenesis by polarized enhancement of Slit/Robo activity at the heart lumen. PLoS Genet. 2013, 9, e1003597. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Altenhein, B.; Paululat, A. The transmembrane receptor Uncoordinated5 (Unc5) is essential for heart lumen formation in Drosophila melanogaster. Dev. Biol. 2011, 350, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, J.; Jacobs, J.R. Talin is required to position and expand the luminal domain of the Drosophila heart tube. Dev. Biol. 2015, 405, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, J.; Vazquez Paz, L.D.; Macmullin, A.; Jacobs, J.R. Integrins are required for cardioblast polarisation in Drosophila. BMC Dev. Biol. 2012, 12, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, A.L.; Cripps, R.M. Purification of cardiac cells from Drosophila embryos. Methods 2012, 56, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hallier, B.; Hoffmann, J.; Roeder, T.; Tögel, M.; Meyer, H.; Paululat, A. The bHLH transcription factor Hand regulates the expression of genes critical to heart and muscle function in Drosophila melanogaster. PLoS ONE 2015, 10, e0134204. [Google Scholar] [CrossRef] [PubMed]
- Salmand, P.A.; Iché-Torres, M.; Perrin, L. Tissue-specific cell sorting from Drosophila embryos: Application to gene expression analysis. Fly (Austin) 2011, 5, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Zeitouni, B.; Sénatore, S.; Severac, D.; Aknin, C.; Sémériva, M.; Perrin, L. Signalling pathways involved in adult heart formation revealed by gene expression profiling in Drosophila. PLoS Genet. 2007, 3, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Cammarato, A.; Ahrens, C.H.; Alayari, N.N.; Qeli, E.; Rucker, J.; Reedy, M.C.; Zmasek, C.M.; Gucek, M.; Cole, R.N.; Van Eyk, J.E.; et al. A mighty small heart: The cardiac proteome of adult Drosophila melanogaster. PLoS ONE 2011, 6, e18497. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.J.; Ringo, J.M.; Dowse, H.B. Morphology of the pupal heart, adult heart, and associated tissues in the fruit fly, Drosophila melanogaster. J. Morphol. 1999, 240, 225–235. [Google Scholar] [CrossRef]
- Iklé, J.; Elwell, J.A.; Bryantsev, A.L.; Cripps, R.M. Cardiac expression of the Drosophila Transglutaminase (CG7356) gene is directly controlled by myocyte enhancer factor-2. Dev. Dyn. 2008, 237, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Levine, R.B.; Ewer, J. Role of the neuropeptide CCAP in Drosophila cardiac function. J. Neurobiol. 2005, 64, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Wasserthal, L.T. Heartbeat reversal and its coordination with accessory pulsatile organs and abdominal movements in Lepidoptera. Experientia 1976, 32, 577–579. [Google Scholar] [CrossRef]
- Wasserthal, L.T. Periodic heartbeat reversals cause cardiogenic inspiration and expiration with coupled spiracle leakage in resting blowflies, Calliphora vicina. J. Exp. Biol. 2014, 217, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Bischoff, J. Heart valve development: Endothelial cell signaling and differentiation. Circ. Res. 2004, 95, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yuan, W.; Bodmer, R.; Wu, X.; Ocorr, K. The role of Pygopus in the differentiation of intracardiac valves in Drosophila. Genesis 2014, 52, 19–28. [Google Scholar] [CrossRef] [PubMed]
- LaBeau, E.M.; Trujillo, D.L.; Cripps, R.M. Bithorax Complex genes control alary muscle patterning along the cardiac tube of Drosophila. Mech. Dev. 2009, 126, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Bataillé, L.; Frendo, J.L.; Vincent, A. Hox control of Drosophila larval anatomy; The alary and thoracic alary-related muscles. Mech. Dev. 2015, 138, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Schaub, C.; Nagaso, H.; Jin, H.; Frasch, M. Org-1, the Drosophila ortholog of Tbx1, is a direct activator of known identity genes during muscle specification. Development 2012, 139, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Boukhatmi, H.; Frendo, J.L.; Enriquez, J.; Crozatier, M.; Dubois, L.; Vincent, A. Tup/Islet1 integrates time and position to specify muscle identity in Drosophila. Development 2012, 139, 3572–3582. [Google Scholar] [CrossRef] [PubMed]
- Weavers, H.; Prieto-Sanchez, S.; Grawe, F.; Garcia-Lopez, A.; Artero, R.; Wilsch-Brauninger, M.; Ruiz-Gomez, M.; Skaer, H.; Denholm, B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 2008, 457, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.R.; Drechsler, M.; Catterson, J.H.; Bodmer, R.; Ocorr, K.; Paululat, A.; Hartley, P.S. Klf15 is critical for the development and differentiation of Drosophila nephrocytes. PLoS ONE 2015, 10, e0134620. [Google Scholar] [CrossRef] [PubMed]
- Tutor, A.S.; Prieto-Sanchez, S.; Ruiz-Gomez, M. Src64B phosphorylates Dumbfounded and regulates slit diaphragm dynamics: Drosophila as a model to study nephropathies. Development 2013, 141, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, Y.; Han, Z. An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J. Am. Soc. Nephrol. 2013, 24, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Shao, H.; Guo, F.; Trimble, R.; Pearce, E.; Abmayr, S.M. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 2009, 136, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.P.; King, R.C. The pericardial cells of Drosophila melanogaster. Q. J. Microscop. Sci. 1965, 106, 261–268. [Google Scholar]
