3.1. Gross Anatomy of the Arterial Valves
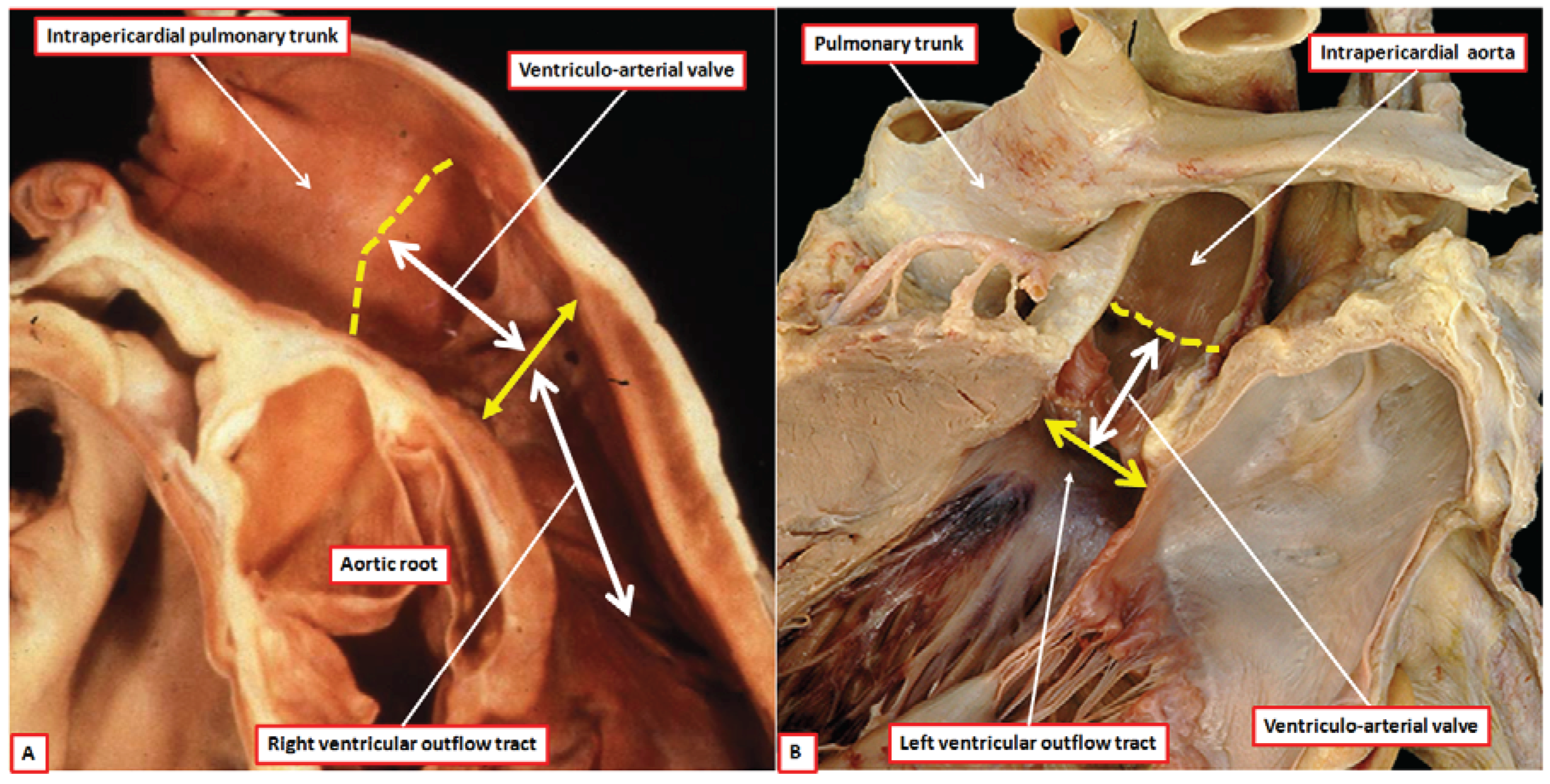
The aortic and pulmonary outflow tracts extend from the distal parts of their respective ventricles to the margins of the pericardial cavity. Within the outflow tracts as thus defined, as we have already discussed, it is possible to recognize three distinct anatomic components, namely the subvalvar ventricular outlets, the arterial valves within their sinuses, and the supravalvar intrapericardial arterial trunks (
Figure 1). At the margins of the pericardial cavity, the intrapericardial trunks become continuous with the extrapericardial ascending aorta and the pulmonary trunk, the latter channel dividing at the margins of the pericardium into the right and left pulmonary arteries. The arterial valves, therefore, are positioned within the intermediate, or middle, zones of the outflow channels [
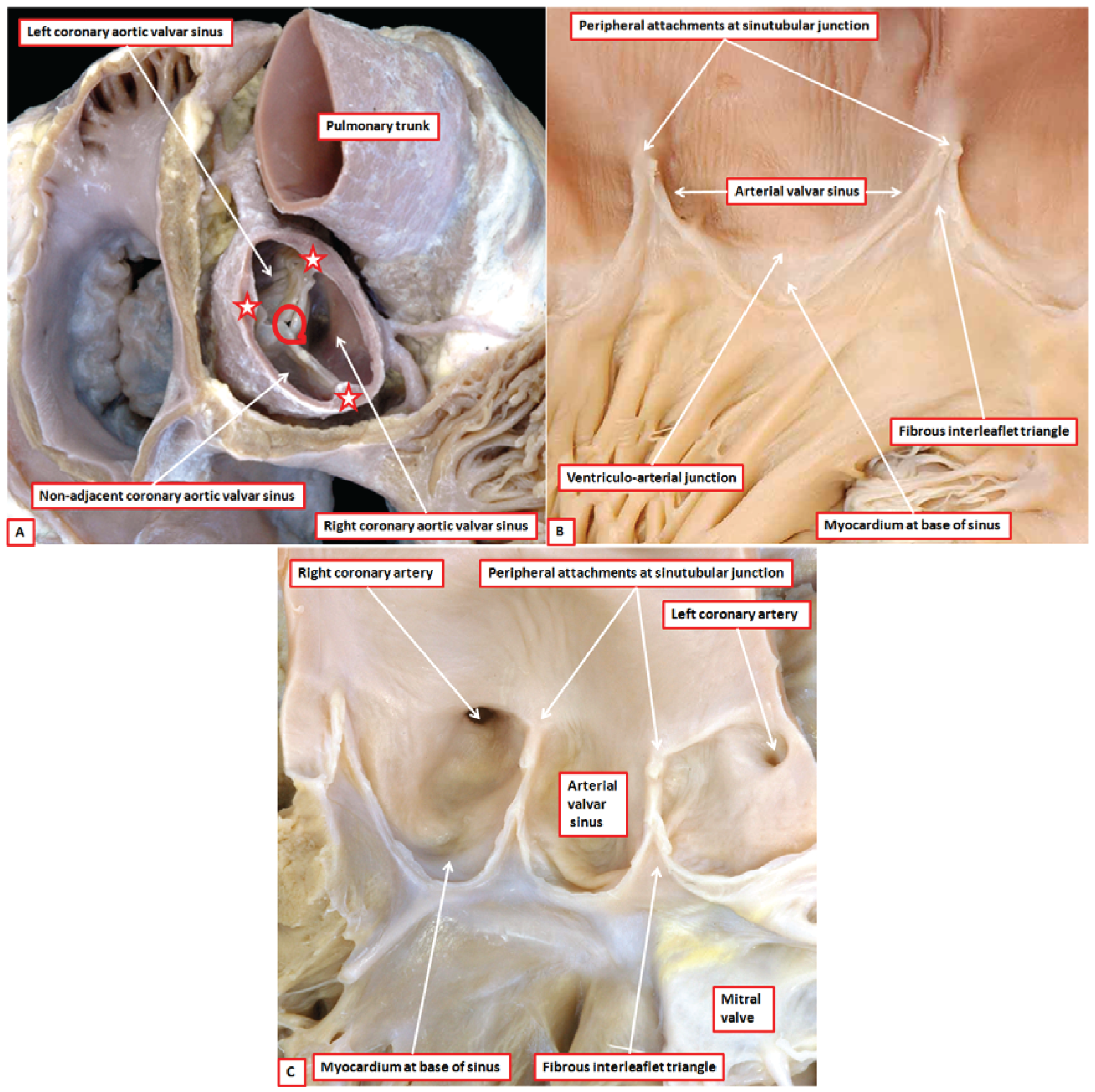
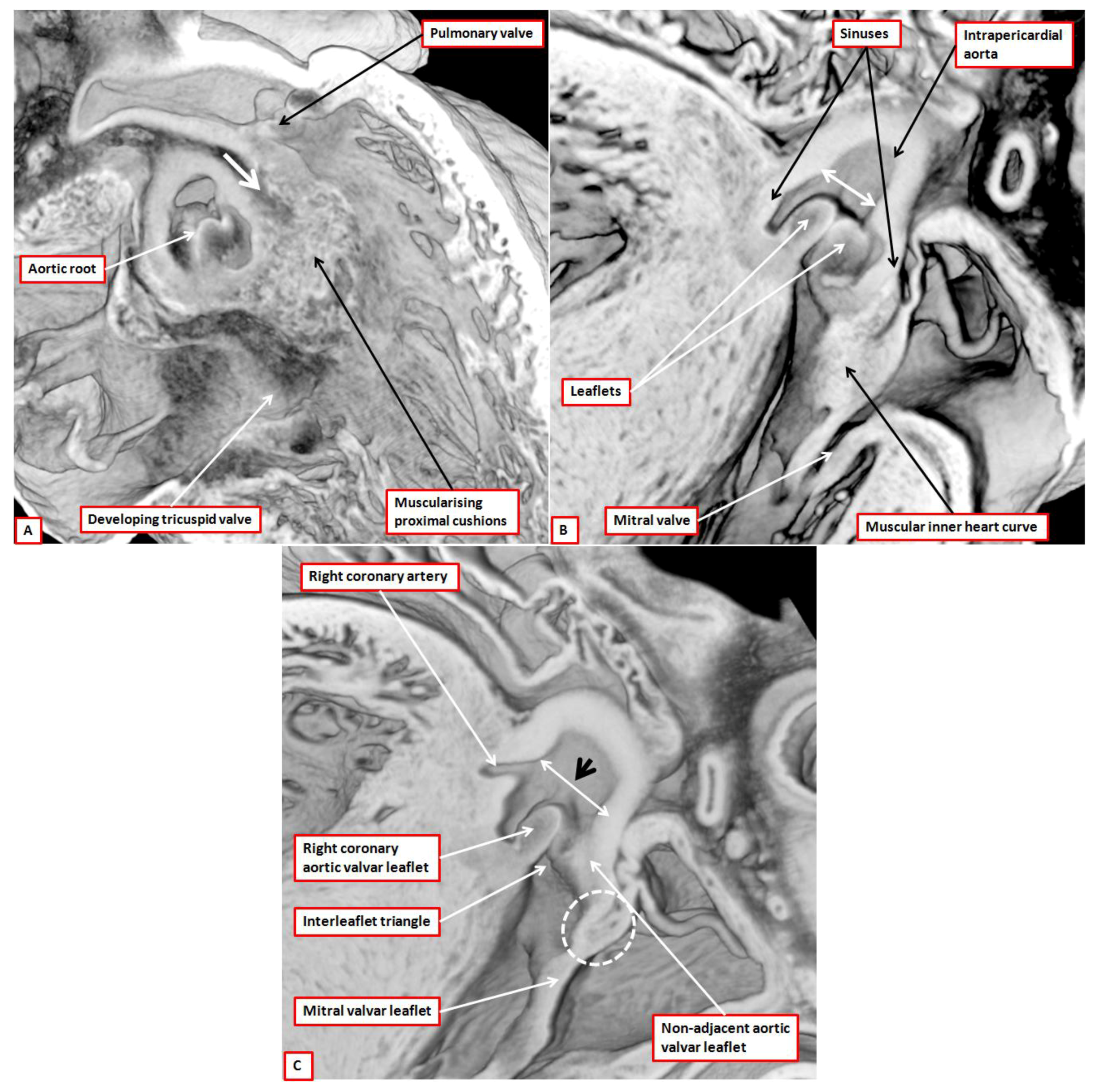
8]. There are two such valves in the normal heart, which are aortic and pulmonary. Abnormal hearts with a common arterial trunk have a solitary valve guarding the ventriculo-arterial junction. The anatomic arrangement of aortic, pulmonary, and common arterial valves is comparable, but complex. The working components are the leaflets, with each normal valve possessing three such entities (
Figure 2A). Each leaflet, which is semilunar in shape, is supported by a parabolic line of attachment within an arterial valvar sinus, with the hinges extending from their basal attachment within the subvalvar ventricular outflow tracts to the sinutubular junction (
Figure 2B). By virtue of the parabolic nature of these hinges, triangles of fibrous tissue are positioned between the sinuses on their ventricular aspect. The triangles extend distally to the level of the sinutubular junction (
Figure 2B,C) [
4]. This arrangement creates an oft-unrecognised discrepancy between the haemodynamic and anatomic ventriculo-arterial junctions. This difference is of importance when considering the different definitions of the “annulus”. The haemodynamic junction is produced by the semilunar hinges of the valvar leaflets (
Figure 2B). The anatomic ventriculo-arterial junction is the locus between the ventricular supporting structures and the fibro-elastic walls of the arterial valvar sinuses of Valsalva [
9]. This junction forms a planar ring, with a condensation at this site pictured as an “annulus” by some molecular biologists [
