Exercise-Induced Cardiac Remodeling: Lessons from Humans, Horses, and Dogs
Abstract
:1. Introduction
1.1. Acute Exercise and Cardiac Loading
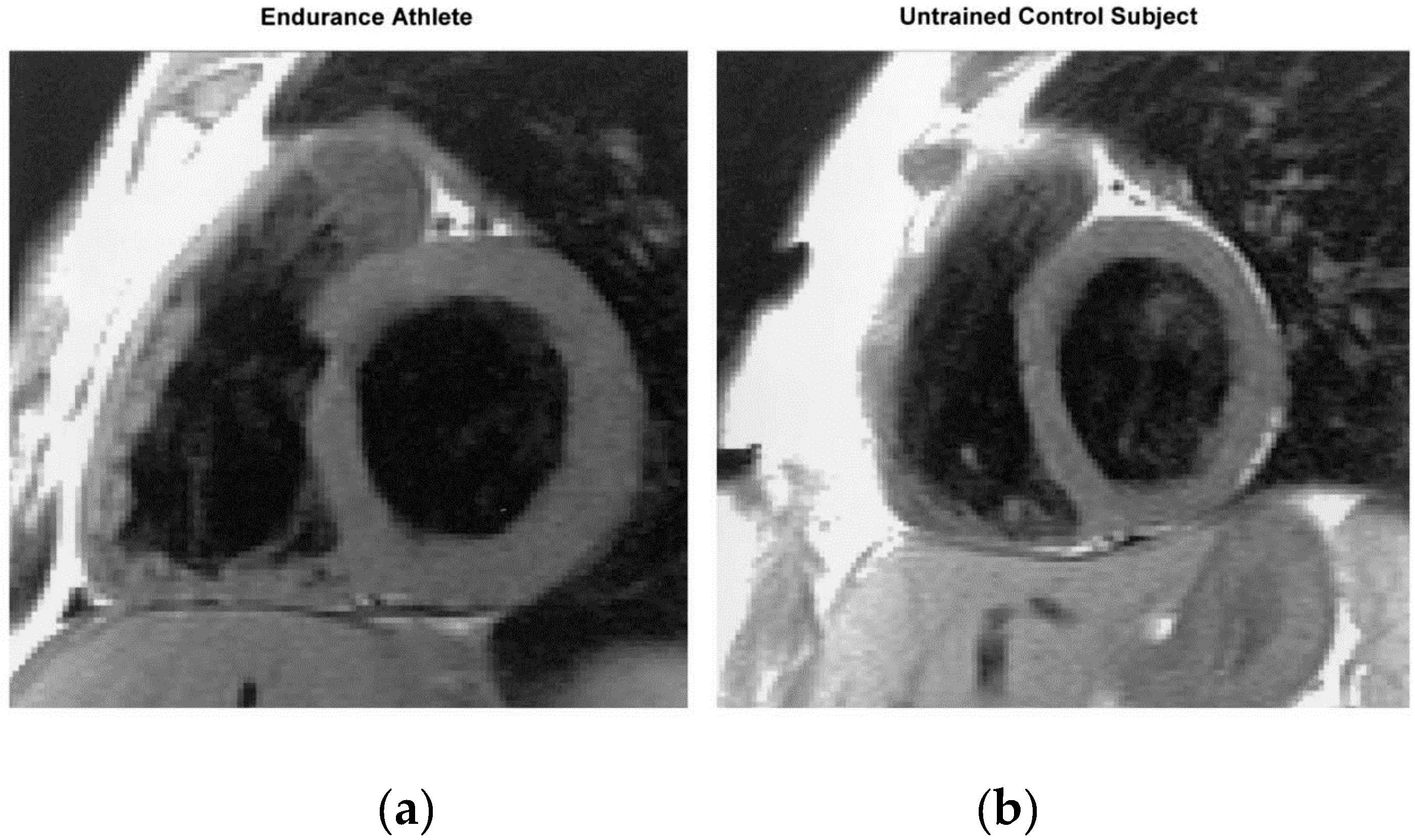
1.2. Athlete’s Heart: Evidence from Humans
1.3. Cardiac Adaptations to Exercise Training beyond the Left Ventricle
1.4. Cardiac Mechanics
2. Summary
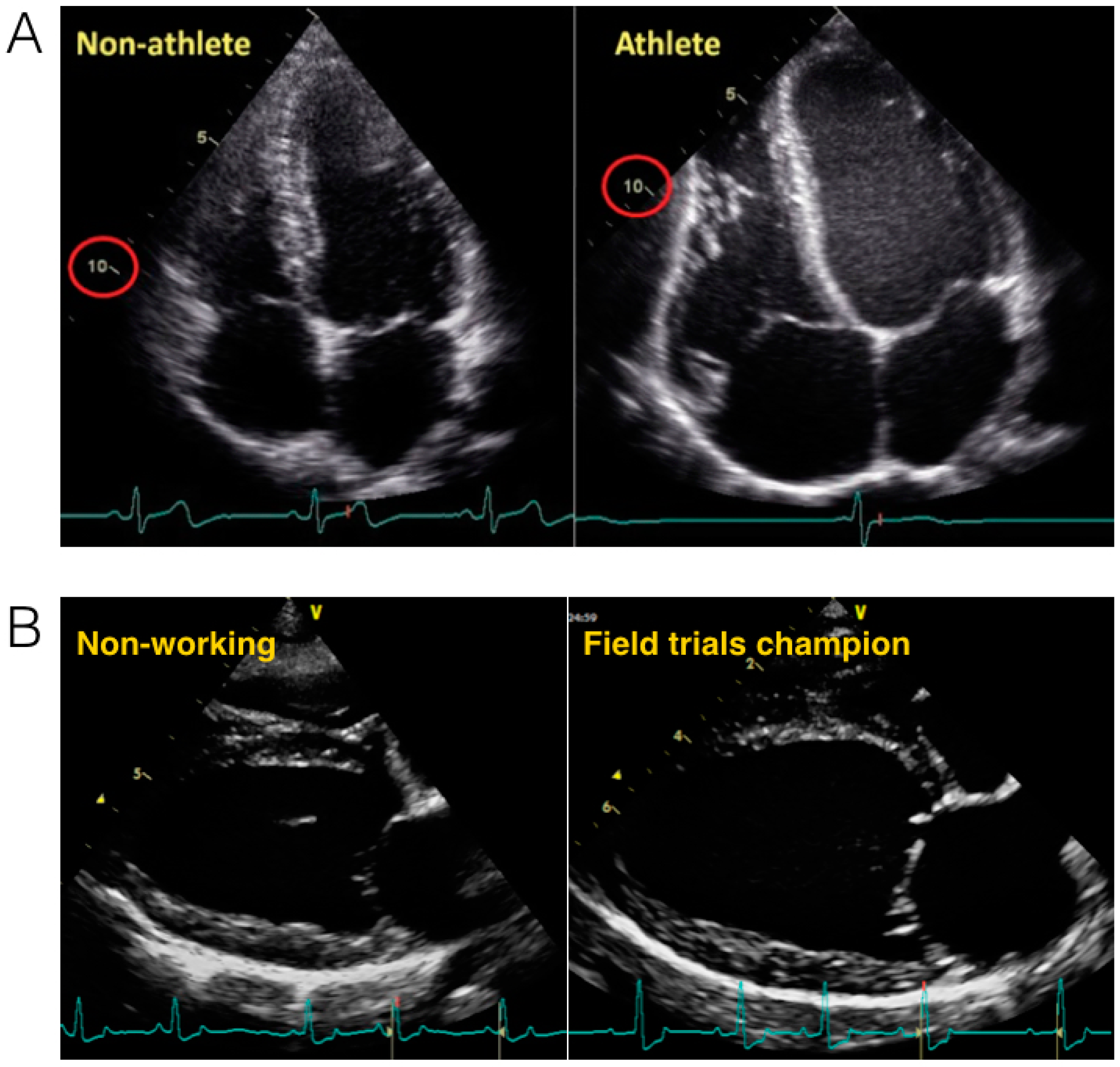
2.1. Cardiac Remodelling in Non-Human Mammalian Athletes
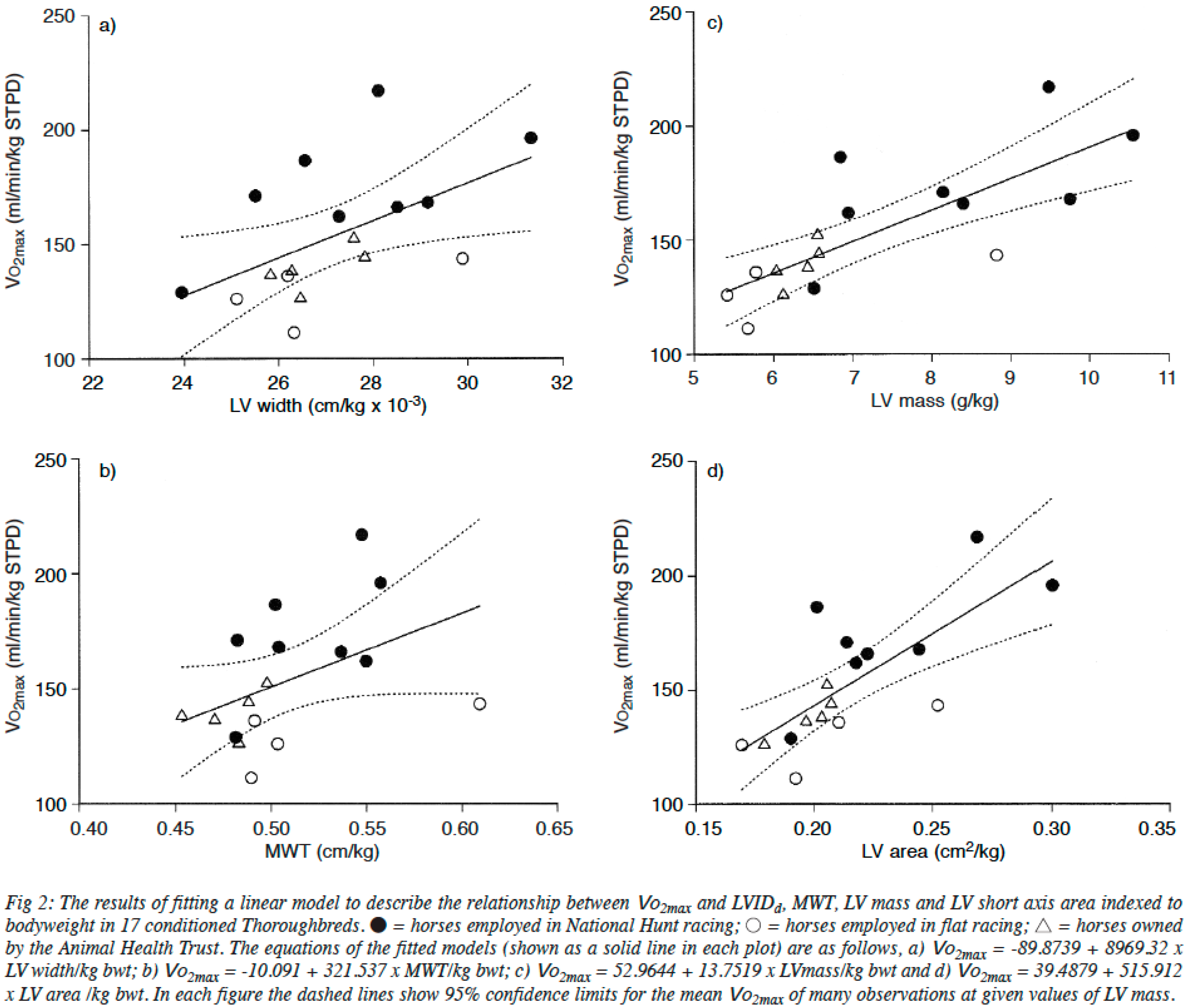
2.2. The Heart of Athletic Horses
2.3. The Athletic Heart in Dogs
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Williams, T.M.; Bengtson, P.; Steller, D.L.; Croll, D.A.; Davis, R.W. The healthy heart: Lessons from nature’s elite athletes. Physiology 2015, 30, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.R. Organ weights in primates and other mammals. Science 1965, 150, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Erickson, H.H. Highly athletic terrestrial mammals: Horses and dogs. Compr. Physiol. 2011, 1, 1–37. [Google Scholar] [PubMed]
- La Gerche, A.; Roberts, T.; Claessen, G. The response of the pulmonary circulation and right ventricle to exercise: Exercise-induced right ventricular dysfunction and structural remodeling in endurance athletes (2013 grover conference series). Pulm. Circ. 2014, 4, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Pelliccia, A. The heart of trained athletes: Cardiac remodeling and the risks of sports, including sudden death. Circulation 2006, 114, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, A.; Sharma, S. Exercise, the athlete’s heart, and sudden cardiac death. Phys. Sportsmed. 2014, 42, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, S.; Sharma, S.; McKenna, W.J. Risk of competitive sport in young athletes with heart disease. Heart 2003, 89, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Corrado, D.; Thiene, G. Cardiovascular causes of sudden death in young individuals including athletes. Cardiol. Rev. 1999, 7, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Distinguishing hypertrophic cardiomyopathy from athlete’s heart physiological remodelling: Clinical significance, diagnostic strategies and implications for preparticipation screening. Br. J. Sports Med. 2009, 43, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Maron, M.S.; Maron, B.J. Assessment of left ventricular hypertrophy in a trained athlete: Differential diagnosis of physiologic athlete’s heart from pathologic hypertrophy. Prog. Cardiovasc. Dis. 2012, 54, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baggish, A.L. Differentiating exercise-induced cardiac adaptations from cardiac pathology: The “grey zone” of clinical uncertainty. Can. J. Cardiol. 