Use of Clinical Video Telehealth as a Tool for Optimizing Medications for Rural Older Veterans with Dementia
Abstract
:1. Introduction
2. Materials and Methods
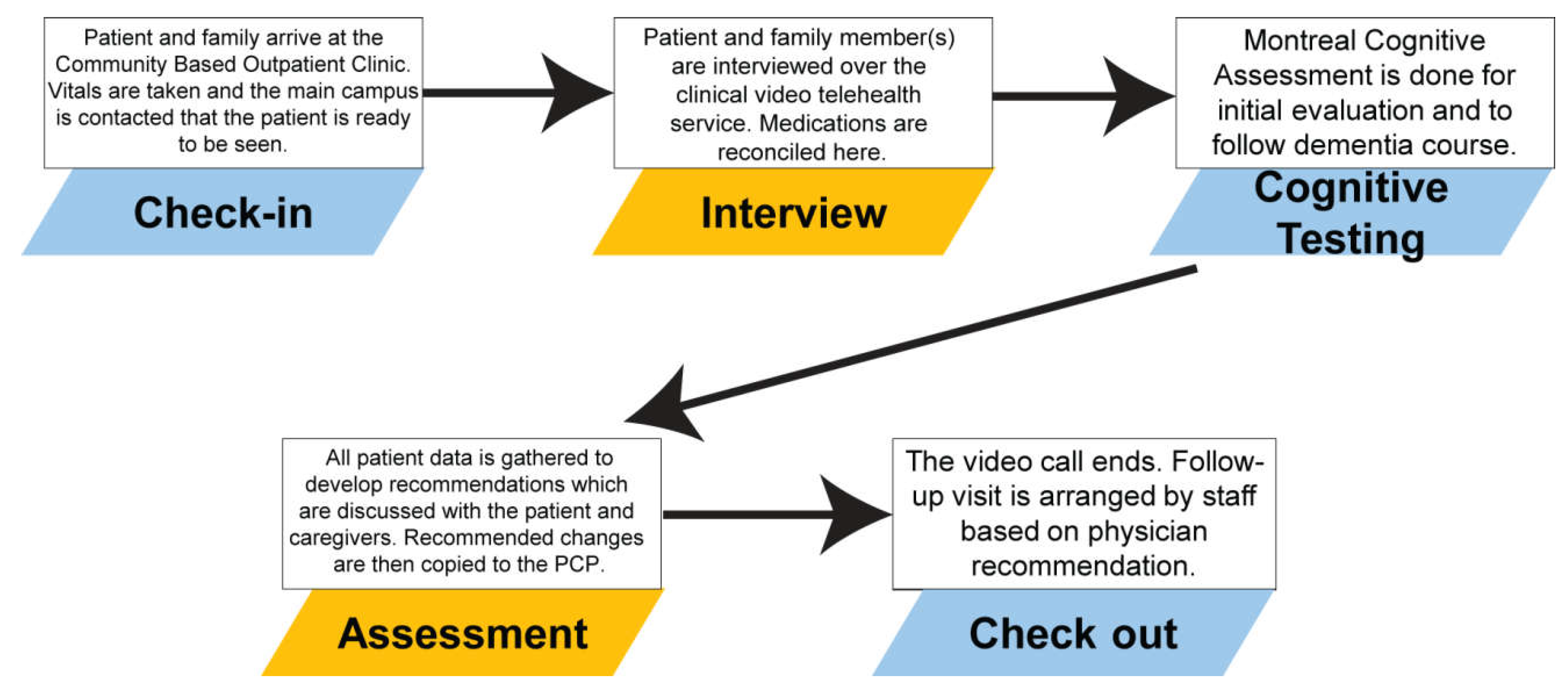
2.1. Teledementia Consult Service
2.2. Teledementia Database
2.3. Study Characteristics
3. Results
3.1. Population Characteristics
3.2. Medication Changes Overall
3.3. Beers Criteria Medications
4. Discussion
4.1. Limitations
4.2. Future Directions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Medication Changes by Type | Initial Consult | Follow-Up Visit | p Value |
|---|---|---|---|
| Type of medication added per encounter | |||
| Anti-dementia | 0.177 | 0.101 | 0.1583 |
| Antidepressants | 0.185 | 0.203 | 0.7703 |
| Pain | 0.054 | 0.000 | 0.0501 |
| Antipsychotics | 0.038 | 0.014 | 0.3492 |
| Anticholinergics | 0.008 | 0.000 | 0.4677 |
| Supplements | 0.200 | 0.101 | 0.0974 |
| Type of medication stopped per encounter | |||
| Anti-dementia | 0.069 | 0.043 | 0.5057 |
| Antidepressants | 0.077 | 0.072 | 0.9209 |
| Pain | 0.031 | 0.000 | 0.1409 |
| Antipsychotics | 0.000 | 0.000 | N/A |
| Anticholinergics | 0.077 | 0.043 | 0.4023 |
| Supplements | 0.092 | 0.101 | 0.8813 |
| Type of medication modified per encounter | |||
| Anti-dementia | 0.285 | 0.203 | 0.2943 |
| Antidepressants | 0.362 | 0.420 | 0.5531 |
| Pain | 0.092 | 0.014 | 0.0844 |
| Antipsychotics | 0.100 | 0.029 | 0.0715 |
| Anticholinergics | 0.085 | 0.043 | 0.3171 |
| Supplements | 0.300 | 0.203 | 0.2770 |
References
- Mohr, N.M.; Young, T.; Harland, K.K.; Skow, B.; Wittrock, A.; Bell, A.; Ward, M.M. Emergency Department Telemedicine Shortens Rural Time-to-Provider and Emergency Department Transfer Times. Telemed. J. E Health 2018. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.T.; Halterman, J.S.; Brown, R.H.; Luo, C.; Randle, S.M.; Hunter, C.R.; Rettiganti, M. Results of an asthma education program delivered via telemedicine in rural schools. Ann. Allergy Asthma Immunol. 2018, 120, 401–408. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: Dementia Fact Sheets; WHO: Geneva, Switzerland, 12 December 2017; Available online: http://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 10 July 2018).