1]. Its ring-like configuration is particularly well seen when the leaflets themselves are absent, as occurs in some congenitally malformed human hearts. The semilunar hinges of the valvar leaflets, however, cross this anatomic ventriculo-arterial junction, and are not supported by it [
9]. It is because of this arrangement that crescents of ventricular myocardium are incorporated at the bases of all the pulmonary valvar sinuses (
Figure 2B). Myocardium is also found at the bases of the two aortic valvar sinuses that give rise to the coronary arteries (
Figure 2C). The third aortic valvar sinus does not usually give rise to a coronary artery, and does not have a myocardial base (
Figure 2A). Usually said to be non-coronary, it is better described as being the non-adjacent sinus, since very rarely it can support a coronary artery. The triangles of fibrous tissue interposed between the valvar sinuses form distal extensions, of varying dimensions, of the subvalvar ventricular outflow tracts. In
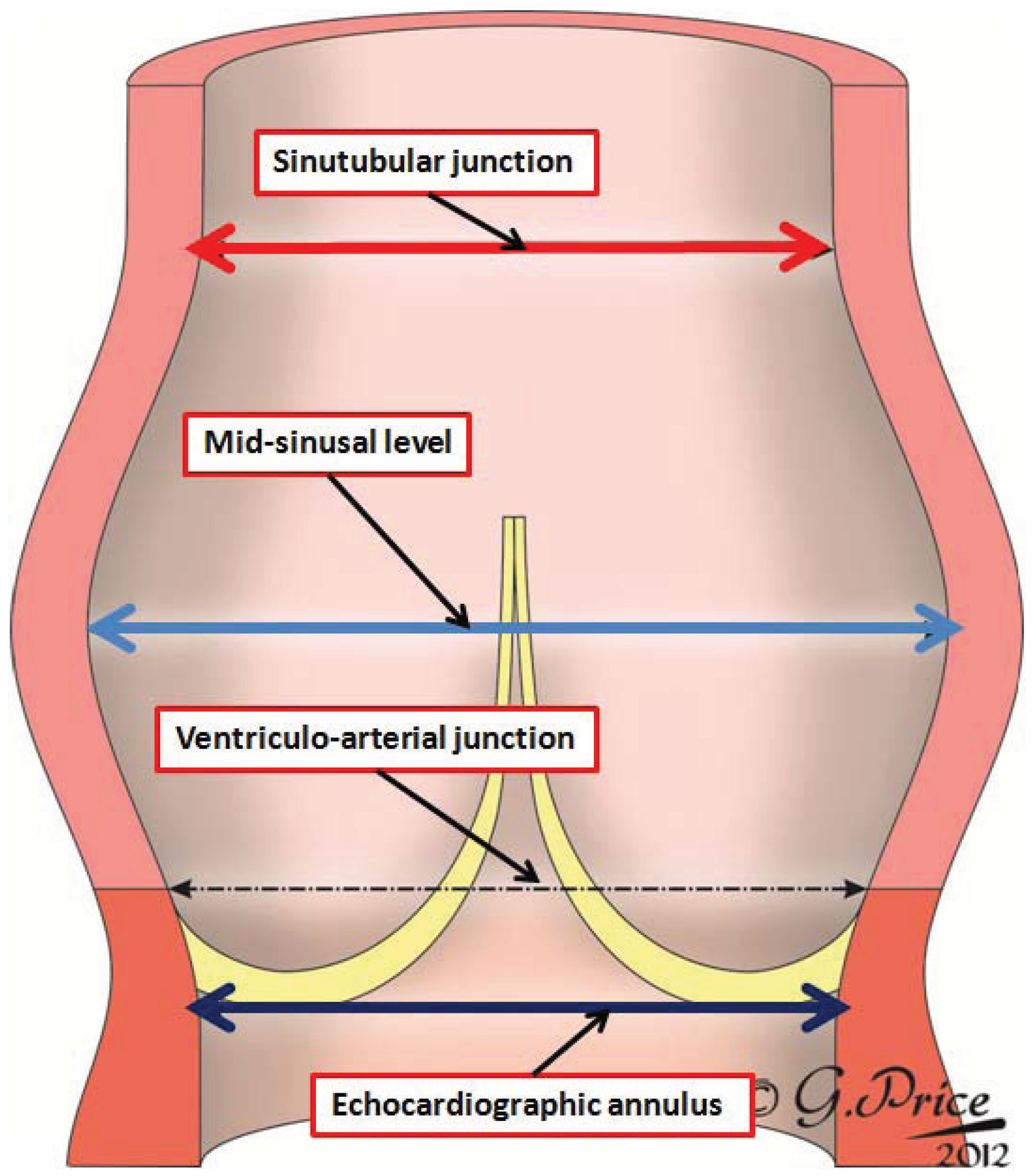
Figure 3, we show a diagrammatic depiction of the three-dimensional arrangement of the parabolic lines of attachment of the leaflets, and their relation to the potential rings described by others within the arterial roots.
Figure 3.
The cartoon shows a three-dimensional representation of the parabolic lines of attachment of the semilunar valvar leaflets (yellow lines). It then shows the location of the annular sinutubular junction (green circle), the circular arterial junction illustrated as the level of the annulus by some molecular biologists (blue circle) [
1], and the virtual basal ring identified by echocardiographers as the valvar annulus (red circle). The original drawing was made by Gemma Price, and we thank her for permitting us to modify it for use in this review.
Figure 3.
The cartoon shows a three-dimensional representation of the parabolic lines of attachment of the semilunar valvar leaflets (yellow lines). It then shows the location of the annular sinutubular junction (green circle), the circular arterial junction illustrated as the level of the annulus by some molecular biologists (blue circle) [
1], and the virtual basal ring identified by echocardiographers as the valvar annulus (red circle). The original drawing was made by Gemma Price, and we thank her for permitting us to modify it for use in this review.
Despite the similarities in make-up, there are also significant differences between the anatomy of the aortic and pulmonary valvar complexes, which collectively are also known as the arterial roots. The roots, therefore, extend from the echocardiographic annulus to the sinutubular junction (
Figure 4).
