Oral Plasmacytoma in a Dog
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Radiologic Findings
3.2. CBC Results
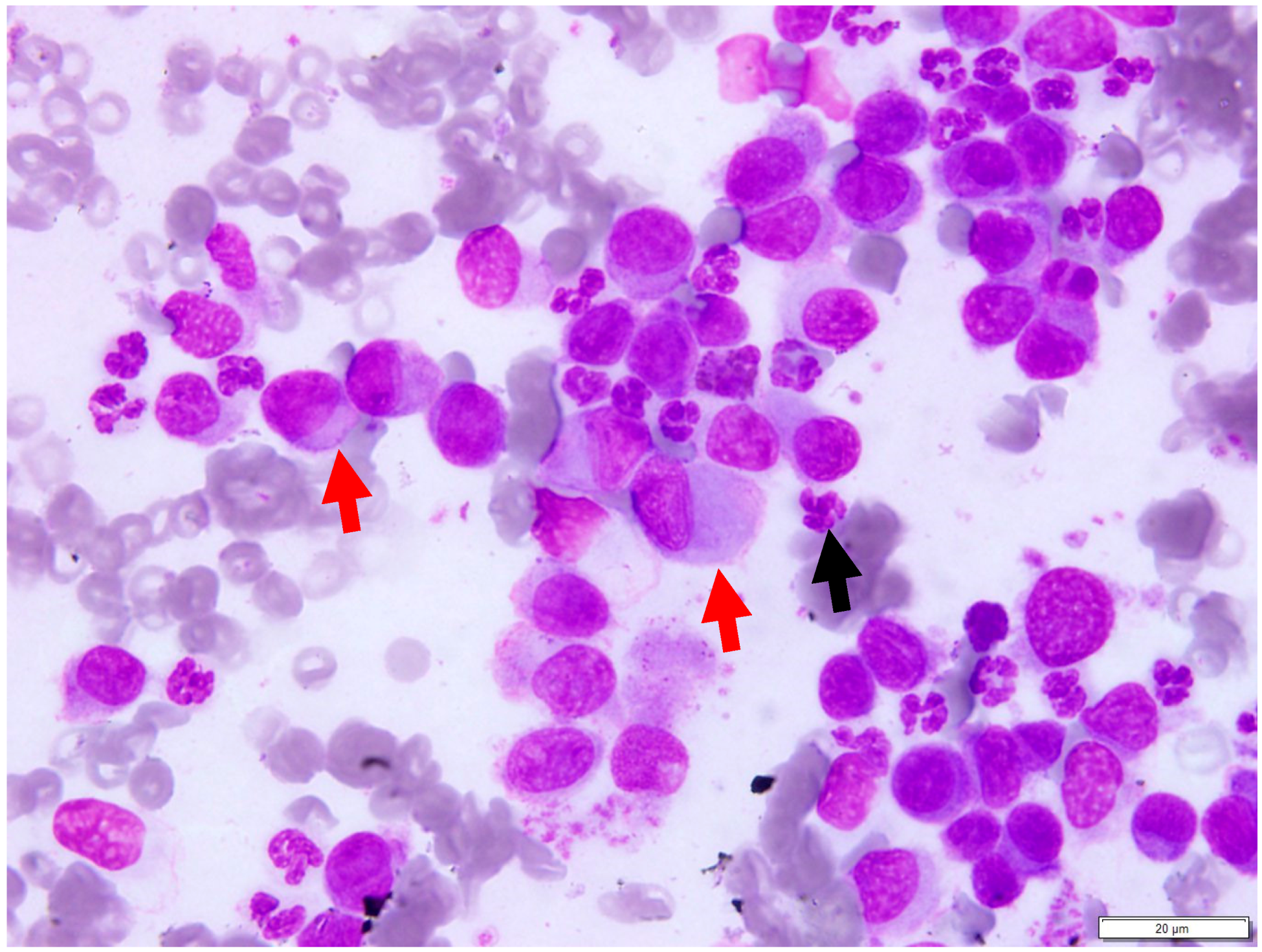
3.3. Cytologic Findings
3.4. Histopathologic Findings
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Ettinger, S.; Feldman, E. Textbook of Veterinary Internal Medicine, 7th ed.; Saunders Elsevier: St Louis, MO, USA, 2010. [Google Scholar]
- Borgatti, A. Plasma Cell Tumors, 6th ed.; Blackwell Publishing Ltd.: Ames, IA, USA, 2010; pp. 516–518. [Google Scholar]
- Kupanoff, P.A.; Popovitch, C.A.; Goldschmidt, M.H. Colorectal plasmacytomas: A retrospective study of nine dogs. J. Am. Anim. Hosp. Assoc. 2006, 42, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Rakich, P.M.; Latimer, K.S.; Weiss, R.; Steffens, W.L. Mucocutaneous plasmacytomas in dogs: 75 cases (1980–1987). J. Am. Vet. Med. Assoc. 1989, 194, 803–810. [Google Scholar] [PubMed]
- Clark, G.N.; Berg, J.; Engler, S.J.; Bronson, R.T. Extramedullary plasmacytomas in dogs: Results of surgical excision in 131 cases. J. Am. Anim. Hosp. Assoc. 1992, 28, 106–111. [Google Scholar]
- Wright, Z.M.; Rogers, K.S.; Mansell, J. Survival data for canine oral extramedullary plasmacytomas: A retrospective analysis (1996–2006). J. Am. Anim. Hosp. Assoc. 2008, 44, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Nofsinger, Y.C.; Mirza, N.; Rowan, P.T.; Lanza, D.; Weinstein, G. Head and neck manifestations of plasma cell neoplasms. Laryngoscope 1997, 107, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.G.; Dittmer, K.E. Tumors of Bone. In Tumors of Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Wiley Blackwell: Ames, IA, USA, 2016; p. 412. [Google Scholar]
- Schrenzel, M.D.; Naydan, D.K.; Moore, P.F. Leukocyte differentiation antigens in canine cutaneous and oral plasmacytomas. Vet. Dermatol. 1998, 9, 33–41. [Google Scholar] [CrossRef]
- Raskin, R.E.; Meyer, D. Canine and Feline Cytology: A Colour Atlas and Interpretation, 2nd ed.; Saunders Elsevier: St. Louis, MO, USA, 2010. [Google Scholar]
