Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan
Abstract
:1. Introduction
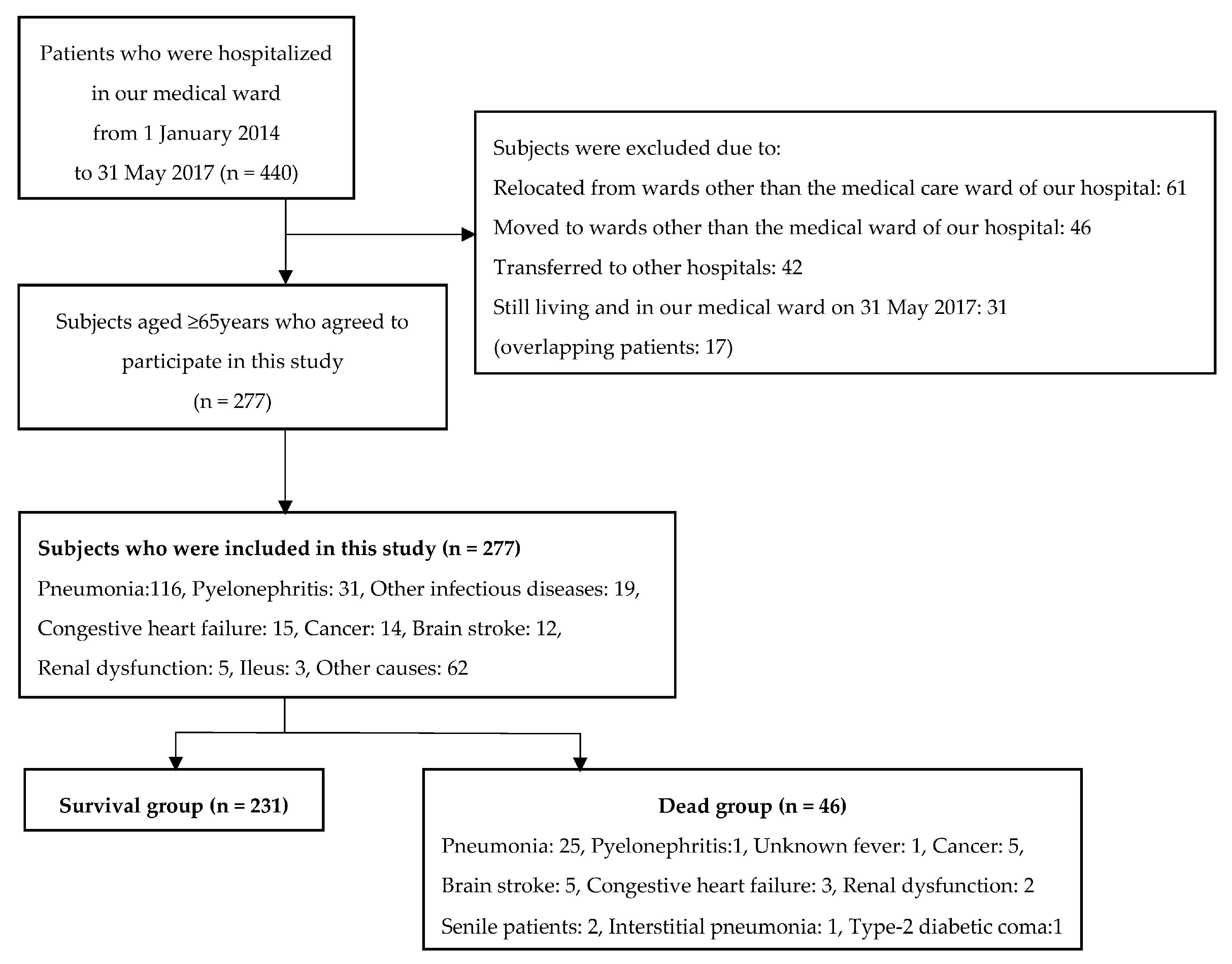
2. Materials and Methods
3. Results
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Teno, J.M.; Harrell, F.E., Jr.; Knaus, W.; Phillips, R.S.; Wu, A.W.; Connors, A., Jr.; Wenger, N.S.; Wagner, D.; Galanos, A.; Desbiens, N.A.; et al. Prediction of survival for older hospitalized patients: The HELP survival model. Hospitalized Elderly Longitudinal Project. J. Am. Geriatr. Soc. 2000, 48 (Suppl. S5), S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Stuck, A.E.; Siu, A.L.; Wieland, G.D.; Adams, J.; Rubenstein, L.Z. Comprehensive geriatric assessment: A meta-analysis of controlled trials. Lancet 1993, 342, 1032–1036. [Google Scholar] [CrossRef]
- Abbatecola, A.M.; Spazzafumo, L.; Corsonello, A.; Sirolla, C.; Bustacchini, S.; Guffanti, E. Development and validation of the HOPE prognostic index on 24-month posthospital mortality and rehospitalization: Italian National Research Center on Aging (INRCA). Rejuv. Res. 2011, 14, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Espaulella, J.; Arnau, A.; Cubí, D.; Amblàs, J.; Yánez, A. Time-dependent prognostic factors of 6-month mortality in frail elderly patients admitted to post-acute care. Age Ageing 2007, 36, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ferrucci, L.; Franceschi, M.; D’Ambrosio, L.P.; Scarcelli, C.; Cascavilla, L.; Paris, F.; Placentino, G.; Seripa, D.; Dallapiccola, B.; et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuv. Res. 2008, 11, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Murakami, T.; Kurumadani, H.; Shimizu, J.; Fujiwara, N. Relationship between criteria for evaluating the degree of independence of disabled elderly persons in performing activities of daily living. Sagyou Ryouhou 2002, 21, 455–462. (In Japanese) [Google Scholar]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [PubMed]
- Kokura, Y.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Higashi, S. High nutritional-related risk on admission predicts less improvement of functional independence measure in geriatric Stroke Patients: A retrospective cohort study. J. Stroke Cerebrovasc. Dis. 2016, 25, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Narumi, T.; Arimoto, T.; Funayama, A.; Kadowaki, S.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013, 62, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hak, E.; Bont, J.; Hoes, A.W.; Verheij, T.J. Prognostic factors for serious morbidity and mortality from community-acquired lower respiratory tract infections among the elderly in primary care. Fam. Pract. 2005, 22, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Ghaderpanahi, M.; Fakhrzadeh, H.; Mirarefin, M.; Badamchizadeh, Z.; Tajalizadekhoob, Y.; Fadayivatan, R.; Philp, I.; Larijani, B. Older people’s mortality index: Development of a practical model for prediction of mortality in nursing homes (Kahrizak Elderly Study). Geriatr. Gerontol. Int. 2012, 12, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, X.; Chen, J.; Wang, H.; Zhang, L.; Dong, B.; Yang, M. Predicting long-term mortality in hospitalized elderly patients using the new ESPEN definition. Sci. Rep. 2017, 7, 4067. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Hertan, H.; Pitchumoni, C.S. Hypoalbuminemia is a poor predictor of survival after percutaneous endoscopic gastrostomy in elderly patients with dementia. Am. J. Gastroenterol. 2000, 95, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Gom, I.; Fukushima, H.; Shiraki, M.; Miwa, Y.; Ando, T.; Takai, K.; Moriwaki, H. Relationship between serum albumin level and aging in community-dwelling self-supported elderly population. J. Nutr. Sci. Vitaminol. Tokyo 2007, 53, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hayakawa, T.; Hozawa, A.; Kadowaki, T.; Murakami, Y.; Kita, Y.; Abbott, R.D.; Okayama, A.; Ueshima, H.; NIPPON DATA80 Research Group. Lower levels of serum albumin and total cholesterol associated with decline in activities of daily living and excess mortality in a 12-year cohort study of elderly Japanese. J. Am. Geriatr. Soc. 2008, 56, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Avelino-Silva, T.J.; Farfel, J.M.; Curiati, J.A.; Amaral, J.R.; Campora, F.; Jacob-Filho, W. Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014, 14, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.M.; Tang, W.H.; Woo, J. Predictors of in-hospital mortality of older patients admitted for community-acquired pneumonia. Age Ageing 2011, 40, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Rosolem, M.M.; Rabello, L.S.; Lisboa, T.; Caruso, P.; Costa, R.T.; Leal, J.V.; Salluh, J.I.; Soares, M. Critically ill patients with cancer and sepsis: Clinical course and prognostic factors. J. Crit. Care 2012, 27, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Nakajo, T.; Okada, S.; Omura, K.; Adachi, K. Evaluation of effects of nutrition intervention on healing of pressure ulcers and nutritional states (randomized controlled trial). Wound Repair Regen. 2011, 19, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Pearte, C.A.; Furberg, C.D.; O’Meara, E.S.; Psaty, B.M.; Kuller, L.; Powe, N.R.; Manolio, T. Characteristics and baseline clinical predictors of future fatal versus nonfatal coronary heart disease events in older adults: The cardiovascular health study. Circulation 2006, 113, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Kim, J.S.; Kwon, S.U.; Yun, S.C.; Koh, J.Y.; Kang, D.W. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch. Neurol. 2008, 65, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Ticinesi, A.; Lauretani, F.; Maggio, M.; Lippi, G.; Prati, B.; Borghi, L.; Meschi, T. The prognostic value of high-sensitivity C-reactive protein and prealbumin for short-term mortality in acutely hospitalized multimorbid elderly patients: A prospective cohort study. J. Nutr. Health Aging 2016, 20, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.C. Fever in the elderly. Clin. Infect. Dis. 2000, 31, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.T. Epidemiology and unique aspects of aging and infectious disease. Clin. Infect. Dis. 2000, 30, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Dewan, S.K.; Zheng, S.B.; Xia, S.J.; Bill, K. Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin. Med. J. Engl. 2012, 125, 3325–3331. [Google Scholar] [PubMed]