2016, 32, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Liguzinski, P.; Korzeniewski, B. Oxygen delivery by blood determines the maximal VO2 and work rate during whole body exercise in humans: In silico studies. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H343–H353. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task force 8: Classification of sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- George, K.P.; Wolfe, L.A.; Burggraf, G.W. The “athletic heart syndrome”. A critical review. Sports Med. 1991, 11, 300–330. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B.; Baggish, A.L. Exercise-induced cardiac remodelling: The need for assessment of regional myocardial function. J. Physiol. 2012, 590, 2829–2830. [Google Scholar] [CrossRef] [PubMed]
- Henschen, S. Eine medizinische sportstudie. Mitt. Med. Klin. Upsala 1899, 2, 15–18. [Google Scholar]
- Darling, M.D. The effects of training. A study of the harvard university crews. Boston Med. Surg. J. 1899, 229–233. [Google Scholar] [CrossRef]
- White, P.D. The pulse after a marathon race. JAMA 1918, 71, 1047–1048. [Google Scholar] [CrossRef]
- Morganroth, J.; Maron, B.J.; Henry, W.L.; Epstein, S.E. Comparative left ventricular dimensions in trained athletes. Ann. Intern. Med. 1975, 82, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Naylor, L.H.; George, K.; O’Driscoll, G.; Green, D.J. The athlete’s heart: A contemporary appraisal of the ‘morganroth hypothesis’. Sports Med. 2008, 38, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.L.; Naylor, L.H.; Carter, H.H.; Buck, C.L.; Dembo, L.; Murray, C.P.; Watson, P.; Oxborough, D.; George, K.P.; Green, D.J. A prospective randomised longitudinal mri study of left ventricular adaptation to endurance and resistance exercise training in humans. J. Physiol. 2011, 589, 5443–5452. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.; Taylor, D.; Teo, K.; Quinney, A.; Humen, D. Left ventricular wall stress during leg-press exercise performed with a brief valsalva maneuver. Chest 2001, 119, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B.; Wang, F.; Isaacs, S.K.; Malhotra, R.; Berkstresser, B.; Kim, J.H.; Hutter, A.M., Jr.; Picard, M.H.; Wang, T.J.; Baggish, A.L. Blood pressure and left ventricular hypertrophy during american-style football participation. Circulation 2013, 128, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Pluim, B.M.; Zwinderman, A.H.; van der Laarse, A.; van der Wall, E.E. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Scharhag, J.; Schneider, G.; Urhausen, A.; Rochette, V.; Kramann, B.; Kindermann, W. Athlete’s heart: Right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J. Am. Coll. Cardiol. 2002, 40, 1856–1863. [Google Scholar] [CrossRef]
- La Gerche, A.; Heidbuchel, H.; Burns, A.T.; Mooney, D.J.; Taylor, A.J.; Pfluger, H.B.; Inder, W.J.; Macisaac, A.I.; Prior, D.L. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med. Sci. Sports Exerc. 2011, 43, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.; Thompson, P.D. A meta-analysis of aortic root size in elite athletes. Circulation 2013, 127, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.; Mujtaba, M.T.; Thompson, P.D. Left atrium size in elite athletes. JACC Cardiovasc. Imaging 2015, 8, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Nottin, S.; Doucende, G.; Schuster, I.; Tanguy, S.; Dauzat, M.; Obert, P. Alteration in left ventricular strains and torsional mechanics after ultralong duration exercise in athletes. Circ. Cardiovasc. Imaging 2009, 2, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Stohr, E.J.; McDonnell, B.; Thompson, J.; Stone, K.; Bull, T.; Houston, R.; Cockcroft, J.; Shave, R. Left ventricular mechanics in humans with high aerobic fitness: Adaptation independent of structural remodelling, arterial haemodynamics and heart rate. J. Physiol. 2012, 590, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B.; DeLuca, J.R.; Wang, F.; Lin, J.; Wasfy, M.M.; Berkstresser, B.; Stohr, E.; Shave, R.; Lewis, G.D.; Hutter, A.M., Jr.; et al. Exercise-induced left ventricular remodeling among competitive athletes: A phasic phenomenon. Circ. Cardiovasc. Imaging 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharp, N.C. Timed running speed of a cheetah (acinonyx jubatus). J. Zool. 1997, 241, 493–494. [Google Scholar] [CrossRef]
- Nowak, R.M. Dogs, Wolves, Coyotes, Jackals, and Foxes: Canidae. In Walker’s Carnivores of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2005; p. 112. [Google Scholar]
- Nielsen, B.D.; Turner, K.K.; Ventura, B.A.; Woodward, A.D.; O’Connor, C.I. Racing speeds of quarter horses, thoroughbreds and arabians. Equine Vet. J. Suppl. 2006, 128–132. [Google Scholar] [CrossRef] [PubMed]