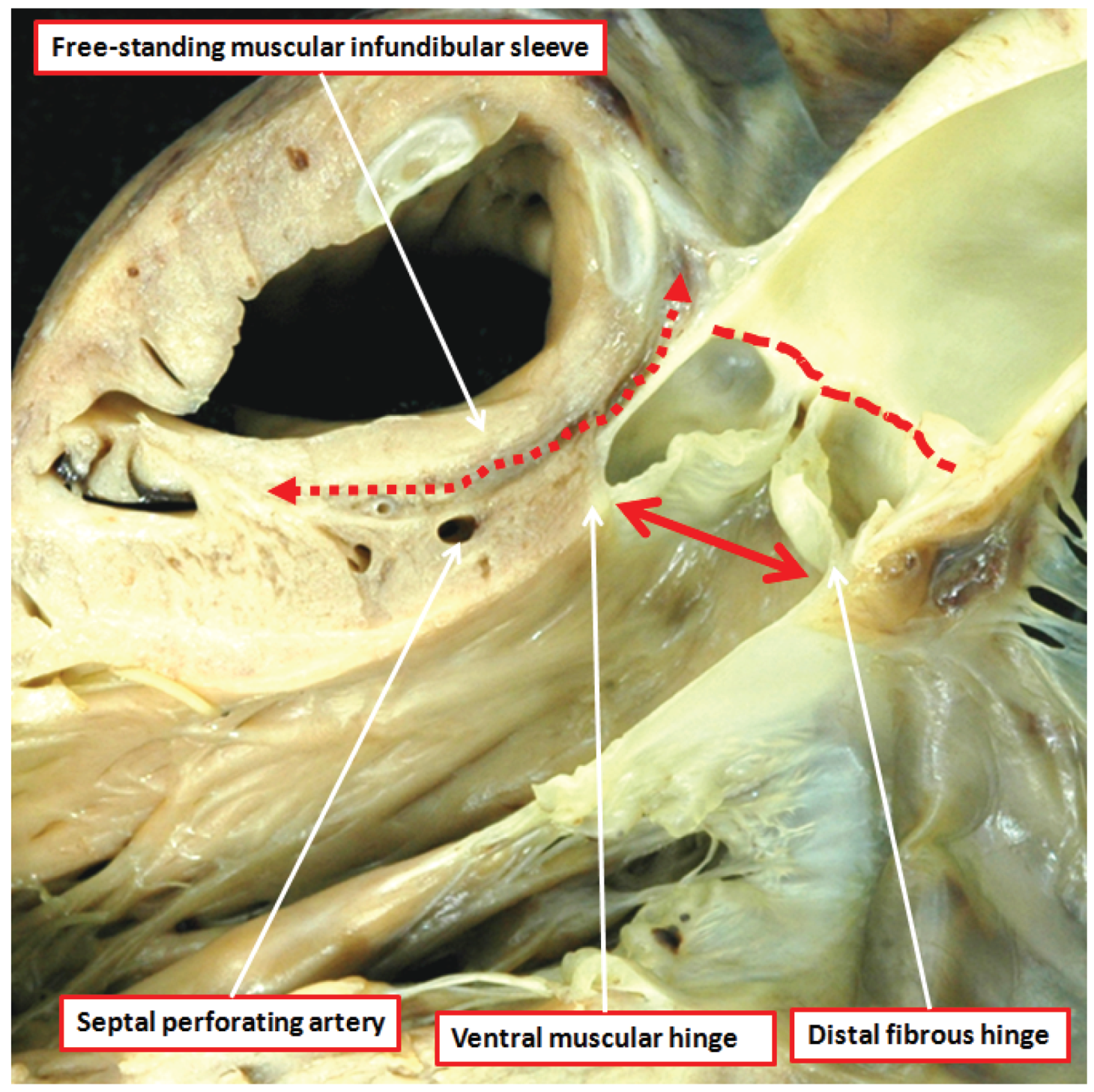
The differences between the two roots become important when considering their development. The pulmonary root is supported exclusively by the musculature of the right ventricular outflow tract (
Figure 1A). This muscle is a free-standing sleeve, which lifts the pulmonary root away from the base of the heart. Its presence makes it possible to remove the root as an autograft for use in the Ross procedure [
10]. Because of the presence of the infundibular sleeve, very little of the outflow musculature in the normal heart is located between the ventricles (
Figure 5) [
11].
Figure 4.
The image shows how the arterial roots extend from the virtual plane identified by echocardiographers as the annulus to the sinutubular junction. Echocardiographers also measure the roots at mid-sinusal level, but do not do so at the level of the arterial junction, albeit that it is this level that is identified by some molecular biologists [
1] as the valvar annulus. The original drawing was made by Gemma Price, and we thank her for permitting us to modify it for use in this review.
Figure 4.
The image shows how the arterial roots extend from the virtual plane identified by echocardiographers as the annulus to the sinutubular junction. Echocardiographers also measure the roots at mid-sinusal level, but do not do so at the level of the arterial junction, albeit that it is this level that is identified by some molecular biologists [
1] as the valvar annulus. The original drawing was made by Gemma Price, and we thank her for permitting us to modify it for use in this review.
The aortic root, in contrast to the pulmonary root, is supported by ventricular muscle only in its ventral aspect (
Figure 5) [
8]. It also differs from the pulmonary root in that two of its valvar sinuses, those adjacent to the pulmonary root, give rise to coronary arteries (
Figure 2A). In the postnatal heart, there is fibrous continuity dorsally between the non-adjacent and left coronary leaflets of the aortic valve, and the aortic leaflet of the mitral valve (
Figure 5). As we will show, when initially transferred to the morphologically left ventricle during its development, the aortic valve has a complete muscular base. Indeed, it is possible on occasion to find normal hearts with completely muscular aortic infundibulums [
12]. The axis of the aortic root is also, in the normally septated heart, aligned at almost right angles to the long axis of the infundibulum and pulmonary root (
Figure 1). In many normal hearts, but not all, it is possible to define a fibrous strand running between the roots at their point of crossing. This is the co-called tendon of the infundibulum [
13].
Figure 5.
The dissection of a postnatal human heart shows how an extracavitary tissue plane (double headed red dashed arrow) interposes between the free-standing infundibular sleeve of the right ventricle and the aortic root. Note also the sinutubular junction (red dashed line) and the virtual echocardiographic annulus (solid double headed red arrow) of the aortic root. The ventral leaflets of the aortic valve have muscular basal hinges, whereas the hinge of the non-adjacent leaflet is in fibrous continuity with the mitral valve.
Figure 5.
The dissection of a postnatal human heart shows how an extracavitary tissue plane (double headed red dashed arrow) interposes between the free-standing infundibular sleeve of the right ventricle and the aortic root. Note also the sinutubular junction (red dashed line) and the virtual echocardiographic annulus (solid double headed red arrow) of the aortic root. The ventral leaflets of the aortic valve have muscular basal hinges, whereas the hinge of the non-adjacent leaflet is in fibrous continuity with the mitral valve.
3.2. Describing the Components of the Arterial Valves
As we have already indicated, there are major discrepancies in the names currently given to the components of the valves [
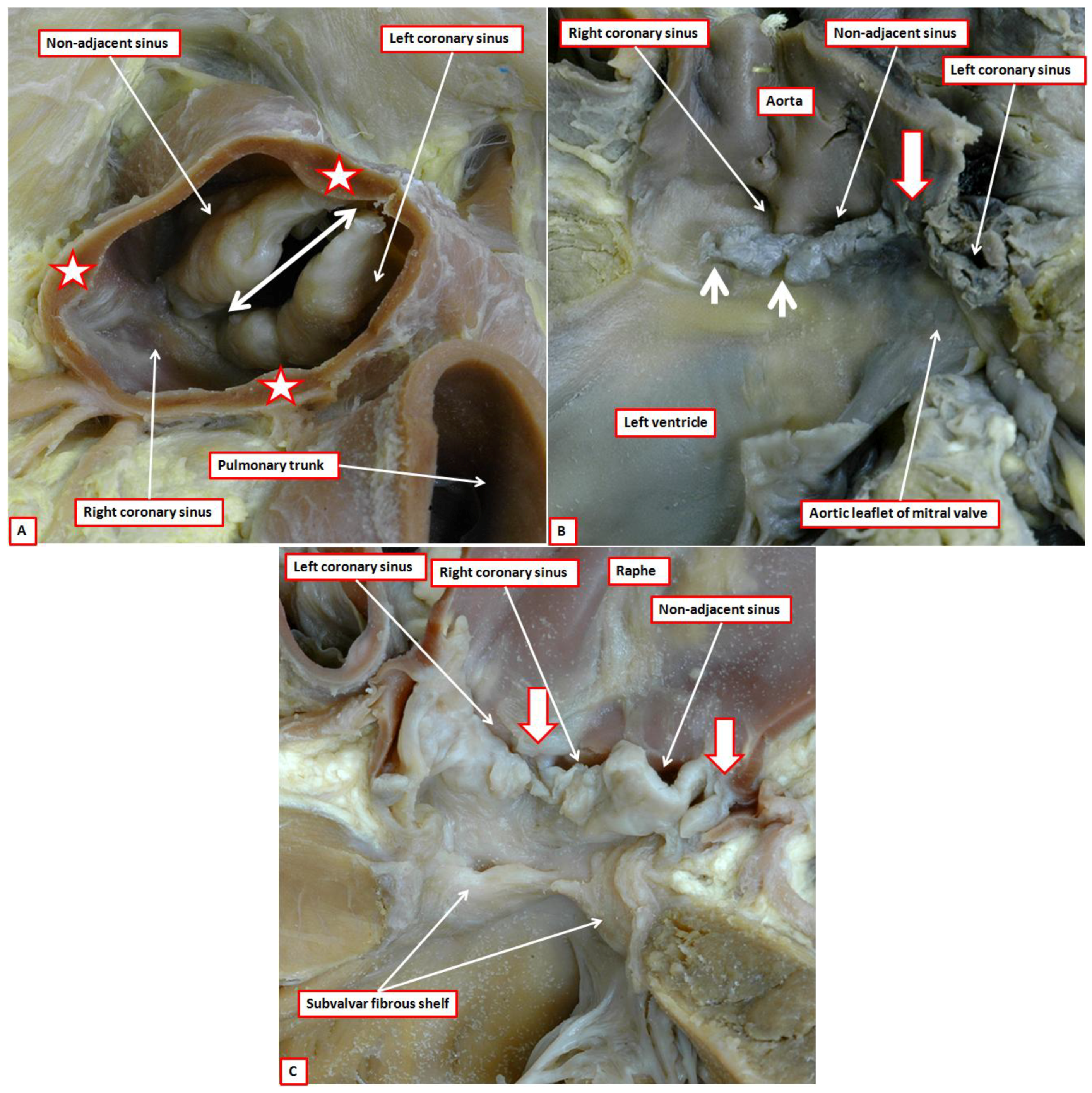
3]. Such variability impacts not only on the understanding of valvar anatomy, but also their development. We describe the working components of both the arterial and the atrioventricular valves as the leaflets. These moving components are frequently described as “cusps” (
Figure 6).