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Jonsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Jonsson, L.; Bond, J.; Prince, M.; Winblad, B.; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013, 9, 1–11.e3. [Google Scholar] [CrossRef] [PubMed]
- Leelakanok, N.; D’Cunha, R.R. Association between polypharmacy and dementia—A systematic review and meta-analysis. Aging Ment. Health 2018. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, R.U.; Norgaard, A.; Jensen-Dahm, C.; Gasse, C.; Wimberley, T.; Waldemar, G. Polypharmacy and Potentially Inappropriate Medication in People with Dementia: A Nationwide Study. J. Alzheimers Dis. 2018, 63, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, S.; Sato, M.; Maeno, T.; Ichinohe, Y. Potentially inappropriate medications with polypharmacy increase the risk of falls in older Japanese patients: 1-year prospective cohort study. Geriatr. Gerontol. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Price, S.D.; Holman, C.D.; Sanfilippo, F.M.; Emery, J.D. Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann. Pharmacother. 2014, 48, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, T.A.; Wallace, A.E.; Weeks, W.B. Impact of rural residence on survival of male veterans affairs patients after age 65. J. Rural Health 2010, 26, 318–324. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Comparative Demographic Estimates: 2013 American Community Survey 1-Year Estimates. Suitland, MD, USA, 2013. Available online: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk (accessed on 2 July 2017).
- Section for Enhancing Geriatric Understanding and Expertise Among Surgical and Medical Specialists (SEGUE), American Geriatrics Society. Retooling for an Aging America: Building the Healthcare Workforce. A white paper regarding implementation of recommendation 4.2 of this Institute of Medicine Report of April 14, 2008, that “All licensure, certification and maintenance of certification for healthcare professionals should include demonstration of competence in care of older adults as a criterion.”. J. Am. Geriatr. Soc. 2011, 59, 1537–1539. [Google Scholar]
- Martin-Khan, M.; Flicker, L.; Wootton, R.; Loh, P.K.; Edwards, H.; Varghese, P.; Byrne, G.J.; Klein, K.; Gray, L.C. The diagnostic accuracy of telegeriatrics for the diagnosis of dementia via video conferencing. J. Am. Med. Dir. Assoc. 2012, 13, 487.e19–487.e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, S.; Gomez-Orozco, C.A.; van Zuilen, M.H.; Levis, S. Providing Dementia Consultations to Veterans Using Clinical Video Telehealth: Results from a Clinical Demonstration Project. Telemed. J. E Health 2018, 24, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, A.E.; Echt, K.V.; Kemp, L.; McGwin, G.; Perkins, M.M.; Mirk, A.K. Academic Detailing with Provider Audit and Feedback Improve Prescribing Quality for Older Veterans. J. Am. Geriatr. Soc. 2018, 66, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Steve, T.A.; Kirk, A.; Crossley, M.; Morgan, D.; D’Arcy, C.; Biem, J.; Forbes, D.; Stewart, N. Medication use in patients presenting to a rural and remote memory clinic. Can. J. Neurol. Sci. 2008, 35, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Verity, R.; Kirk, A.; Morgan, D.; Karunanayake, C. Trends in Medication Use Over 11 Years in Patients Presenting to a Rural and Remote Memory Clinic. Can. J. Neurol. Sci. 2016, 43, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Van Ast, P.; Larson, A. Supporting rural carers through telehealth. Rural Remote Health 2007, 7, 634. [Google Scholar] [PubMed]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, M.A.; van Marum, R.J.; Egberts, A.C.; Jansen, P.A. Relationship between polypharmacy and underprescribing. Br. J. Clin. Pharmacol. 2008, 65, 130–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, B.B.; Homer, M.C.; Morone, N.; Edmonds, N.; Rossi, M.I. Creation of an Interprofessional Teledementia Clinic for Rural Veterans: Preliminary Data. J. Am. Geriatr. Soc. 2017, 65, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Hedeen, A.N.; Heagerty, P.J.; Fortney, J.C.; Borowsky, S.J.; Walder, D.J.; Chapko, M.K. VA community-based outpatient clinics: Quality of care performance measures. Med. Care 2002, 40, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2227–2246. [Google Scholar] [Green Version]
