Metastatic Squamous Cell Carcinoma in a Northern Brown Bandicoot (Isoodon macrourus)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Animal Details
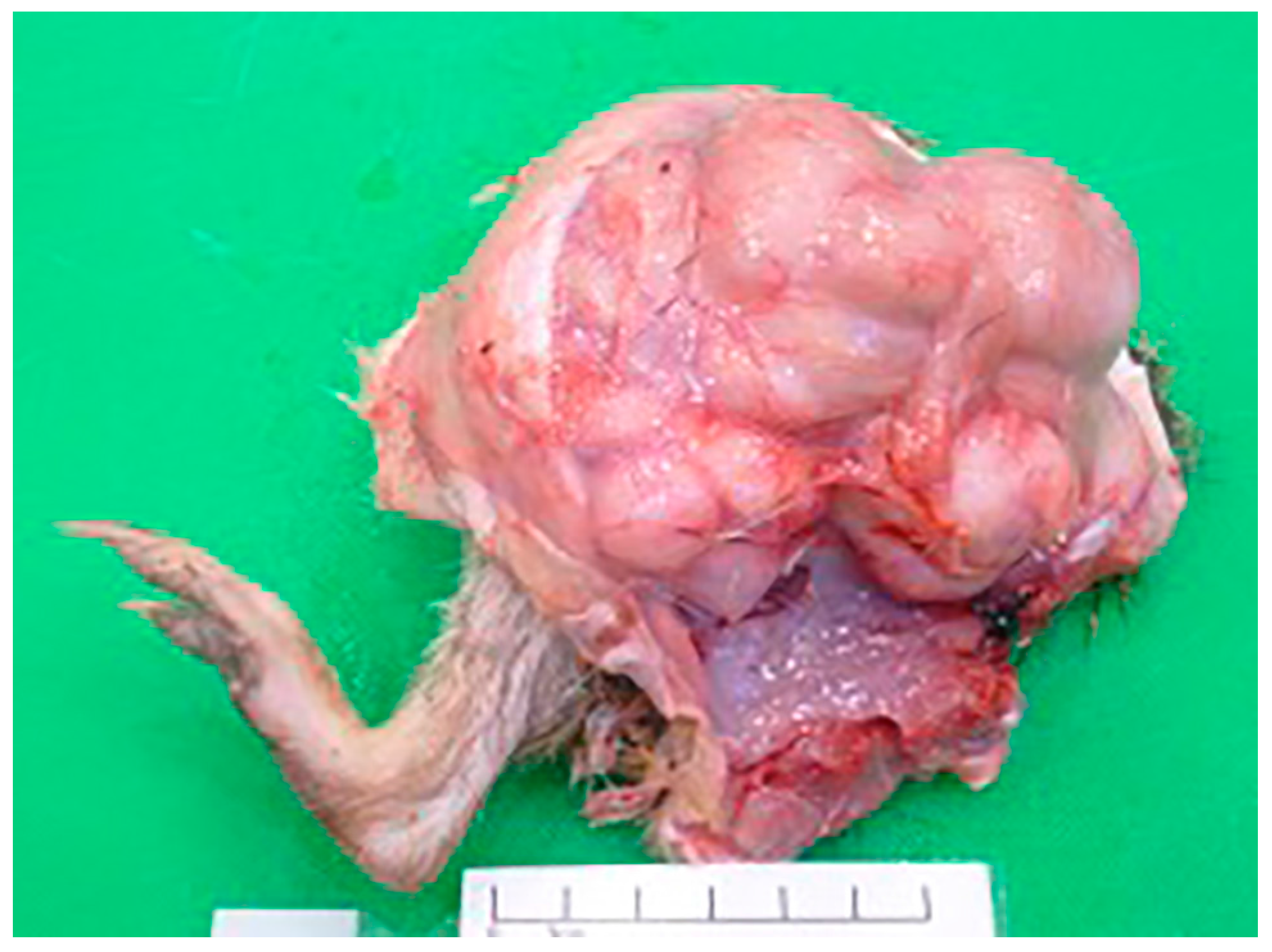
3.2. Post-Mortem Examination
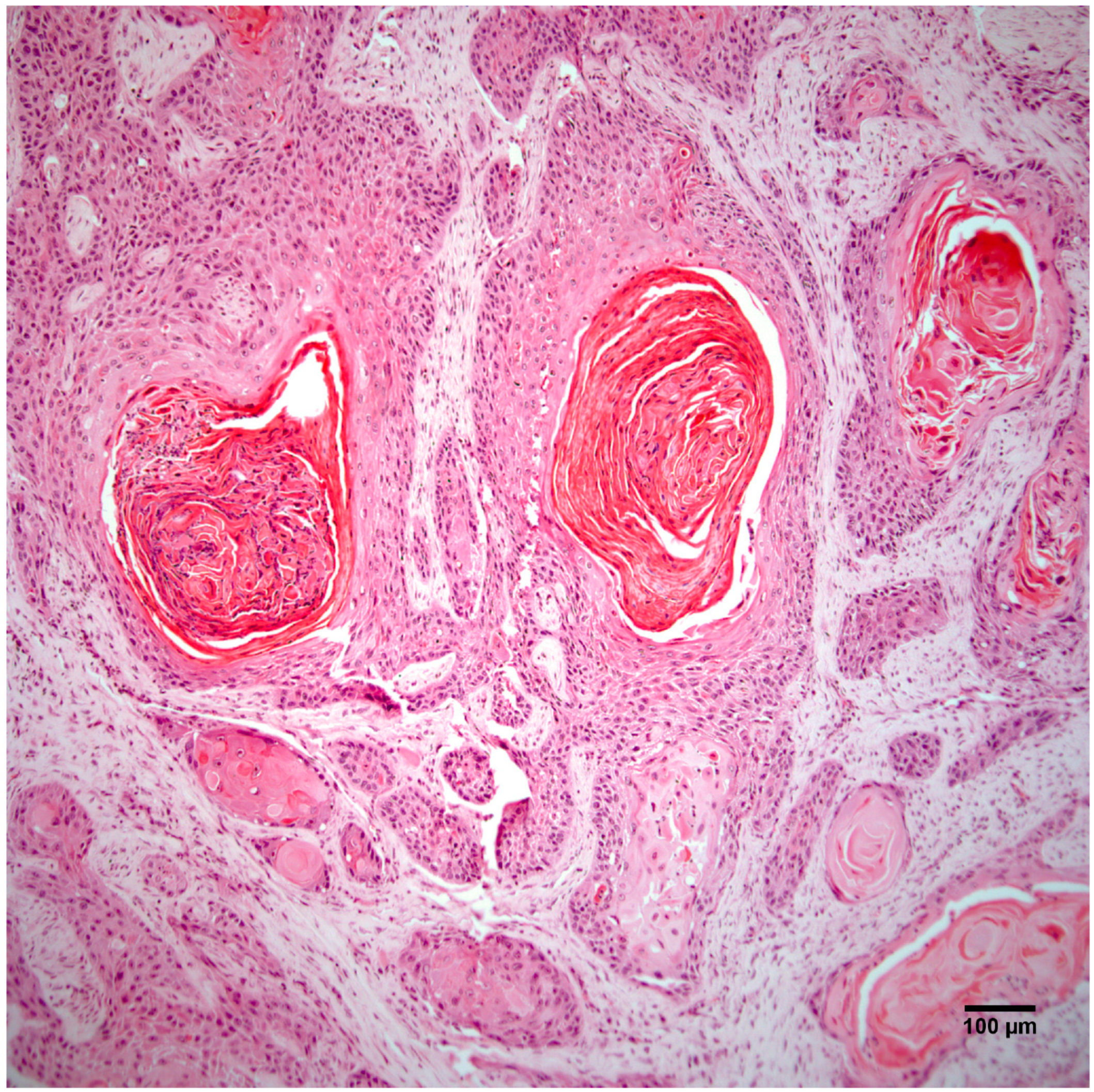
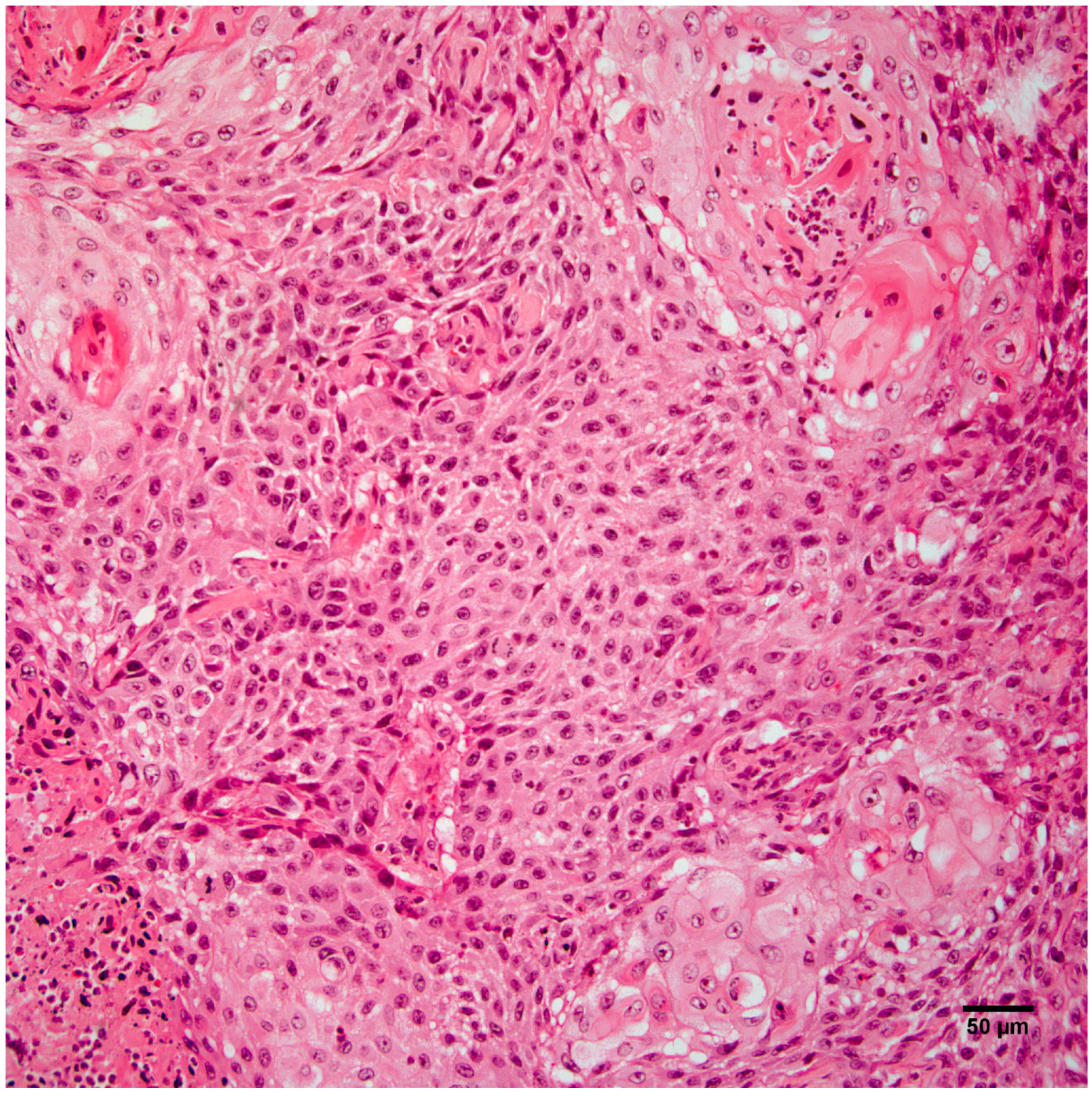
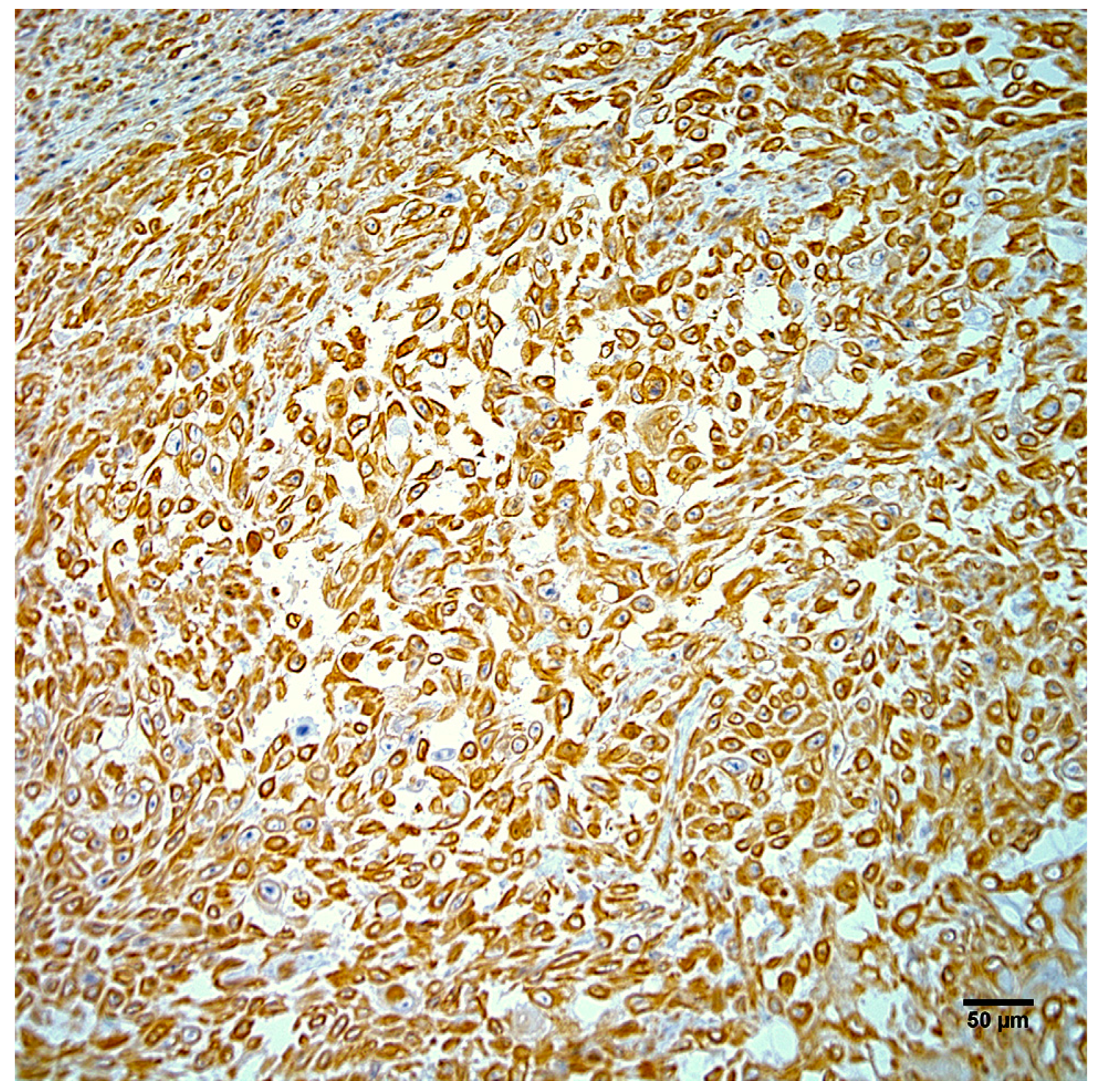
3.3. Histopathology