Some choose to distinguish between “cusps” in the arterial valves, and “leaflets” in the atrioventricular valves. This makes little sense, since the atrioventricular valves themselves are described as being bicuspid, or mitral, and tricuspid. “Cusp”, however, is also used by some to describe the valvar sinuses. This can create considerable confusion. In reality, the word is poorly suited for description of either the leaflets or the sinuses. This is because, when used in the vernacular sense, a “cusp” is an elevation, or else describes the crossing point of two curves. We presume that anatomists, when introducing the term, noted the likeness, when viewed in closed position from their ventricular aspect, of the arterial valves to the surfaces of the molar or premolar teeth (
Figure 4). Such historical precedence does not help resolve the ongoing difficulties in description [
3]. When the moving parts of the valves are described as leaflets, it is an easy matter to distinguish them from the sinuses. This usage also permits direct comparisons to be made with the moving parts of the atrioventricular valves.
Another problem then emerges in describing the zones of apposition between the leaflets. This creates problems for both the arterial and atrioventricular valves. Clinicians describe the peripheral attachments of the zones of apposition between adjacent leaflets as the “commissures”. If we approached the debate from the anatomical stance, the entirety of the zone of apposition should be considered to represent the commissure. To avoid confusion, we suggest that the areas where the leaflets meet should be described as their zones of apposition. The peripheral extents of these zones of apposition between the leaflets, at their points of attachment at the sinutubular junction, can then continue to be described as the commissures. It is the zones of apposition that are of greatest significance when considering valvar function (
Figure 2A).
Figure 6.
The image shows the undersurface of the closed aortic valve of a postnatal human heart as viewed from the ventricular apex. Note that the ventral leaflets of the valve (stars) are hinged from the septal myocardium (open white arrow with red borders), while the dorsal parts are in fibrous continuity with the aortic leaflet of the mitral valve (double headed red dashed arrow). It was presumably the resemblance of the leaflets, when viewed in their closed position, to the surfaces of the molar and premolar teeth that prompted anatomists do describe them as “cusps”.
Figure 6.
The image shows the undersurface of the closed aortic valve of a postnatal human heart as viewed from the ventricular apex. Note that the ventral leaflets of the valve (stars) are hinged from the septal myocardium (open white arrow with red borders), while the dorsal parts are in fibrous continuity with the aortic leaflet of the mitral valve (double headed red dashed arrow). It was presumably the resemblance of the leaflets, when viewed in their closed position, to the surfaces of the molar and premolar teeth that prompted anatomists do describe them as “cusps”.
The greatest problem in properly describing the arterial roots is created by the use of “annulus”. To recap, in the clinical arena, many surgeons tend to use “annulus” to describe the overall crown-like arrangement of the hingepoints of the leaflets (
Figure 3) [
14]. Some surgeons, in contrast, follow the approach taken by their angiographers and echocardiographers, and use the word to describe the diameter of the entrance to the arterial roots (
Figure 3 and
Figure 4) [
3]. Since the echocardiographic annulus is a virtual line, it cannot be used as an anatomical landmark. There is no structure that forms a continuous ring at the site of this “annulus”. The circular locus best seen in the arterial roots is the anatomic ventriculo-arterial junction. This junction is truly annular, and it is at this location that some molecular biologists illustrate the presence of a discrete anatomical structure that they label as the “annulus” [
1,
15]. This ring, if it exists, cannot be coincident throughout its extent with the valvar hingelines, which represent the haemodynamic ventriculo-arterial junction (
Figure 2B,C). Our current studies now permit us to update the developmental concept, ensuring that it is anatomically accurate.
3.3. Development of the Arterial Valves and their Supporting Sinuses
There can be no question regarding the importance, and the overall validity, of the investigation carried out by Kramer in the first half of the twentieth century [
1]. A significant part of his investigation was to demonstrate the development of the intercalated cushions [
2]. The intercalated cushions are formed in the intermediate part of the outflow tract (
Figure 7).
From the outset of their appearance, the intercalated cushions occupy the eventual location of the off-set arterial valves (
Figure 1A). When first formed, the developing outflow tract has exclusively muscular walls. At this early stage, it is supported exclusively above the cavity of the developing right ventricle, and is lined throughout its extent by endocardial jelly. Shortly thereafter, at E10.5, there is ingrowth at its distal margin of non-myocardial tissues derived from the second heart field. Non-myocardial spurs, when first seen, extend to greater extent cranially and caudally, but they rapidly rotate to form right-sided and left-sided tongues. The persisting distal margin of the myocardial walls then takes on a fishmouth configuration (
Figure 8A), with the tongues of non-myocardial tissue filling in the angles of the jaws of the fishmouth (
Figure 8B).
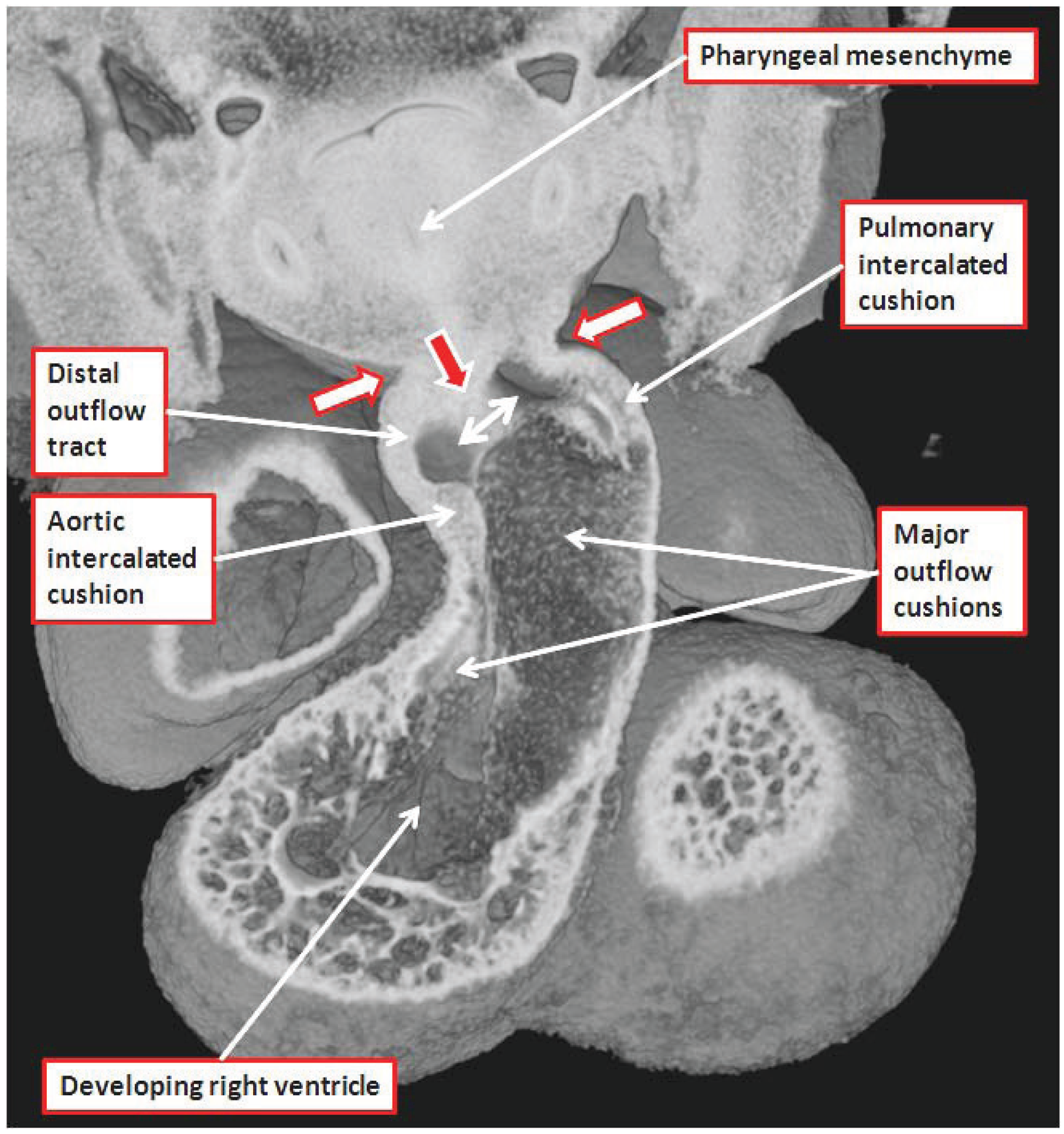
Figure 7.
The image is a section through an episcopic dataset prepared from a developing mouse at E11.5. The cut passes through the long axis of the dogleg bend within the outflow tract, and shows how the intercalated cushions are appearing at the inner and outer angles of the dogleg. Note that the walls of the distal outflow tract merge with the pharyngeal mesenchyme at the margins of the pericardial cavity (white arrows with red borders), and that a ventral protrusion from the dorsal wall of the aortic sac is growing toward the distal aspects of the major outflow cushions (red arrow with white borders). The space between the protrusion and the cushions is the embryonic aortopulmonary foramen. The major outflow cushions, still to fuse at this stage, spiral through the intermediate and proximal parts of the outflow tract. The section has largely bisected the septal cushion. Note that the arrow identifying the pharyngeal mesenchyme points also to the developing trachea, which develops in the central part of the mesenchyme.
Figure 7.
The image is a section through an episcopic dataset prepared from a developing mouse at E11.5. The cut passes through the long axis of the dogleg bend within the outflow tract, and shows how the intercalated cushions are appearing at the inner and outer angles of the dogleg. Note that the walls of the distal outflow tract merge with the pharyngeal mesenchyme at the margins of the pericardial cavity (white arrows with red borders), and that a ventral protrusion from the dorsal wall of the aortic sac is growing toward the distal aspects of the major outflow cushions (red arrow with white borders). The space between the protrusion and the cushions is the embryonic aortopulmonary foramen. The major outflow cushions, still to fuse at this stage, spiral through the intermediate and proximal parts of the outflow tract. The section has largely bisected the septal cushion. Note that the arrow identifying the pharyngeal mesenchyme points also to the developing trachea, which develops in the central part of the mesenchyme.