- Lim, H.-Y.; Wang, W.; Chen, J.; Ocorr, K.; Bodmer, R. ROS Regulate Cardiac Function via a Distinct Paracrine Mechanism. Cell Rep. 2014, 7, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tögel, M.; Pass, G.; Paululat, A. The Drosophila wing hearts originate from pericardial cells and are essential for wing maturation. Dev. Biol. 2008, 318, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, M.; Schmidt, A.; Paululat, A. The ADAMTS-like protein Lonely heart mediates fibrillar Pericardin matrix formation essential for cardiac integrity in Drosophila melanogaster. PLoS Genet. 2013, 9, e1003616. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Panz, M.; Zmojdzian, M.; Jagla, K.; Paululat, A. Neprilysin 4, a novel endopeptidase from Drosophila melanogaster, displays distinct substrate specificities and exceptional solubility states. J. Exp. Biol. 2009, 212, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Wolfstetter, G.; Holz, A. The role of LamininB2 (LanB2) during mesoderm differentiation in Drosophila. Cell. Mol. Life Sci. 2012, 69, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Hollfelder, D.; Frasch, M.; Reim, I. Distinct functions of the laminin β LN domain and collagen IV during cardiac extracellular matrix formation and stabilization of alary muscle attachments revealed by EMS mutagenesis in Drosophila. BMC Dev. Biol. 2014, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Didangelos, A.; Yin, X.; Mandal, K.; Saje, A.; Smith, A.; Xu, Q.; Jahangiri, M.; Mayr, M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: A proteomics approach. Mol. Cell Proteomics 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Fuhrmann, A.; Cammarato, A.; Engler, A.J. In situ mechanical analysis of myofibrillar perturbation and aging on soft, bilayered Drosophila myocardium. Biophys. J. 2011, 101, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Spenlehauer, A.; Sessions, A.O.; Trujillo, A.S.; Fuhrmann, A.; Fu, Z.; Venkatraman, V.; Pohl, D.; Tuler, J.; Wang, M.; et al. Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Zambon, A.C.; Fuhrmann, A.; Bernstein, S.I.; Bodmer, R.; Engler, A.J.; Cammarato, A. Measuring passive myocardial stiffness in Drosophila melanogaster to investigate diastolic dysfunction. J. Cell. Mol. Med. 2012, 16, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.; Wang, S.; Rotstein, B.; Paululat, A. Matricellular proteins in development: Perspectives from the Drosophila heart. Matrix Biol. 2014, 37, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Pass, G.; Tögel, M.; Krenn, H.; Paululat, A. The circulatory organs of insect wings: Prime examples for the origin of evolutionary novelties. Zool. Anz. 2015, 256, 82–95. [Google Scholar] [CrossRef]
- Wirkner, C.S.; Tögel, M.; Pass, G. The Arthropod Circulatory System. In Arthopod Biology and Evolution: Molecules, Development, Morphology; Minelli, A., Boxshall, G., Fusco, G., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2013; pp. 343–391. [Google Scholar]
- Lehmacher, C.; Tögel, M.; Pass, G.; Paululat, A. The Drosophila wing hearts consist of syncytial muscle cells that resemble adult somatic muscles. Arthropod Struct. Dev. 2009, 38, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Tögel, M.; Pass, G.; Paululat, A. In vivo imaging of Drosophila wing heart development during pupal stages. Int. J. Dev. Biol. 2013, 57, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tögel, M.; Meyer, H.; Lehmacher, C.; Heinisch, J.J.; Pass, G.; Paululat, A. The bHLH transcription factor hand is required for proper wing heart formation in Drosophila. Dev. Biol. 2013, 381, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.R.; Gamliel, A.; Wessells, R.J.; Taghli-Lamallem, O.; Jepsen, K.; Ocorr, K.; Korenberg, J.R.; Peterson, K.L.; Rosenfeld, M.G.; Bodmer, R.; et al. Over-expression of DSCAM and COL6A2 cooperatively generates congenital heart defects. PLoS Genet. 2011, 7, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Kumsta, C.; Kaushik, G.; Diop, S.B.; Ding, Y.; Bisharat-Kernizan, J.; Catan, H.; Cammarato, A.; Ross, R.S.; Engler, A.J.; et al. A dual role for integrin-linked kinase and β1-integrin in modulating cardiac aging. Aging Cell 2014, 13, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Ocorr, K.; Bodmer, R.; Oldham, S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Diop, S.B.; Bodmer, R. Drosophila as a model to study the genetic mechanisms of obesity-associated heart dysfunction. J. Cell. Mol. Med. 2012, 16, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Neely, G.G.; Kuba, K.; Cammarato, A.; Isobe, K.; Amann, S.; Zhang, L.; Murata, M.; Elmen, L.; Gupta, V.; Arora, S.; et al. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell 2010, 141, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Sénatore, S.; Rami Reddy, V.; Sémériva, M.; Perrin, L.; Lalevée, N. Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet. 2010, 6, e1001088. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotstein, B.; Paululat, A. On the Morphology of the Drosophila Heart. J. Cardiovasc. Dev. Dis. 2016, 3, 15. https://doi.org/10.3390/jcdd3020015
Rotstein B, Paululat A. On the Morphology of the Drosophila Heart. Journal of Cardiovascular Development and Disease. 2016; 3(2):15. https://doi.org/10.3390/jcdd3020015
Chicago/Turabian StyleRotstein, Barbara, and Achim Paululat. 2016. "On the Morphology of the Drosophila Heart" Journal of Cardiovascular Development and Disease 3, no. 2: 15. https://doi.org/10.3390/jcdd3020015