- Zalacain, R.; Torres, A.; Celis, R.; Blanquer, J.; Aspa, J.; Esteban, L.; Menéndez, R.; Blanquer, R.; Borderías, L. Commmity-acquired pneumonia in the elderly: Spanish multicentre study. Eur. Respir. J. 2003, 21, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Miller, S.C.; Teno, J.M.; Kiely, D.K.; Davis, R.B.; Shaffer, M.L. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA 2010, 304, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
| Self-supported | Rank J | Some disabilities, but daily living is mostly independent; capable of going outdoors unassisted. |
| 1 | Goes outdoors with means of transportation, etc. | |
| 2 | Goes out near home. | |
| Quasi-bedridden | Rank A | Indoor living predominantly independent, but unable to go out without assistance. |
| 1 | Goes out with assistance, spending most of the time during the daytime out of bed. | |
| 2 | Does not go out frequently, repeating cycles of lying down on and getting up from the bed during the daytime. | |
| Bedridden | Rank B | Some assistance needed for indoor living, also lies in bed for much of the daytime, although sitting position is possible. |
| 1 | Uses a wheelchair without assistance, takes meals, and excretes/urinates off the bed. | |
| 2 | Uses a wheelchair with assistance. | |
| Rank C | Bedridden all day, requires assistance with excretion/urination, meals, and dressing/undressing. | |
| 1 | Capable of changing posture in bed. | |
| 2 | Unable to change posture in bed without assistance. |
| Rank I | Has some type of dementia, but almost independent in terms of daily living at home and in society. |
| Rank II | Some daily life-disturbing symptoms, behaviors and problems in communication seen but can lead daily life independently if kept watched by someone. |
| IIa | Condition II, mentioned above, seen outside home. |
| IIb | Condition II, mentioned above, seen at home. |
| Rank III | Daily life-disturbing symptoms, behaviors, and problems in communication that require assistance. |
| IIIa | Condition III, mentioned above, seen primarily during the daytime. |
| IIIb | Condition III, mentioned above, seen primarily at night. |
| Rank IV | Daily life-disturbing symptoms, behaviors, and problems in communication frequently require assistance. |
| Rank M | Marked psychiatric symptoms/related symptoms or serious physical disorders require expert management. |
| Variable | Survived (n = 231) | Died (n = 46) | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Sex | 0.878 | 0.443–1.753 | 0.745 | ||
| Male | 98 | 21 | |||
| Female | 133 | 25 | |||
| Hospitalization source | |||||
| Home | 28 | 4 | |||
| nursing home | 150 | 26 | |||
| other hospital | 53 | 16 | |||
| Independence degree of daily living for the disabled elderly | 0.200 | ||||
| JA | 12 | 0 | |||
| B | 73 | 4 | |||
| C | 146 | 42 | |||
| Independence degree of daily living for the demented elderly | 0.029 * | ||||
| I | 39 | 0 | |||
| II | 38 | 3 | |||
| III | 73 | 9 | |||
| IV/M | 81 | 34 | |||