![Jcdd 01 00177 g007]()
By the time that the fishmouth arrangement has become evident, the endocardial jelly has been invaded proximally by cells derived from the endocardium, having undergone endocardial to mesenchymal transformation, and distally by migration of neural crest cells, thus, forming the major outflow tract cushions. At this stage these cushions, which spiral through the outflow tract, terminate distally at the myocardial margins (
Figure 8A). The myocardium, therefore, is more extensive cranially and caudally than at the right and left sides. A protrusion then enters the cavity of the distal outflow tract in oblique fashion from the dorsal wall of the aortic sac, extending from right and caudal to left and cranial, and separating the origins of the fourth and sixth pairs of pharyngeal arch arteries (
Figure 7). Van Mierop and colleagues [
16] identified this protrusion appropriately as the embryonic aortopulmonary septum. It has now become popular to assign the outflow cushions, rather than the protrusion, the role of forming the “aortopulmonary septal complex” [
17,
18]. This description is misleading, since the major role of the cushions is to separate the developing arterial roots and the proximal outflow tracts. Although they then play a crucial role in separating the intermediate and proximal arterial channels [
7], their septal activity is transient. In the postnatal heart, there are no septal structures interposed between the either the intrapericardial arterial trunks or the arterial roots, and very little, if any, muscular tissue to be found between the ventricular outlets (
Figure 5).
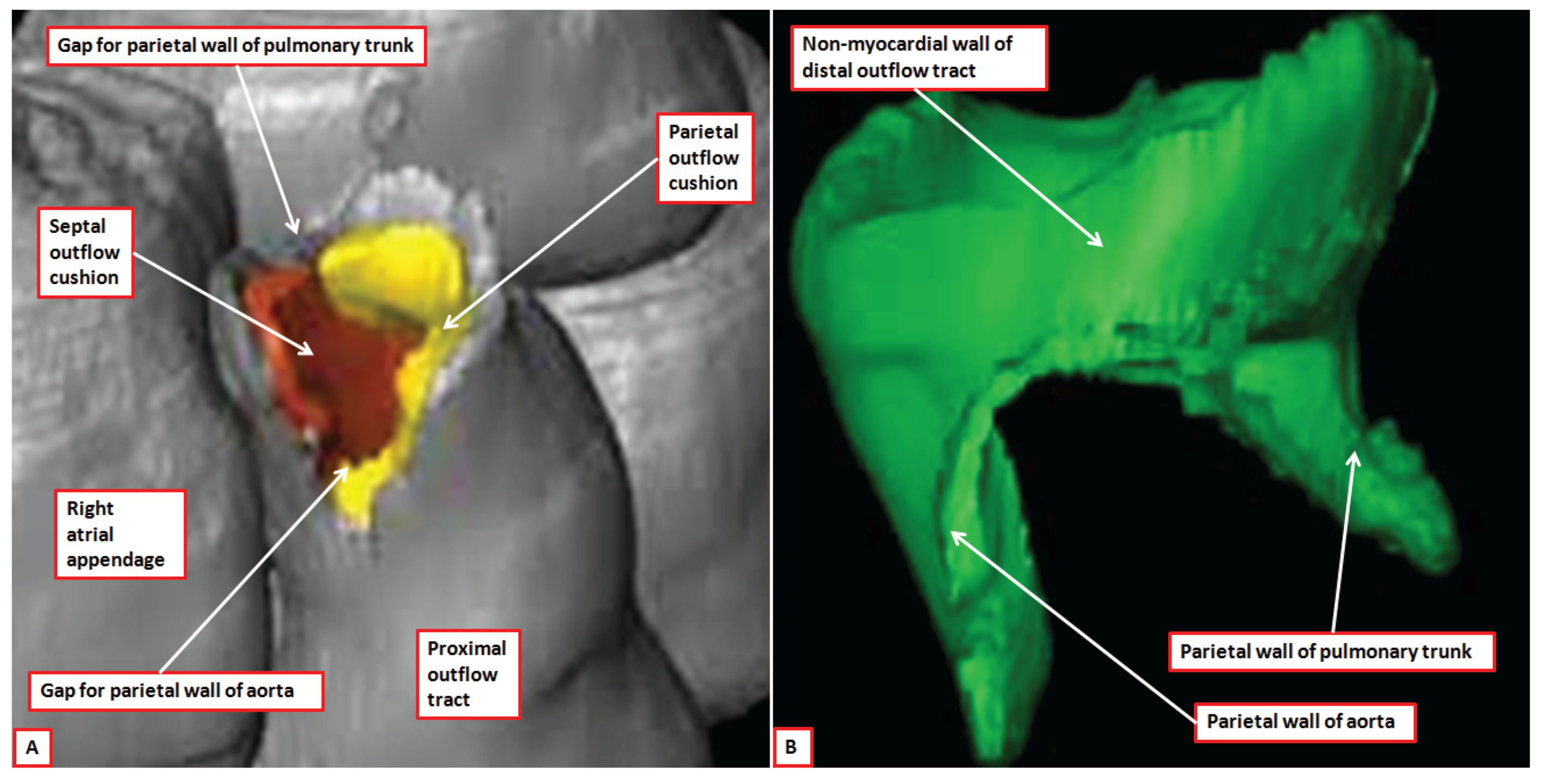
Figure 8.
The images show reconstructed aspects of the outflow tract of a developing mouse at E11.5. The reconstructions were segmented so that all myocardium, determined using a myocardial-specific marker, was shown in silver, and non-myocardial tissues, lacking the marker, were shown in green (Details provided in Reference [
7]). The septal outflow cushion was shown in brown, and the parietal cushion in yellow. Panel (
A) shows a superior view of the distal margins of the muscular outflow tract having digitally removed the non-myocardial distal outflow tract. The distal myocardial border has an obvious fishmouth appearance, with the distal margins of the major outflow cushions confluent with the muscular margins. Panel (
B) shows the non-myocardial walls of the distal outflow tract viewed from the front. The two non-myocardial tongues fill the spaces formed by the angles of the jaws of the muscular fishmouth. They will become the parietal walls of the aorta and the pulmonary trunk.
Figure 8.
The images show reconstructed aspects of the outflow tract of a developing mouse at E11.5. The reconstructions were segmented so that all myocardium, determined using a myocardial-specific marker, was shown in silver, and non-myocardial tissues, lacking the marker, were shown in green (Details provided in Reference [
7]). The septal outflow cushion was shown in brown, and the parietal cushion in yellow. Panel (
A) shows a superior view of the distal margins of the muscular outflow tract having digitally removed the non-myocardial distal outflow tract. The distal myocardial border has an obvious fishmouth appearance, with the distal margins of the major outflow cushions confluent with the muscular margins. Panel (
B) shows the non-myocardial walls of the distal outflow tract viewed from the front. The two non-myocardial tongues fill the spaces formed by the angles of the jaws of the muscular fishmouth. They will become the parietal walls of the aorta and the pulmonary trunk.
It is the growth of the ventral protrusion from the dorsal wall of the aortic sac, and its fusion with the distal ends of the outflow cushions, therefore, which serves initially to separate the cavities of the intrapericardial aorta and pulmonary trunk [
7]. The core of the protrusion is derived from the second heart field, but it is covered by material derived from the neural crest [
7,
19]. The outflow cushions themselves are also packed by such cells derived from the neural crest [
7]. Thus, fusion of these components, which produces separation of the intrapericardial arterial channels, and closure of the embryonic aortopulmonary foramen, is dependent on the interplay between the cells derived from the second heart field and the neural crest [
7]. Initially functioning as a septum, the tissues derived from the neural crest become less significant with the passage of time. Eventually, they are seen as no more than an indistinct line in the tissues interposed between the newly formed and separate walls of the intrapericardial arterial trunks (Compare
Figure 9A and
Figure 9B).
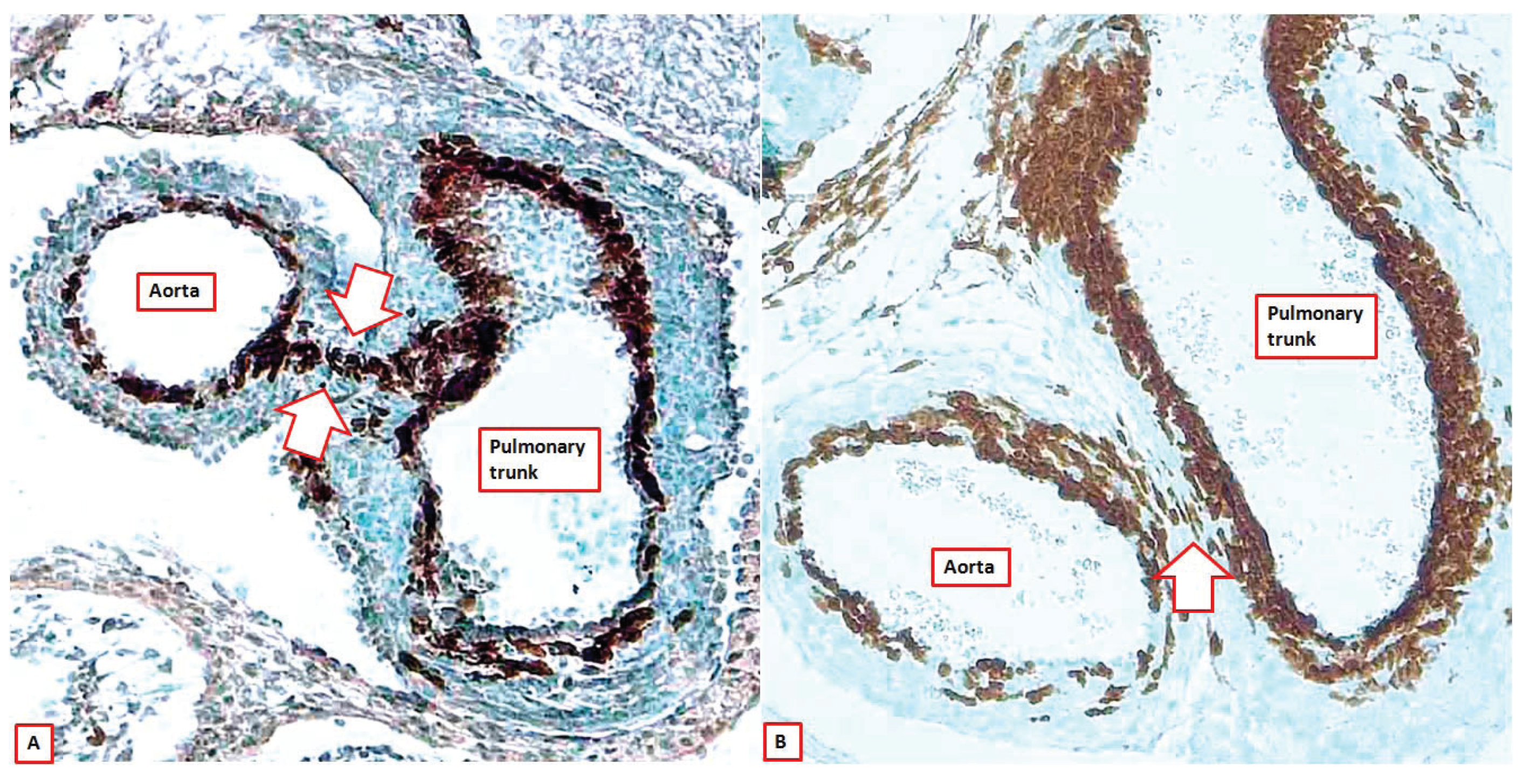
Figure 9.
