TLR-4 and CD14 Genotypes and Soluble CD14: Could They Predispose to Coronary Atherosclerosis?
Abstract
:1. Introduction
2. Methods
2.1. Patients—DNA Extraction
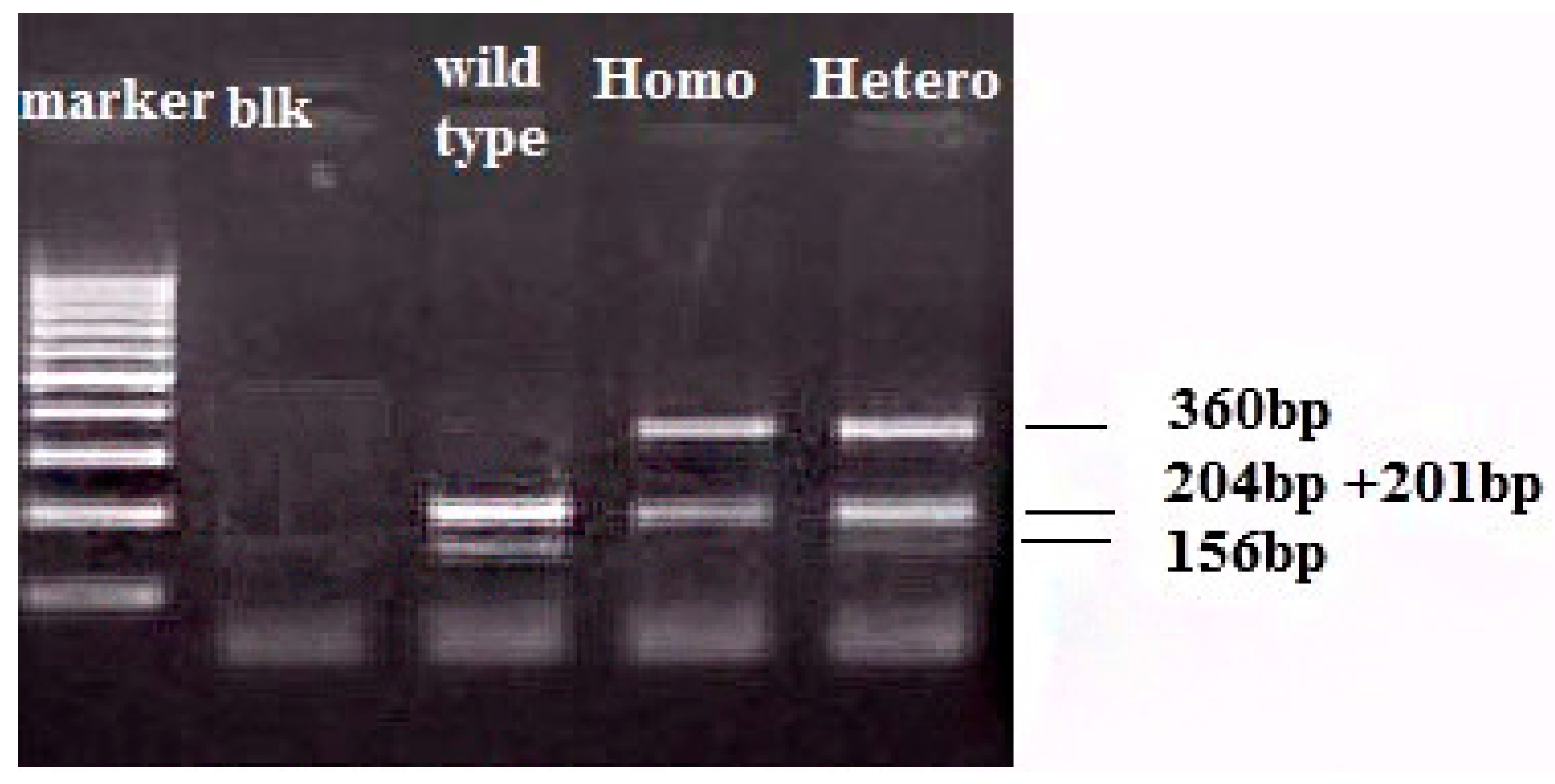
2.2. TLR-4 and CD14 Genotyping
2.3. ELISA for sCD14
2.4. Statistical Analysis
3. Results
3.1. TLR-4 Results
3.2. CD14—sCD14 Results
4. Discussion
4.1. TLR-4 Polymorphisms and CAD
4.2. CD14 Polymorphisms, sCD14 and CAD
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hawe, E.; Talmud, P.J.; Miller, G.J.; Humphries, S.E.; Second Northwick Park Heart Study. Family history is a coronary heart disease risk factor in the second northwick park heart study. Ann. Hum. Genet. 2003, 67, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rollins, J.; Paigen, B.; Wang, X. Genetic and genomic insights into the molecular basis of atherosclerosis. Cell Metab. 2007, 6, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the american heart association. Circulation 2012, 125, e2–e220. [Google Scholar] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.E.; Zhou, Y.F.; Zhu, J. Infection and atherosclerosis: Emerging mechanistic paradigms. Circulation 1999, 100, e20–e28. [Google Scholar] [CrossRef] [PubMed]
- Liao, W. Endotoxin: Possible roles in initiation and development of atherosclerosis. J. Lab. Clin. Med. 1996, 128, 452–460. [Google Scholar] [CrossRef]
- Dunne, A.; O’Neill, L.A. The interleukin-1 receptor/toll-like receptor superfamily: Signal transduction during inflammation and host defense. Sci. STKE 2003, 2003, re3. [Google Scholar] [CrossRef] [PubMed]
- Armant, M.A.; Fenton, M.J. Toll-like receptors: A family of pattern-recognition receptors in mammals. Genome Biol. 2002, 3, reviews3011.1–reviews3011.6. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Arbour, N.C.; Lorenz, E.; Schutte, B.C.; Zabner, J.; Kline, J.N.; Jones, M.; Frees, K.; Watt, J.L.; Schwartz, D.A. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 2000, 25, 187–191. [Google Scholar] [PubMed]
- Zanoni, I.; Granucci, F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell. Infect. Microbiol. 2013, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Gioannini, T.L.; Teghanemt, A.; Zhang, D.; Coussens, N.P.; Dockstader, W.; Ramaswamy, S.; Weiss, J.P. Isolation of an endotoxin-MD-2 complex that produces toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl. Acad. Sci. USA 2004, 101, 4186–4191. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 controls the LPS-induced endocytosis of toll-like receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Ostuni, R.; Barresi, S.; Di Gioia, M.; Broggi, A.; Costa, B.; Marzi, R.; Granucci, F. CD14 and NFAT mediate lipopolysaccharide-induced skin edema formation in mice. J. Clin. Investig. 2012, 122, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Haziot, A.; Chen, S.; Ferrero, E.; Low, M.G.; Silber, R.; Goyert, S.M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 1988, 141, 547–552. [Google Scholar] [PubMed]