| History of bone fracture | 97 | 17 | 0.810 | 0.394–1.624 | 0.623 |
| History of brain stroke | 117 | 15 | 0.473 | 0.224–0.959 | 0.0349 * |
| History of heart failure | 80 | 20 | 1.450 | 0.719–2.891 | 0.313 |
| History of malignant disease | 46 | 14 | 1.755 | 0.798–3.721 | 0.120 |
| Intake of steroid | 15 | 3 | 1.005 | 0.179–3.775 | 1.000 |
| Intake of sleeping pill | 57 | 6 | 0.459 | 0.151–1.168 | 0.122 |
| Intake of psychotropic drug | 40 | 11 | 1.449 | 0.632–3.342 | 0.301 |
| Intake of dementia treatment drug | 42 | 5 | 0.550 | 0.160–1.551 | 0.285 |
| Variable | Survived (n = 231) | Died (n = 46) | p-Value |
|---|---|---|---|
| Age (y.o.) | 84.1 ± 8.6 | 88.8 ± 6.3 | 0.0001 * |
| Height (cm) | 150.5 ± 10.2 | 148.1 ± 9.4 | 0.180 |
| Weight (kg) | 46.1 ± 9.6 | 42.0 ± 9.7 | 0.021 * |
| BMI (kg/m2) | 20.4 ± 3.8 | 19.1 ± 3.8 | 0.048 * |
| The highest body temperature (°C) | 37.4 ± 0.9 | 37.1 ± 0.8 | 0.038 * |
| Systolic blood pressure (mmHg) | 133.3 ± 22.5 | 135.0 ± 23.5 | 0.718 |
| Heart rate (beats/min) | 81.0 ± 14.9 | 83.5 ± 21.7 | 0.607 |
| Respiratory rate (times/min) | 20.1 ± 6.5 | 21.5 ± 7.3 | 0.082 |
| SpO2 (%) | 93.2 ± 7.2 | 91.4 ± 5.0 | 0.002 * |
| Oxygen flow (L/min) | 0.5 ± 1.1 | 1.5 ± 2.3 | 0.001 * |
| White blood Cell (×103/μL) | 8.6 ± 4.1 | 9.0 ± 4.2 | 0.445 |
| Hematocrit (%) | 33.8 ± 5.0 | 33.1 ± 5.0 | 0.282 |
| Hemoglobin (g/dL) | 11.5 ± 1.8 | 11.1 ± 1.7 | 0.235 |
| Platelet (×104/μL) | 24.8 ± 12.6 | 22.5 ± 11.0 | 0.317 |
| C-reactive protein (mg/dL) | 4.2 ± 4.9 | 4.6 ± 5.2 | 0.217 |
| Blood urea nitrogen (mg/dL) | 19.3 ± 11.1 | 33.7 ± 34.7 | 0.012 * |
| Creatinine (mg/dL) | 0.89 ± 0.54 | 1.24 ± 1.03 | 0.010 * |
| Serum sodium (mEq/L) | 136.9 ± 5.1 | 135.7 ± 7.2 | 0.019 * |
| Serum potassium (mEq/L) | 4.1 ± 0.7 | 4.3 ± 0.9 | 0.263 |
| Total protein (g/dL) | 6.5 ± 0.8 | 6.4 ± 0.8 | 0.555 |
| Serum albumin (g/dL) | 3.2 ± 0.6 | 2.9 ± 0.6 | 0.001 * |
| GNRI | 84.5 ± 9.7 | 77.9 ± 10.5 | 0.0003 * |
| CONUT | 4.8 ± 2.6 | 6.2 ± 2.6 | 0.002 * |
| Cholinesterase (IU/L) | 190.4 ± 63.9 | 152.8 ± 60.7 | 0.0002 * |
| Total cholesterol (mg/dL) | 161.4 ± 43.8 | 150.4 ± 51.3 | 0.085 |
| Blood glucose (mg/dL) | 128.5 ± 47.5 | 163.6 ± 203.4 | 0.370 |
| HbA1c (NGSP) (%) | 6.5 ± 10.3 | 5.9 ± 1.3 | 0.976 |
| Variable | Odds Ratio (Adjusted) | 95% Confidence Interval (Adjusted) | p-Value |
|---|---|---|---|
| Age (y.o.) | 1.09 (1.07) | 1.04–1.15 (1.00–1.14) | 0.027 |
| Blood urea nitrogen (mg/dL) | 1.04 (1.04) | 1.02–1.06 (1.02–1.07) | <0.001 |
| Serum albumin (g/dL) | 0.37 (0.39) | 0.21–0.66 (0.19–0.78) | 0.006 |
| Independence degree of daily living for the demented elderly | 3.06 (2.76) | 1.85–5.08 (1.63–4.68) | <0.001 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, S. Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan. Geriatrics 2017, 2, 32. https://doi.org/10.3390/geriatrics2040032
Abe S. Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan. Geriatrics. 2017; 2(4):32. https://doi.org/10.3390/geriatrics2040032
Chicago/Turabian StyleAbe, Shuichi. 2017. "Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan" Geriatrics 2, no. 4: 32. https://doi.org/10.3390/geriatrics2040032