The images show sections across the intrapericardial arterial trunks from two Wnt1-cre mice, with the brown colouring showing the location of the cells derived from the neural crest. As can be seen, at E12.5 (panel (A)), the neural crest cells form the sub-endothelial regions of both arterial trunks, but also show the line of fusion between the distal parts of the outflow cushions and the protrusion from the dorsal wall of the aortic sac (seam between white arrows). By E15.5 (panel (B)), this seam is no more than an oblique strand in the extracardiac connective tissue that separates the walls of the intrapericardial arterial trunks (white arrow).
Figure 9.
The images show sections across the intrapericardial arterial trunks from two Wnt1-cre mice, with the brown colouring showing the location of the cells derived from the neural crest. As can be seen, at E12.5 (panel (A)), the neural crest cells form the sub-endothelial regions of both arterial trunks, but also show the line of fusion between the distal parts of the outflow cushions and the protrusion from the dorsal wall of the aortic sac (seam between white arrows). By E15.5 (panel (B)), this seam is no more than an oblique strand in the extracardiac connective tissue that separates the walls of the intrapericardial arterial trunks (white arrow).
It is the ventral protrusion from the dorsal wall of the aortic sac, therefore, that is largely responsible for separating the intrapericardial components of the arterial trunks. As already emphasized, although initially described as the aortopulmonary septum by Van Mierop and colleagues [
16], some investigators have considered the cushions to provide an “aortopulmonary septal complex” [
17,
18]. The cushions, however, separate the intermediate and proximal parts of the outflow tract. Our preference, therefore, is to describe the structure initially separating the distal aortic channel from that of the pulmonary trunk as the ventral protrusion, even though it does produce the embryonic aortopulmonary septum.
It is the closure of the aortopulmonary foramen, and transformation of the distal outflow tract into the intrapericardial arterial trunks, that sets the stage for formation of the arterial valves.
As already emphasized, the process of valvar formation is heralded by the appearance of the intercalated cushions in the intermediate part of the outflow tract (
Figure 7) [
2]. By the time the intercalated cushions are appearing, the distal parts of the major outflow cushions are themselves beginning to fuse so as to separate the intermediate part of the outflow tract, still enclosed within its muscular walls, into its aortic and pulmonary components. It is the position of the intercalated cushions between the unfused peripheral parts of the major cushions that defines the position of the valvar primordiums (
Figure 10).
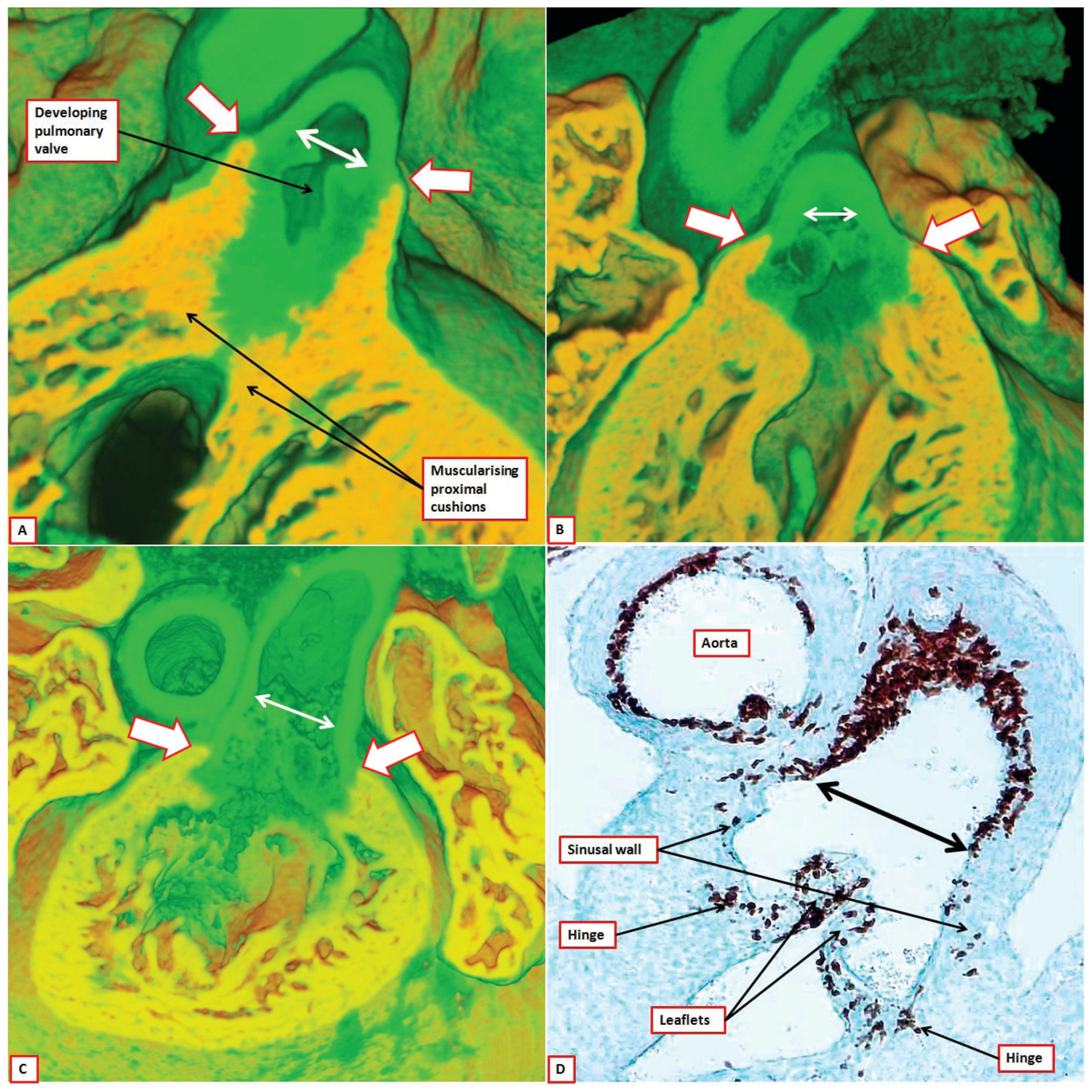
Figure 10.
The images are from an episcopic dataset prepared from a developing mouse at E12.5. Panel (A) shows an overall short-axis section through the atrial chambers, taken obliquely across the intermediate part of the outflow tract so as to cut through both the intercalated cushions. Panel (B), which is a magnified image of the boxed area in panel (A), shows how the intercalated cushions interpose between the unfused parietal margins of the major outflow cushions, thus forming the primordiums of the arterial valves.
Figure 10.
The images are from an episcopic dataset prepared from a developing mouse at E12.5. Panel (A) shows an overall short-axis section through the atrial chambers, taken obliquely across the intermediate part of the outflow tract so as to cut through both the intercalated cushions. Panel (B), which is a magnified image of the boxed area in panel (A), shows how the intercalated cushions interpose between the unfused parietal margins of the major outflow cushions, thus forming the primordiums of the arterial valves.
Through E12.5, and continuing through the next days of development, there is then a gradual excavation of the distal faces of the intercalated cushions, along with the peripheral ends of the major outflow cushions. Concomitant with this excavation, which produces the semilunar valvar leaflets, there is further growth proximally from the tissues derived from the second heart field. These non-myocardial tissues initially grow within the walls of the myocardial turret. This means that the distal borders of the remodeling cushions remain at what will become the level of the sinutubular junction. The processes are often considered to represent “lengthening of the valvar leaflets”. In reality, the continuing ingrowth of the non-myocardial tissues to form the valvar sinuses produces no more than an apparent lengthening of the leaflets. The process is better analyzed in terms of excavation of the outflow cushions. There is, nonetheless, continuing movement of the distal myocardial boundary towards the ventricular apex (
Figure 11).
Figure 11.
The images are from developing mice at E13.5 (panel (
A)), 14.5 (panel (
B)), and 15.5 (panels (
C) and (
D)). The sections in panels (
A) through (
C) are from episcopic datasets prepared from mice in which the myocardium had been marked with a cre to myosin light chain. Panel (
D) is a section from a Wnt1-cre mouse, with the brown stained tissues derived from the neural crest. The panels show how, over the period of development from E13.5 through E15.5, there is gradual excavation of the outflow cushions combined with regression of the distal myocardial border (white arrows with red borders) and ingrowth on non-myocardial tissues to form the valvar sinuses. The end-result is to elongate the distance from the hinge of the developing valvar leaflets to their distal attachment at the sinutubular junction (double headed white and black arrows). The semilunar hinges themselves join together at the distal sinutubular junction (see
Figure 2).
Figure 11.