- Goyert, S.M.; Ferrero, E.M.; Seremetis, S.V.; Winchester, R.J.; Silver, J.; Mattison, A.C. Biochemistry and expression of myelomonocytic antigens. J. Immunol. 1986, 137, 3909–3914. [Google Scholar] [PubMed]
- Antal-Szalmas, P. Evaluation of CD14 in host defence. Eur. J. Clin. Investig. 2000, 30, 167–179. [Google Scholar] [CrossRef]
- Labeta, M.O.; Durieux, J.J.; Fernandez, N.; Herrmann, R.; Ferrara, P. Release from a human monocyte-like cell line of two different soluble forms of the lipopolysaccharide receptor, CD14. Eur. J. Immunol. 1993, 23, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Kravchenko, V.; Kirkland, T.N.; Han, J.; Mackman, N.; Moriarty, A.; Leturcq, D.; Tobias, P.S.; Ulevitch, R.J. Glycosyl-phosphatidylinositol-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc. Natl. Acad. Sci. USA 1993, 90, 9930–9934. [Google Scholar] [CrossRef] [PubMed]
- Unkelbach, K.; Gardemann, A.; Kostrzewa, M.; Philipp, M.; Tillmanns, H.; Haberbosch, W. A new promoter polymorphism in the gene of lipopolysaccharide receptor CD14 is associated with expired myocardial infarction in patients with low atherosclerotic risk profile. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Hubacek, J.A.; Rothe, G.; Pit’ha, J.; Skodova, Z.; Stanek, V.; Poledne, R.; Schmitz, G. C(−260)→T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation 1999, 99, 3218–3220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.E.; Hetherington, C.J.; Tan, S.; Dziennis, S.E.; Gonzalez, D.A.; Chen, H.M.; Tenen, D.G. Sp1 is a critical factor for the monocytic specific expression of human CD14. J. Biol. Chem. 1994, 269, 11425–11434. [Google Scholar] [PubMed]
- LeVan, T.D.; Bloom, J.W.; Bailey, T.J.; Karp, C.L.; Halonen, M.; Martinez, F.D.; Vercelli, D. A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J. Immunol. 2001, 167, 5838–5844. [Google Scholar] [CrossRef] [PubMed]
- Vainas, T.; Stassen, F.R.; Bruggeman, C.A.; Welten, R.J.; van den Akker, L.H.; Kitslaar, P.J.; Pena, A.S.; Morre, S.A. Synergistic effect of Toll-like receptor 4 and CD14 polymorphisms on the total atherosclerosis burden in patients with peripheral arterial disease. J. Vasc. Surg. 2006, 44, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ameziane, N.; Beillat, T.; Verpillat, P.; Chollet-Martin, S.; Aumont, M.C.; Seknadji, P.; Lamotte, M.; Lebret, D.; Ollivier, V.; de Prost, D. Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler. Thromb. Vasc. Biol. 2003, 23, e61–e64. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Lorenz, E.; Reindl, M.; Wiedermann, C.J.; Oberhollenzer, F.; Bonora, E.; Willeit, J.; Schwartz, D.A. Toll-like receptor 4 polymorphisms and atherogenesis. N. Engl. J. Med. 2002, 347, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, E.; Frees, K.L.; Schwartz, D.A. Determination of the TLR4 genotype using allele-specific PCR. BioTechniques 2001, 31, 22–24. [Google Scholar] [PubMed]
- Gazouli, M.; Mantzaris, G.; Kotsinas, A.; Zacharatos, P.; Papalambros, E.; Archimandritis, A.; Ikonomopoulos, J.; Gorgoulis, V.G. Association between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World J. Gastroenterol. 2005, 11, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Sole, X.; Guino, E.; Valls, J.; Iniesta, R.; Moreno, V. Snpstats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Breslin, J.W.; Wu, M.H.; Guo, M.; Reynoso, R.; Yuan, S.Y. Toll-like receptor 4 contributes to microvascular inflammation and barrier dysfunction in thermal injury. Shock 2008, 29, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.J.; Niu, P.P.; Cong, J.Z.; Zhang, B.K.; Zhao, M. TLR4 signaling: A potential therapeutic target in ischemic coronary artery disease. Int. Immunopharmacol. 2014, 23, 54–59. [Google Scholar] [PubMed]
- Cieri, E.; Lenti, M.; De Rango, P.; Isernia, G.; Marucchini, A.; Cao, P. Functional ability in patients with critical limb ischaemia is unaffected by successful revascularisation. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 256–263. [Google Scholar] [PubMed]
- Edfeldt, K.; Swedenborg, J.; Hansson, G.K.; Yan, Z.Q. Expression of Toll-like receptors in human atherosclerotic lesions: A possible pathway for plaque activation. Circulation 2002, 105, 1158–1161. [Google Scholar] [PubMed]
- Yang, I.A.; Holloway, J.W.; Ye, S. TLR4 Asp299Gly polymorphism is not associated with coronary artery stenosis. Atherosclerosis 2003, 170, 187–190. [Google Scholar] [CrossRef]
- Edfeldt, K.; Bennet, A.M.; Eriksson, P.; Frostegard, J.; Wiman, B.; Hamsten, A.; Hansson, G.K.; De Faire, U.; Yan, Z.Q. Association of hypo-responsive toll-like receptor 4 variants with risk of myocardial infarction. Eur. Heart J. 2004, 25, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Ismailoglu, Z.; Batar, B.; Unal, S.; Onaran, I.; Karadag, B.; Ongen, Z. The effect of genetic polymorphisms of TLR2 and TLR4 in turkish patients with coronary artery disease. Gene 2015, 558, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Kolek, M.J.; Carlquist, J.F.; Muhlestein, J.B.; Whiting, B.M.; Horne, B.D.; Bair, T.L.; Anderson, J.L. Toll-like receptor 4 gene Asp299Gly polymorphism is associated with reductions in vascular inflammation, angiographic coronary artery disease, and clinical diabetes. Am. Heart J. 2004, 148, 1034–1040. [Google Scholar] [PubMed]