- McKeever, K.H.; Hinchcliff, K.W.; Reed, S.M.; Robertson, J.T. Plasma constituents during incremental treadmill exercise in intact and splenectomised horses. Equine Vet. J. 1993, 25, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Betros, C.L.; McKeever, K.H.; Kearns, C.F.; Malinowski, K. Effects of ageing and training on maximal heart rate and VO2max. Equine Vet. J. Suppl. 2002, 34, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Young, L.E.; Marlin, D.J.; Deaton, C.; Brown-Feltner, H.; Roberts, C.A.; Wood, J.L. Heart size estimated by echocardiography correlates with maximal oxygen uptake. Equine Vet. J. Suppl. 2002, 34, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Young, L.E.; Rogers, K.; Wood, J.L. Left ventricular size and systolic function in thoroughbred racehorses and their relationships to race performance. J. Appl. Physiol. (1985) 2005, 99, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, M.M.; Durando, M.M.; Holbrook, T.C.; Payton, M.E.; Birks, E.K. Comparison of echocardiographic measurements in elite and nonelite arabian endurance horses. Am. J. Vet. Res. 2014, 75, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.S.T.; Sugimoto, O. Relationship between training and heart in thoroughbred racehorse. Exp. Rep. Eq. Hlth. Lab. 1974, 11, 89–93. [Google Scholar]
- Young, L.E. Cardiac responses to training in 2-year-old thoroughbreds: An echocardiographic study. Equine Vet. J. Suppl. 1999, 30, 195–198. [Google Scholar] [CrossRef]
- Buhl, R.; Ersboll, A.K. Echocardiographic evaluation of changes in left ventricular size and valvular regurgitation associated with physical training during and after maturity in standardbred trotters. J. Am. Vet. Med. Assoc. 2012, 240, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.P.; Ducharme, N.G.; Gleed, R.D.; Mitchell, L.; Soderholm, L.V.; Erickson, B.K.; Erb, H.N. Do thoroughbred and standardbred horses have similar increases in pulmonary vascular pressures during exertion? Can. J. Vet. Res. 2003, 67, 291–296. [Google Scholar] [PubMed]
- Lightfoot, G.; Jose-Cunilleras, E.; Rogers, K.; Newton, J.R.; Young, L.E. An echocardiographic and auscultation study of right heart responses to training in young national hunt thoroughbred horses. Equine Vet. J. Suppl. 2006, 36, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, K.W.; McKeever, K.H.; Schmall, L.M.; Kohn, C.W.; Muir, W.W., 3rd. Renal and systemic hemodynamic responses to sustained submaximal exertion in horses. Am. J. Physiol. 1990, 258, R1177–R1183. [Google Scholar] [PubMed]