The images are from developing mice at E13.5 (panel (
A)), 14.5 (panel (
B)), and 15.5 (panels (
C) and (
D)). The sections in panels (
A) through (
C) are from episcopic datasets prepared from mice in which the myocardium had been marked with a cre to myosin light chain. Panel (
D) is a section from a Wnt1-cre mouse, with the brown stained tissues derived from the neural crest. The panels show how, over the period of development from E13.5 through E15.5, there is gradual excavation of the outflow cushions combined with regression of the distal myocardial border (white arrows with red borders) and ingrowth on non-myocardial tissues to form the valvar sinuses. The end-result is to elongate the distance from the hinge of the developing valvar leaflets to their distal attachment at the sinutubular junction (double headed white and black arrows). The semilunar hinges themselves join together at the distal sinutubular junction (see
Figure 2).
In addition, as the myocardial border moves proximally relative to the developing sinutubular junction, so the tissues filling the gaps between the excavating sinuses form the walls of the intersinusal arterial roots. With further ongoing maturation, these columns become thinned, and will eventually form the fibrous interleaflet triangles. Further differences can then be noted in the contributions made by the neural crest to the developing leaflets of the arterial valves. Cells derived from the neural crest pack the major outflow cushions, which then fuse so as to separate the developing aortic and pulmonary roots. As with the intrapericardial arterial trunks, these separating tissues become decreasingly significant with the passage of time. By the stage at which the sinutubular junctions become recognizable, at E15.5, the aortic and pulmonary roots are completely separated one from the other (
Figure 12A), with their long axes at right angles, as in the postnatal heart (
Figure 1).
Figure 12.
The images are from episcopic datasets prepared from developing mice at E 15.5. Panels (A) and (B) are taken from the same dataset, with panel (A) cut to replicate the oblique subcostal echocardiographic section, and panel (B) showing the parasternal long axis equivalent. Panel (A) shows how the axes of the valves are at right angles to each other, and that the pulmonary valve has been lifted away from the base of the heart by the formation of the free-standing infundibular sleeve, which now produces a tissue plane between the pulmonary valve and the aortic root (white arrow). Panel (B) shows that the aortic valvar leaflets, in this mouse, remain supported by a completely muscular ventricular outflow tract. The leaflets now co-apt below the level of the sinutubular junction (double headed white arrow). Panel (C) shows a dataset from a more mature embryo at E15.5, again in parasternal long axis equivalent section. The hinges of the leaflets now come together at the sinutubular junction (black arrowhead transecting double headed white arrow). The muscular tissue of the inner heart curve is attenuating in the area between the hinges of the aortic and mitral valves (area within dotted white oval). The wall of the outflow tract separating the semilunar hinges is now recognizable as an interleaflet triangle. It will become fibrous in the postnatal heart. Note the origin of the right coronary artery within the valvar sinus.
Figure 12.
The images are from episcopic datasets prepared from developing mice at E 15.5. Panels (A) and (B) are taken from the same dataset, with panel (A) cut to replicate the oblique subcostal echocardiographic section, and panel (B) showing the parasternal long axis equivalent. Panel (A) shows how the axes of the valves are at right angles to each other, and that the pulmonary valve has been lifted away from the base of the heart by the formation of the free-standing infundibular sleeve, which now produces a tissue plane between the pulmonary valve and the aortic root (white arrow). Panel (B) shows that the aortic valvar leaflets, in this mouse, remain supported by a completely muscular ventricular outflow tract. The leaflets now co-apt below the level of the sinutubular junction (double headed white arrow). Panel (C) shows a dataset from a more mature embryo at E15.5, again in parasternal long axis equivalent section. The hinges of the leaflets now come together at the sinutubular junction (black arrowhead transecting double headed white arrow). The muscular tissue of the inner heart curve is attenuating in the area between the hinges of the aortic and mitral valves (area within dotted white oval). The wall of the outflow tract separating the semilunar hinges is now recognizable as an interleaflet triangle. It will become fibrous in the postnatal heart. Note the origin of the right coronary artery within the valvar sinus.
![Jcdd 01 00177 g012]()
By this stage, the cells derived from the neural crest have populated the adjacent leaflets of both the developing aortic and pulmonary valves, with neural crest cells also forming their hinges with the supporting ventricular myocardium (
Figure 11D). Apart from the hinge, which corresponds with the eventual semilunar line of attachment of the leaflets, we are unable to identify a discrete structure at the circular ventriculo-arterial junction, although such an entity has been illustrated in cartoons produced by some who have investigated valvar development [
1,
15]. We have shown, however, that the non-adjacent leaflet of the aortic valve, and its supporting hinge, is similarly populated with cells derived from the neural crest. The non-adjacent leaflet of the pulmonary valve, in contrast, contains significantly fewer cells of neural crest origin [
20]. By E13.5, the developing aortic root has become committed to the left ventricle by fusion of the proximal outflow cushions with the apical muscular ventricular septum [
21]. As late as E15.5, the inner heart curvature remains still as a muscular fold interposed between the leaflets of the developing aortic and mitral valves (
Figure 12B). It is only in the days leading up to birth in the mouse that this muscle regresses, permitting the eventual complete formation of aortic-to-mitral valvar fibrous continuity. Myocardium, therefore, is removed from the aortic root during the final stages of its development. Muscle, in contrast, is added to the developing pulmonary root by myocardialisation of the proximal outflow cushions to form the subpulmonary muscular infundibular sleeve (
Figure 13) [
22,
23].
In this regard, the proximal outflow cushions have still to fuse at the stage when the distal parts of the major outflow cushions have themselves fused with the ventral protrusion to separate the intrapericardial trunks. The proximal cushions are similarly unfused at the time point when the intermediate parts of the cushions fuse to separate the developing aortic and pulmonary roots. When the proximal cushions do fuse, they create an arch above the cavity of the developing right ventricle, the proximal part of the outflow tract remaining supported by the right ventricle at this stage [
21]. Concomitant with commitment of the aorta to the left ventricle, the arch formed by the leading edge of the proximal cushions, subsequent to their fusion, is brought into better alignment with the crest of the apical muscular septum [
21]. It is only when the aorta has shifted across to the left ventricle that the remaining embryonic interventricular communication can be closed by the rightward margins of the atrioventricular endocardial cushions, thus forming the membranous part of the ventricular septum [
21,
24]. The face of the muscularised proximal cushions then becomes converted into the sleeve of free-standing infundibular musculature as the core of the cushions, initially packed with cells derived from the neural crest, is transformed into the tissue plane that separates the newly formed infundibulum from the aortic root (
Figure 12A). The cells derived from the neural crest persist and become the tendon of the infundibulum. The end result is that the leaflets of the pulmonary valve, derived from the ventral parts of the intermediate major cushions and the ventral intercalated cushion, are lifted away from the ventricular base by the newly formed infundibulum (
Figure 12A). The leaflets of the aortic valve, in contrast, which are derived from the dorsal parts of the major cushions along with the dorsal intercalated cushion, are placed in fibrous continuity with the aortic leaflet of the mitral valve only subsequent to the attenuation of the muscular inner heart curvature. The origin of the interleaflet triangles (
Figure 12C) has still to be established, as do the mechanics underscoring the effective proximal migration of the distal myocardial border.
Figure 13.
The image, from an episcopic dataset, is a short axis section across the cardiac base of an embryonic mouse at E13.5, viewed from the atrial aspect. The mouse was genetically modified to show the location of the myocardial components, which are colored yellow. At this stage, the developing valvar components are contained within a myocardial turret. New myocardium, however, is entering the proximal outflow cushions, and will eventually form the free-standing infundibular sleeve of the right ventricle.
Figure 13.
The image, from an episcopic dataset, is a short axis section across the cardiac base of an embryonic mouse at E13.5, viewed from the atrial aspect. The mouse was genetically modified to show the location of the myocardial components, which are colored yellow. At this stage, the developing valvar components are contained within a myocardial turret. New myocardium, however, is entering the proximal outflow cushions, and will eventually form the free-standing infundibular sleeve of the right ventricle.
3.4. Abnormal Development of the Arterial Valves and Their Sinuses
Bicuspid arterial valves are the commonest cardiovascular congenital malformations [
25]. Either the aortic valve or the pulmonary valve can develop with two, as opposed to three, leaflets. In the most severe form of congenital aortic valvar disease, the so-called unicommissural and unicuspid variant, the valve effectively has but a solitary zone of apposition (
Figure 14A,B), but so does the bifoliate valve (
Figure 14C).
It is often difficult, therefore, for clinicians to establish the number of leaflets in congenitally malformed arterial valves. The key to analysis is to establish the extent of formation of the interleaflet triangles, with two such triangles formed in the presence of bifoliate valves, and a solitary triangle extending to the level of the sinutubular junction in the setting of the unicuspid valves (
Figure 14) [
26]. The unicuspid variant is very much more common in aortic as opposed to pulmonary position. It cannot be coincidental that the persisting zone of apposition almost always points back towards the aortic leaflet of the mitral valve (
Figure 14B). The morphology of the bifoliate and unifoliate valves also demonstrates the annular paradox, since the hinges of the valves are much closer to a true ring in absence of formation of the interleaflet triangles (
Figure 14).
Figure 14.
The images show grossly dysplastic aortic valves that produced critical stenosis in early childhood. Panel (A) shows a valve with a solitary zone of apposition between the dysplastic remnants of the leaflets (double headed white arrow). It is possible to recognize three thickenings at the sinutubular junction (stars). Opening the valve (panel (B)) reveals that the leaflets are attached in annular fashion, with two rudimentary interleaflet triangles (white arrows) corresponding to the thickenings at the sinutubular junction. This is the so-called unicuspid and unicommissural variant, with the zone of apposition at the apex of the solitary interleaflet triangle pointing back to the aortic leaflet of the mitral valve (white arrow with red borders). In the heart shown in panel (C), two zones of apposition, at the apex of dual interleaflet triangles, extend to the sinutubular junction (white arrows with red borders). There is a raphe in one of the remaining leaflets. This is a bicuspid valve, with the conjoined leaflet formed from the right coronary and non-adjacent leaflets. Note the presence of an additional subvalvar fibrous shelf, which accentuates the valvar stenosis.
Figure 14.