- Cheong, C.K.; Lim, K.H.; Jang, J.W.; Jhoo, J.H. The effect of telemedicine on the duration of treatment in dementia patients. J. Telemed. Telecare 2015, 21, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jhoo, J.H.; Jang, J.W. The effect of telemedicine on cognitive decline in patients with dementia. J. Telemed. Telecare 2017, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bryant, L.; Garnham, B.; Tedmanson, D.; Diamandi, S. Tele-social work and mental health in rural and remote communities in Australia. Int. Soc. Work 2018, 61, 143–155. [Google Scholar] [CrossRef]
- Burton, R.L.; O’Connell, M.E. Telehealth Rehabilitation for Cognitive Impairment: Randomized Controlled Feasibility Trial. JMIR Res. Protoc. 2018, 7, e43. [Google Scholar] [CrossRef] [PubMed]
- Durrani, H.; Khoja, S. A systematic review of the use of telehealth in Asian countries. J. Telemed. Telecare 2009, 15, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sarfo, F.S.; Adamu, S.; Awuah, D.; Ovbiagele, B. Tele-neurology in sub-Saharan Africa: A systematic review of the literature. J. Neurol. Sci. 2017, 380, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.H.; Jhoo, J.H.; Lee, K.U.; Kim, K.W.; Lee, D.Y.; Woo, J.I. A telemedicine system as a care modality for dementia patients in Korea. Alzheimer Dis. Assoc. Disord. 2000, 14, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on the Future Health Care Workforce for Older Americans. Retooling for an Aging America: Building the Health Care Workforce; National Academies Press: Washington, DC, USA, 2008.
| Characteristics | Initial Consult | Follow-Up Visit | p Value |
|---|---|---|---|
| Gender, n (%) | N/A | ||
| Male | 130 (100) | 69 (100) | |
| Race, n (%) | 0.952 | ||
| Caucasian | 122 (93.9) | 64 (92.8) | |
| Black | 5 (3.8) | 3 (4.3) | |
| Declined to state | 3 (2.3) | 2 (2.9) | |
| Ethnicity, n (%) | 0.089 | ||
| Hispanic | 1 (0.8) | 0 (0) | |
| Not hispanic | 127 (97.7) | 65 (92.8) | |
| Declined to state | 2 (1.5) | 5 (7.2) | |
| Seen by geriatric psychiatry, n (%) | 0.0002 ** | ||
| Yes | 65 (50) | 53 (76.8) | |
| No | 65 (50) | 16 (23.2) |
| Characteristics | Initial Consult | Follow-Up Visit | Combined |
|---|---|---|---|
| CVT Encounters | 130 | 69 | 199 |
| Overall medication events | 230 | 78 | 308 |
| Total medication add events, n (%) | 95 (41.3) | 30 (38.5) | 125 (40.6) |
| Total medication stop events, n (%) | 89 (38.7) | 28 (35.9) | 117 (38.0) |
| Total medication modification events, n (%) | 46 (20.0) | 20 (25.6) | 66 (21.4) |
| Type of Medication Change Per Encounter | Initial Consult (N = 130) | Follow-Up Visit (N = 69) | p Value |
|---|---|---|---|
| Medications added | 0.731 | 0.435 | 0.0009 ** |
| Medications stopped | 0.685 | 0.406 | 0.0704 |
| Medications modified | 0.354 | 0.290 | 0.4653 |
| Total medication changes | 1.769 | 1.130 | 0.0079 ** |
| Net medications changed | 0.046 | 0.029 | 0.9158 |
| Type of Medication Change | Initial Consult (N = 130) | Follow-Up Visit (N = 69) | p Value |
|---|---|---|---|
| 2015 Beers Criteria Table 1 PIMs | |||
| Medications stopped (per encounter) | 0.208 | 0.001 | 0.025 * |
| Medications modified (per encounter) | 0.230 | 0.029 | 0.001 ** |
| All 2015 Beers Criteria medications | |||
| Medications stopped (per encounter) | 0.323 | 0.150 | 0.044 * |
| Medications modified (per encounter) | 0.338 | 0.202 | 0.123 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.; Homer, M.; Rossi, M.I. Use of Clinical Video Telehealth as a Tool for Optimizing Medications for Rural Older Veterans with Dementia. Geriatrics 2018, 3, 44. https://doi.org/10.3390/geriatrics3030044
Chang W, Homer M, Rossi MI. Use of Clinical Video Telehealth as a Tool for Optimizing Medications for Rural Older Veterans with Dementia. Geriatrics. 2018; 3(3):44. https://doi.org/10.3390/geriatrics3030044
Chicago/Turabian StyleChang, Woody, Marcia Homer, and Michelle I. Rossi. 2018. "Use of Clinical Video Telehealth as a Tool for Optimizing Medications for Rural Older Veterans with Dementia" Geriatrics 3, no. 3: 44. https://doi.org/10.3390/geriatrics3030044