- Withrow, S.J.; Vail, D. Withrow & MacEwen’s Small Animal Clinical Oncology, 4th ed.; Saunders Elsevier: St. Louis, MO, USA, 2001. [Google Scholar]
- Platz, S.J.; Breuer, W.; Pfleghaar, S.; Minkus, G.; Hermanns, W. Prognostic value of histopathological grading in canine extramedullary plasmacytomas. Vet. Pathol. 1999, 36, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Cangul, I.T.; Wijnen, M.; Van Garderen, E.; van den Ingh, T.S. Clinico-pathological aspects of canine cutaneous and mucocutaneous plasmacytomas. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.J.; West, K.H.; Jackson, M.L.; Kidney, B.A. Immunohistochemical and pathology. histochemical stains for differentiating canine cutaneous round cell tumors. Veterinary 2005, 42, 437–445. [Google Scholar]
- Ramos-Vara, J.A.; Miller, M.A.; Valli, V.E. Immunohistochemical detection of multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine plasmacytoma: Comparison with CD79a and CD20. Vet. Pathol. 2007, 44, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.L.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis; Blackwell Science: Oxford, UK, 2005. [Google Scholar]
- Ramos-Vara, J.A.; Miller, M.A.; Pace, L.W.; Linke, R.P.; Common, R.S.; Watson, G.L. Intestinal multinodular A lambda-amyloid deposition associated with extramedullary plasmacytoma in three dogs: Clinicopathological and immunohistochemical studies. J. Comp. Pathol. 1998, 119, 239–249. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Mehdipour, M.; Rohani, B.; Esmaeili, V. Extramedullary Plasmacytoma of the Oral Cavity in a Young Man: A Case Report. J. Dent. (Shiraz) 2016, 17, 155–158. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pargass, I.; Bally, A.; Suepaul, R. Oral Plasmacytoma in a Dog. Vet. Sci. 2017, 4, 68. https://doi.org/10.3390/vetsci4040068
Pargass I, Bally A, Suepaul R. Oral Plasmacytoma in a Dog. Veterinary Sciences. 2017; 4(4):68. https://doi.org/10.3390/vetsci4040068
Chicago/Turabian StylePargass, Indira, Alissa Bally, and Rod Suepaul. 2017. "Oral Plasmacytoma in a Dog" Veterinary Sciences 4, no. 4: 68. https://doi.org/10.3390/vetsci4040068