- Patteson, M.W.; Cripps, P.J. A survey of cardiac auscultatory findings in horses. Equine Vet. J. 1993, 25, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kriz, N.G.; Hodgson, D.R.; Rose, R.J. Prevalence and clinical importance of heart murmurs in racehorses. J. Am. Vet. Med. Assoc. 2000, 216, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Young, L.E.; Wood, J.L. Effect of age and training on murmurs of atrioventricular valvular regurgitation in young thoroughbreds. Equine Vet. J. 2000, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Navas de Solis, C. Exercising arrhythmias and sudden cardiac death in horses: Review of the literature and comparative aspects. Equine Vet. J. 2016, 48, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Lyle, C.H.; Uzal, F.A.; McGorum, B.C.; Aida, H.; Blissitt, K.J.; Case, J.T.; Charles, J.T.; Gardner, I.; Horadagoda, N.; Kusano, K.; et al. Sudden death in racing thoroughbred horses: An international multicentre study of post mortem findings. Equine Vet. J. 2011, 43, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.A.; Macdonald, D.W.; O’Brien, S.J. From wild animals to domestic pets, an evolutionary view of domestication. Proc. Natl. Acad. Sci. USA 2009, 106, 9971–9978. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J.; Rush, J.E.; MacGregor, J.; Ross, J.N., Jr.; Brewer, B.; Rand, W.M. M-mode echocardiographic ratio indices in normal dogs, cats, and horses: A novel quantitative method. J. Vet. Intern. Med. 2003, 17, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Haggstrom, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of m-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.; Shave, R.; Rishniw, M.; Harris, J.; Patteson, M. Reference intervals for transthoracic echocardiography in the english springer spaniel: A prospective, longitudinal study. J. Small Anim. Pract. 2016, 57, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Tipton, C.M.; Carey, R.A.; Eastin, W.C.; Erickson, H.H. A submaximal test for dogs: Evaluation of effects of training, detraining, and cage confinement. J. Appl. Physiol. 1974, 37, 271–275. [Google Scholar] [PubMed]