- Liu, F.; Lu, W.; Qian, Q.; Qi, W.; Hu, J.; Feng, B. Frequency of TLR 2, 4, and 9 gene polymorphisms in chinese population and their susceptibility to type 2 diabetes and coronary artery disease. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Reismann, P.; Lichy, C.; Rudofsky, G.; Humpert, P.M.; Genius, J.; Si, T.D.; Dorfer, C.; Grau, A.J.; Hamann, A.; Hacke, W.; et al. Lack of association between polymorphisms of the Toll-like receptor 4 gene and cerebral ischemia. J. Neurol. 2004, 251, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zalai, C.V.; Kolodziejczyk, M.D.; Pilarski, L.; Christov, A.; Nation, P.N.; Lundstrom-Hobman, M.; Tymchak, W.; Dzavik, V.; Humen, D.P.; Kostuk, W.J.; et al. Increased circulating monocyte activation in patients with unstable coronary syndromes. J. Am. College Cardiol 2001, 38, 1340–1347. [Google Scholar] [CrossRef]
- Lee, W.H.; Lee, Y.; Jeong, J.O.; Lee, S.Y.; Choi, Y.H.; Park, J.E. Activation of CD14 on circulating monocytes in patients with acute coronary syndrome. Int. J. Cardiol. 2001, 80, 135–142. [Google Scholar] [CrossRef]
- Shimada, K.; Watanabe, Y.; Mokuno, H.; Iwama, Y.; Daida, H.; Yamaguchi, H. Common polymorphism in the promoter of the CD14 monocyte receptor gene is associated with acute myocardial infarction in japanese men. Am. J. Cardiol. 2000, 86, 682–684. [Google Scholar] [CrossRef]
- Elghannam, H.; Tavackoli, S.; Ferlic, L.; Gotto, A.M., Jr.; Ballantyne, C.M.; Marian, A.J. A prospective study of genetic markers of susceptibility to infection and inflammation, and the severity, progression, and regression of coronary atherosclerosis and its response to therapy. J. Mol. Med. 2000, 78, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Risley, P.; Jerrard-Dunne, P.; Sitzer, M.; Buehler, A.; von Kegler, S.; Markus, H.S. Promoter polymorphism in the endotoxin receptor (CD14) is associated with increased carotid atherosclerosis only in smokers: The carotid atherosclerosis progression study (CAPS). Stroke 2003, 34, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Zee, R.Y.; Lindpaintner, K.; Struk, B.; Hennekens, C.H.; Ridker, P.M. A prospective evaluation of the CD14 C(-260)T gene polymorphism and the risk of myocardial infarction. Atherosclerosis 2001, 154, 699–702. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhong, B.L.; Zhu, W.L.; Xie, S.L.; Qiu, L.X.; Zhu, L.G.; Wang, Y.; Lei, L. CD14 C-260T gene polymorphism and ischemic heart disease susceptibility: A huge review and meta-analysis. Genet. Med. 2009, 11, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Khuseyinova, N.; Hoffmann, M.M.; Marz, W.; Frohlich, M.; Hoffmeister, A.; Brenner, H.; Rothenbacher, D. CD14 C(-260)→T polymorphism, plasma levels of the soluble endotoxin receptor CD14, their association with chronic infections and risk of stable coronary artery disease. J. Am. College Cardiol. 2002, 40, 34–42. [Google Scholar] [CrossRef]
- Amar, J.; Ruidavets, J.B.; Bal Dit Sollier, C.; Bongard, V.; Boccalon, H.; Chamontin, B.; Drouet, L.; Ferrieres, J. Soluble CD14 and aortic stiffness in a population-based study. J. Hypertens. 2003, 21, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Morange, P.E.; Tiret, L.; Saut, N.; Luc, G.; Arveiler, D.; Ferrieres, J.; Amouyel, P.; Evans, A.; Ducimetiere, P.; Cambien, F.; et al. TLR4/Asp299Gly, CD14/C-260T, plasma levels of the soluble receptor CD14 and the risk of coronary heart disease: The prime study. Eur. J. Hum. Genet. 2004, 12, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Ridker, P.M.; Libby, P.; Kwiatkowski, D.J. Atherosclerosis: The path from genomics to therapeutics. J. Am. College Cardiol. 2007, 49, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | N (%) |
|---|---|
| Mean Age in Years (SD) | 49.53 (6.9) |
| Age Group | |
| ≤50 years | 86 (43) |
| >50 years | 114 (57) |
| Gender | |
| Men | 163 (81.5) |
| Women | 37 (18.5) |
| Severity of atherosclerosis | |
| Moderate | 89 (44.5) |
| Severe | 111 (55.5) |
| Smoking | |
| Yes | 50 (25) |
| No | 150 (75) |
| Family History | |
| Yes | 101 (50.5) |
| No | 99 (49.5) |
| Dyslipidemia | |
| Yes | 127 (63.5) |
| No | 73 (36.5) |
| Hypertension | |
| Yes | 94 (47) |
| No | 106 (53) |
| Diabetes | |
| Yes | 28 (14) |
| No | 172 (86) |
| BMI | |
| ≤30 kg/m2 | 92 (46) |
| >30 kg/m2 | 108 (54) |
| Polymorphisms of TLR-4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Results | Frequency | Results | Frequency | ||||||
| ASP/ASP | ASP/GLY | GLY/GLY | ASP/ASP | ASP/GLY | GLY/GLY | A ALLELE | G ALLELE | A ALLELE | G ALLELE | |
| A | 100 | 0 | 0 | 100.0% | 0.0% | 0.0% | 200 | 0 | 100.00% | 0.00% |
| B | 97 | 3 | 0 | 97.0% | 3.0% | 0.0% | 197 | 3 | 98.50% | 1.50% |
| A+B | 197 | 3 | 0 | 98.5% | 1.5% | 0.0% | 397 | 3 | 99.25% | 0.75% |
| C | 95 | 4 | 1 | 95.0% | 4.0% | 1.0% | 194 | 6 | 97.00% | 3.00% |
| Polymorphisms of TLR-4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Results | Frequency | Results | Frequency | ||||||
| THR/THR | THR/ILE | ILE/ILE | THR/THR | THR/ILE | ILE/ILE | T ALLELE | I ALLELE | T ALLELE | I ALLELE | |