The images show grossly dysplastic aortic valves that produced critical stenosis in early childhood. Panel (A) shows a valve with a solitary zone of apposition between the dysplastic remnants of the leaflets (double headed white arrow). It is possible to recognize three thickenings at the sinutubular junction (stars). Opening the valve (panel (B)) reveals that the leaflets are attached in annular fashion, with two rudimentary interleaflet triangles (white arrows) corresponding to the thickenings at the sinutubular junction. This is the so-called unicuspid and unicommissural variant, with the zone of apposition at the apex of the solitary interleaflet triangle pointing back to the aortic leaflet of the mitral valve (white arrow with red borders). In the heart shown in panel (C), two zones of apposition, at the apex of dual interleaflet triangles, extend to the sinutubular junction (white arrows with red borders). There is a raphe in one of the remaining leaflets. This is a bicuspid valve, with the conjoined leaflet formed from the right coronary and non-adjacent leaflets. Note the presence of an additional subvalvar fibrous shelf, which accentuates the valvar stenosis.
![Jcdd 01 00177 g014]()
The presence of raphes in the so-called conjoined leaflets of bifoliate valves is strong evidence that the valves were initially formed on the basis of a trifoliate template, but that fusion of two of the components occurred during fetal maturation. The elegant studies of Sans-Coma and colleagues [
27] proved this to be the case for the Syrian hamster, in which the aortic coronary valvar leaflets fuse to produce the bifoliate arrangement. Their studies with the eNOS knock-out mouse then showed that fusion between the aortic intercalated cushion and the right coronary aortic cushion accounts for the second commonest variant of bicuspid aortic valve [
28]. On occasion, nonetheless, it is known that bicuspid aortic valves can be found in the absence of a raphe in either of the sinuses. It is possible that such bicuspid and bisinuate valves may represent failure of formation of an intercalated cushion. Similar abnormal mechanisms could account for the comparable variants noted in bicuspid pulmonary valves. It is noteworthy that two-thirds of patients with tetralogy of Fallot are described as having bicuspid pulmonary valves.
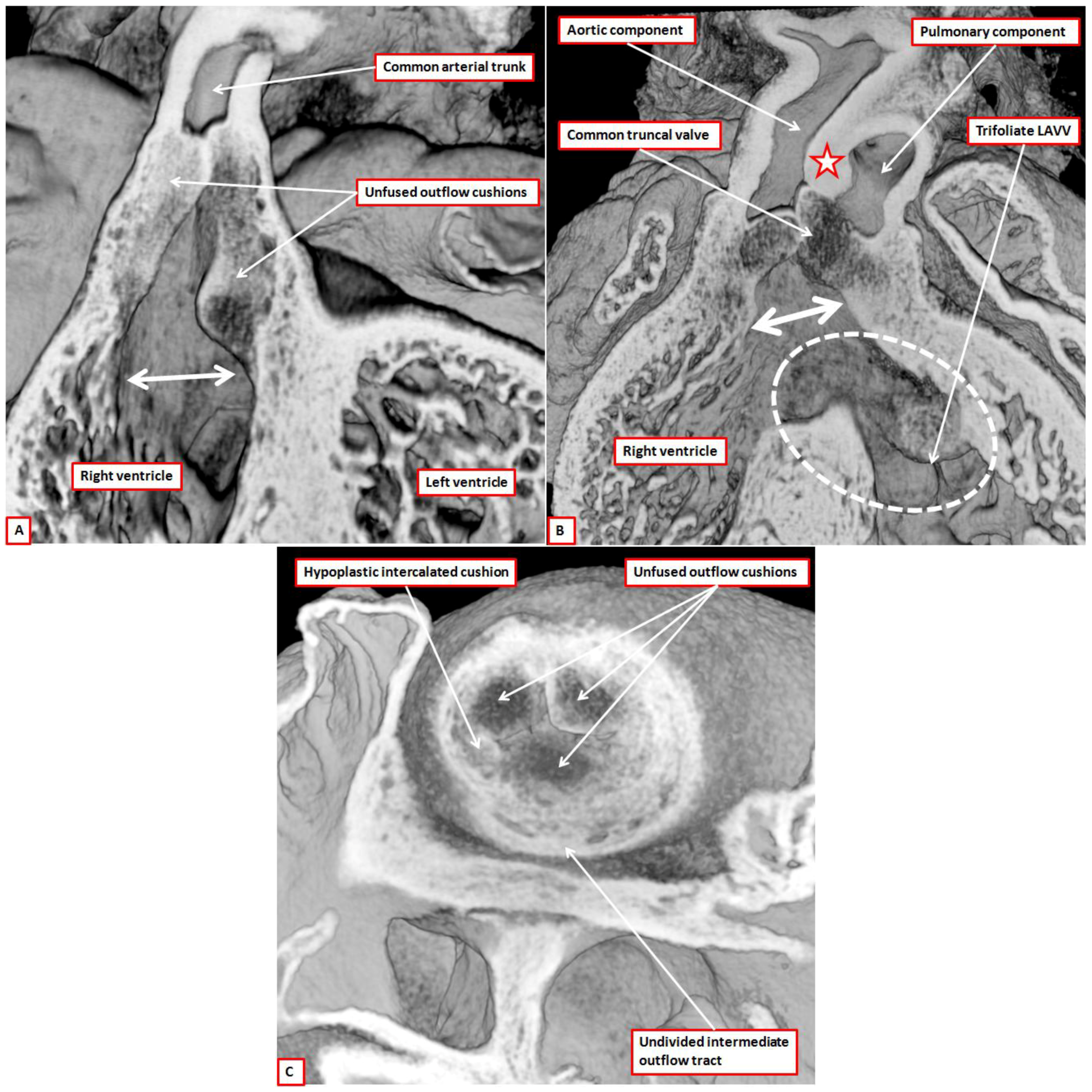
The other major malformation associated with abnormal arterial valves is common arterial trunk. This entity was long since shown to be the consequence of failure of fusion of the major outflow cushions [
29]. The images obtained from investigation of genetically modified mice, including those that are Tbx1 null, confirm that the major cushions have failed to fuse (
Figure 15A,B). They also show that the pulmonary intercalated cushion is hypoplastic when the truncal valve has only three leaflets (
Figure 15C), providing support in this regard for those who have previously investigated the Tbx1 null mouse [
30,
31,
32].
We include the images shown in
Figure 15 solely to demonstrate failure of fusion of the major cushions, and to support our notion that excavation of the distal margins of the cushions is responsible for formation of the leaflets. We make no claims regarding the mechanisms of abnormal development. In the clinical setting, it is well recognized that, although patients with common arterial trunk can be found with four valvar leaflets and sinuses, the commonest pattern is for the truncal valve to be trifoliate and trisinuate [
33]. This led some authorities to argue that the valve was no more than an aortic valve, the pulmonary component of the outflow tract having failed to form [
34]. The site of origin of the coronary arteries in the setting of common arterial trunk, however, is evidence against this concept [
33]. The developmental evidence, furthermore, initially produced by Van Mierop and colleagues [
29], provides strong evidence that it is failure of fusion of the major outflow cushions that is the major culprit in the morphogenesis of common trunk (
Figure 15A). Our ongoing studies in the Tbx1 knock-out mouse confirm in this regard the previous studies from the group working in Marseilles [
32], showing that the pulmonary intercalated cushion is often grossly hypoplastic, thus, setting the scene for formation of truncal valves with only three leaflets (
Figure 15C). The particular origin of the coronary arteries from the valvar sinuses, again initially described by the group from Marseilles [
32], also lends credence to the notion that the subpulmonary component of the outflow tract is often hypoplastic when there is common arterial trunk.
Figure 15.
The images are taken from episcopic datasets prepared from mice with, in panels (A) and (C), knock-out of the Tbx1 gene, and in panel (B), floxed perturbation of the furin enzyme. The images are shown solely to demonstrate the failure of fusion of the outflow cushions (Panels (A) and (B)), and the consequence for formation of the leaflets of the common truncal valve (Panel (C)). The hearts from the mice null for the Tbx1 gene were both obtained at E12.5. As is seen in panel A, the major cushions remain unfused thoughout the length of the outflow tract, with preservation of an extensive length of muscular inner heart curve (double headed white arrow). The undivided outflow tract is supported exclusively by the developing right ventricle. In the heart from the mouse with the perturbed furin enzyme, obtained at E14.5, there has been separation of the distal outflow tract by growth of the aortopulmonary septum (star), with balanced arrangement of the pulmonary and systemic components of the distal outflow. There remains a common muscular infundibulum, with lack of fusion of the proximal outflow cushions (double headed white arrow), but there is also a common atrioventricular junction (white dashed oval). Shunting across the atrioventricular septal defect, however, is exclusively at ventricular level. Note the trifoliate arrangement of the left ventricular component of the atrioventricular valve (LAVV). Panel (C) is a short axis section of the undivided outflow tract viewed from above in the Tbx1 null mouse. There are three major cushions, the putative pulmonary intercalated cushion being grossly hypoplastic.
Figure 15.
The images are taken from episcopic datasets prepared from mice with, in panels (A) and (C), knock-out of the Tbx1 gene, and in panel (B), floxed perturbation of the furin enzyme. The images are shown solely to demonstrate the failure of fusion of the outflow cushions (Panels (A) and (B)), and the consequence for formation of the leaflets of the common truncal valve (Panel (C)). The hearts from the mice null for the Tbx1 gene were both obtained at E12.5. As is seen in panel A, the major cushions remain unfused thoughout the length of the outflow tract, with preservation of an extensive length of muscular inner heart curve (double headed white arrow). The undivided outflow tract is supported exclusively by the developing right ventricle. In the heart from the mouse with the perturbed furin enzyme, obtained at E14.5, there has been separation of the distal outflow tract by growth of the aortopulmonary septum (star), with balanced arrangement of the pulmonary and systemic components of the distal outflow. There remains a common muscular infundibulum, with lack of fusion of the proximal outflow cushions (double headed white arrow), but there is also a common atrioventricular junction (white dashed oval). Shunting across the atrioventricular septal defect, however, is exclusively at ventricular level. Note the trifoliate arrangement of the left ventricular component of the atrioventricular valve (LAVV). Panel (C) is a short axis section of the undivided outflow tract viewed from above in the Tbx1 null mouse. There are three major cushions, the putative pulmonary intercalated cushion being grossly hypoplastic.
![Jcdd 01 00177 g015]()