- Barnard, R.J.; Duncan, H.W.; Baldwin, K.M.; Grimditch, G.; Buckberg, G.D. Effects of intensive exercise training on myocardial performance and coronary blood flow. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 49, 444–449. [Google Scholar] [PubMed]
- Ritzer, T.F.; Bove, A.A.; Carey, R.A. Left ventricular performance characteristics in trained and sedentary dogs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 130–138. [Google Scholar] [PubMed]
- Mackintosh, I.C.; Dormehl, I.C.; van Gelder, A.L.; du Plessis, M. Blood volume, heart rate, and left ventricular ejection fraction changes in dogs before and after exercise during endurance training. Am. J. Vet. Res. 1983, 44, 1960–1962. [Google Scholar] [PubMed]
- Stepien, R.L.; Hinchcliff, K.W.; Constable, P.D.; Olson, J. Effect of endurance training on cardiac morphology in alaskan sled dogs. J. Appl. Physiol. 1998, 85, 1368–1375. [Google Scholar] [PubMed]
- Wyatt, H.L.; Mitchell, J.H. Influences of physical training on the heart of dogs. Circ. Res. 1974, 35, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.A.; Ritzer, T.F.; Bove, A.A. Effect of endurance training on myocardial myosin adenosine triphosphatase activity of the dog. Med. Sci. Sports 1979, 11, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Franklin, D.; Higgins, C.B.; Patrick, T.; Braunwald, E. Left ventricular response to severe exertion in untethered dogs. J. Clin. Investig. 1972, 51, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Riedhammer, H.H.; Rafflenbeul, W.; Weihe, W.H.; Krayenbuhl, H.P. Left ventricle contractile function in trained dogs with cardial hypertrophy. Basic Res. Cardiol. 1976, 71, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Edmunds, G.; Atwell, R.B. Echocardiographic values in the greyhound. Aust. Vet. J. 1993, 70, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.P.; Truex, R.C.; Knowles, J.O. Comparative observations of the hearts of mongrel and greyhound dogs. Anat. Rec. 1964, 149, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Carew, T.E.; Covell, J.W. Left ventricular function in exercise-induced hypertrophy in dogs. Am. J. Cardiol. 1978, 42, 82–88. [Google Scholar] [CrossRef]
- Seckerdieck, M.; Holler, P.; Smets, P.; Wess, G. Simpson’s method of discs in salukis and whippets: Echocardiographic reference intervals for end-diastolic and end-systolic left ventricular volumes. J. Vet. Cardiol. 2015, 17, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Price, J.M.; Alpert, J.S.; Rippe, J.M. Hemodynamics and left ventricular function: A comparison between adult racing greyhounds and greyhounds completely untrained from birth. Basic Res. Cardiol. 