| A | 100 | 0 | 0 | 100.00% | 0.00% | 0.00% | 200 | 0 | 100.00% | 0.00% |
| B | 100 | 0 | 0 | 100.00% | 0.00% | 0.00% | 200 | 0 | 100.00% | 0.00% |
| A + B | 200 | 0 | 0 | 100.00% | 0.00% | 0.00% | 400 | 0 | 100.00% | 0.00% |
| C | 99 | 0 | 1 | 99.00% | 0.00% | 1.00% | 198 | 2 | 99.00% | 1.00% |
| Polymorphisms of CD14 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Results | Frequency | Results | Frequency | ||||||
| CC | CT | TT | CC | CT | TT | C ALLELE | T ALLELE | C ALLELE | T ALLELE | |
| A | 53 | 44 | 3 | 53.00% | 44.00% | 3.00% | 150 | 50 | 75.00% | 25.00% |
| B | 62 | 31 | 7 | 62.00% | 31.00% | 7.00% | 155 | 45 | 77.50% | 22.50% |
| A + B | 115 | 75 | 10 | 57.50% | 37.50% | 5.00% | 305 | 95 | 76.25% | 23.75% |
| C | 43 | 40 | 17 | 43.00% | 40.00% | 17.00% | 126 | 74 | 63.00% | 37.00% |
| Model | Genotype | Controls | Patients | OR (95% CI) | p-Value | AIC |
|---|---|---|---|---|---|---|
| Codominant | C/C | 43% | 57.9% | 1.00 | 0.0031 | 325.8 |
| C/T | 40% | 37.2% | 0.69 (0.40–1.20) | |||
| T/T | 17% | 4.8% | 0.21 (0.08–0.055) | |||
| Dominant | C/C | 43% | 57.9% | 1.00 | 0.021 | 330 |
| C/C-C/T | 57% | 42.1% | 0.55 (0.33–0.92) | |||
| Recessive | C/C-C/T | 83% | 95.2% | 1.00 | 0.0017 | 325.5 |
| T/T | 17% | 4.8% | 0.25 (0.10–0.62) | |||
| Overdominant | C/C-T/T | 60% | 62.8% | 1.00 | 0.66 | 335.1 |
| C/T | 40% | 37.2% | 0.89 (0.53–1.50) | |||
| Log-additive | - | - | - | 0.54 (0.36–0.80) | 0.0017 | 325.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinidou, M.K.; Goutas, N.; Vlachodimitropoulos, D.; Chaidaroglou, A.; Stefanou, D.; Poumpouridou, N.; Mastorakou, R.; Gazouli, M.; Kyparissopoulos, D.; Spiliopoulou, C. TLR-4 and CD14 Genotypes and Soluble CD14: Could They Predispose to Coronary Atherosclerosis? J. Cardiovasc. Dev. Dis. 2016, 3, 9. https://doi.org/10.3390/jcdd3010009
Konstantinidou MK, Goutas N, Vlachodimitropoulos D, Chaidaroglou A, Stefanou D, Poumpouridou N, Mastorakou R, Gazouli M, Kyparissopoulos D, Spiliopoulou C. TLR-4 and CD14 Genotypes and Soluble CD14: Could They Predispose to Coronary Atherosclerosis? Journal of Cardiovascular Development and Disease. 2016; 3(1):9. https://doi.org/10.3390/jcdd3010009
Chicago/Turabian StyleKonstantinidou, Maria Kalliopi, Nikos Goutas, Dimitrios Vlachodimitropoulos, Antigoni Chaidaroglou, Demetrios Stefanou, Nikoleta Poumpouridou, Renata Mastorakou, Maria Gazouli, Dimitrios Kyparissopoulos, and Chara Spiliopoulou. 2016. "TLR-4 and CD14 Genotypes and Soluble CD14: Could They Predispose to Coronary Atherosclerosis?" Journal of Cardiovascular Development and Disease 3, no. 1: 9. https://doi.org/10.3390/jcdd3010009