1986, 81, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, R.A.; Labuc, R.H.; Robertson, I.D. Echocardiographic parameters in training compared with non-training greyhounds. Vet. Radiol. Ultrasound 1998, 39, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.H.; Peterson, L.H.; Detweiler, D.K. Comparison of arterial hemodynamics in the mongrel dog and the racing greyhound. Am. J. Physiol. 1976, 230, 211–218. [Google Scholar] [PubMed]
- McKeever, K.H.; Schurg, W.A.; Convertino, V.A. Exercise training-induced hypervolemia in greyhounds: Role of water intake and renal mechanisms. Am. J. Physiol. 1985, 248, R422–R425. [Google Scholar] [PubMed]
- Hinchcliff, K.W.; Reinhart, G.A.; Burr, J.R.; Schreier, C.J.; Swenson, R.A. Metabolizable energy intake and sustained energy expenditure of alaskan sled dogs during heavy exertion in the cold. Am. J. Vet. Res. 1997, 58, 1457–1462. [Google Scholar] [PubMed]
- Hinchcliff, K.W.; Constable, P.D.; Farris, J.W.; Schmidt, K.E.; Hamlin, R.L. Electrocardiographic characteristics of endurance-trained alaskan sled dogs. J. Am. Vet. Med. Assoc. 1997, 211, 1138–1141. [Google Scholar] [PubMed]
- Van Citters, R.L.; Franklin, D.L. Cardiovascular performance of alaska sled dogs during exercise. Circ. Res. 1969, 24, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; Jensen-Urstad, M.; van Hall, G.; Holmberg, H.C.; Rosdahl, H.; Saltin, B. Maximal muscular vascular conductances during whole body upright exercise in humans. J. Physiol. 2004, 558, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T.; Timmons, J.A. Genomics and genetics in the biology of adaptation to exercise. Compr. Physiol. 2011, 1, 1603–1648. [Google Scholar] [PubMed]



© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shave, R.; Howatson, G.; Dickson, D.; Young, L. Exercise-Induced Cardiac Remodeling: Lessons from Humans, Horses, and Dogs. Vet. Sci. 2017, 4, 9. https://doi.org/10.3390/vetsci4010009
Shave R, Howatson G, Dickson D, Young L. Exercise-Induced Cardiac Remodeling: Lessons from Humans, Horses, and Dogs. Veterinary Sciences. 2017; 4(1):9. https://doi.org/10.3390/vetsci4010009
Chicago/Turabian StyleShave, Rob, Glyn Howatson, Dave Dickson, and Lesley Young. 2017. "Exercise-Induced Cardiac Remodeling: Lessons from Humans, Horses, and Dogs" Veterinary Sciences 4, no. 1: 9. https://doi.org/10.3390/vetsci4010009