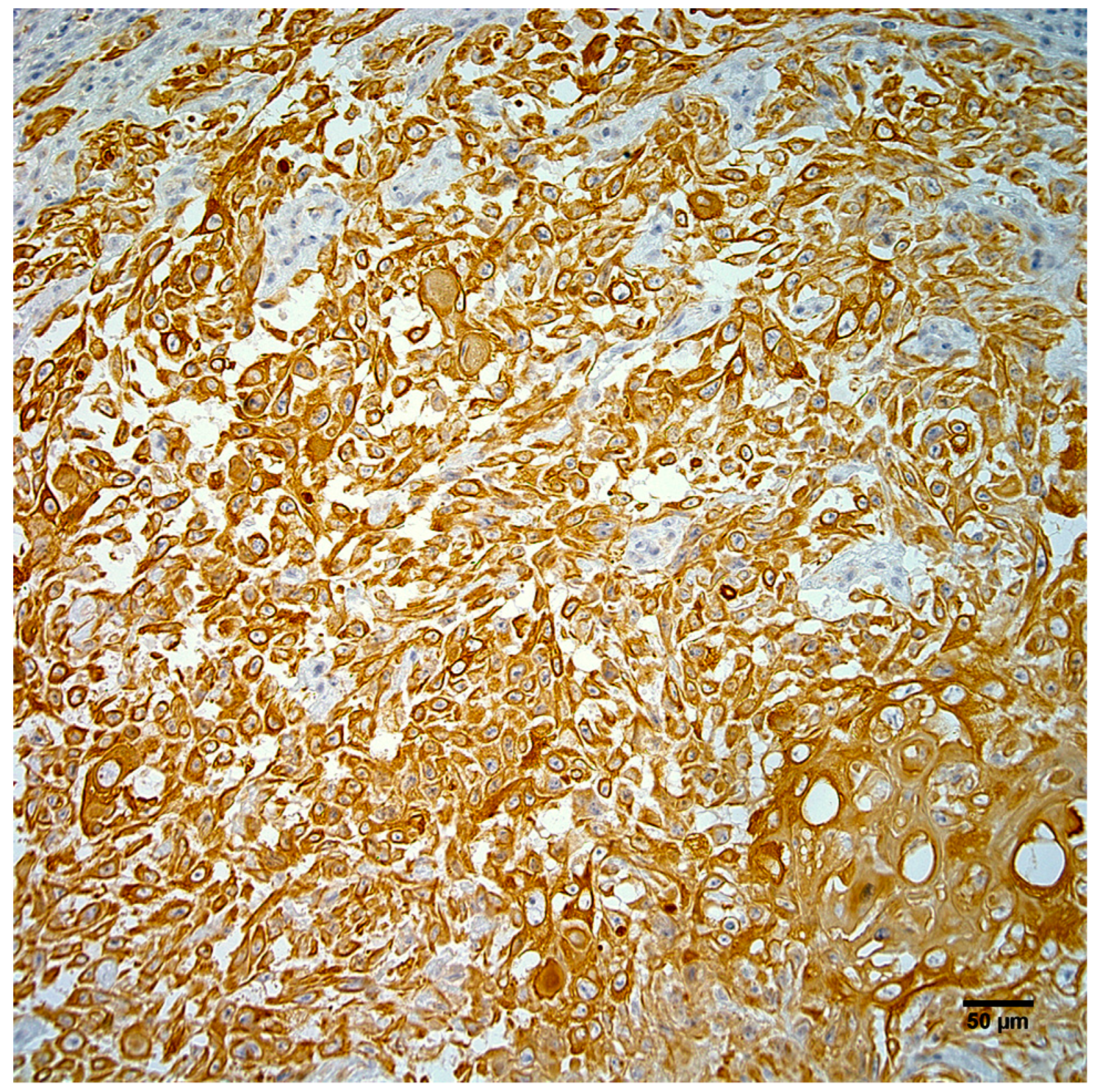
3.4. Immunohistochemistry and In Situ Hybridization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duncan, C.; Backus, L.; Lynn, T.; Powers, B.; Salman, M. Passive, opportunistic wildlife disease surveillance in the Rocky Mountain Region, USA. Transbound. Emerg. Dis. 2008, 55, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Martineau, D.; Lemberger, K.; Dallaire, A.; Labelle, P.; Lipscomb, T.P.; Michel, P.; Mikaelian, I. Cancer in wildlife, a case study: Beluga from the St. Lawrence estuary, Québec, Canada. Environ. Health Perspect. 2002, 110, 285–292. [Google Scholar] [CrossRef] [PubMed]
- McAloose, D.; Newton, A.L. Wildlife cancer: A conservation perspective. Nat. Rev. Cancer 2009, 9, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Bodley, K.B.; Booth, R.J.; Samuel, J.; Wilkie, J.S. Disseminated haemangiosarcoma in an eastern barred bandicoot (Perameles. gunnii). Aust. Vet. J. 2000, 78, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Ladds, P.W. Pathology of Australian Native Wildlife; CSIRO Publishing: Collingwood, Australia, 2009; pp. 445–446. [Google Scholar]
- Bennett, M.D.; Woolford, L.; O’Hara, A.J.; Warren, K.S.; Nicholls, P.K. In situ hybridization to detect bandicoot papillomatosis carcinomatosis virus type 1 in biopsies from endangered western barred bandicoots (Perameles bougainville). J. Gen. Virol. 2008, 89, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.D.; Woolford, L.; Stevens, H.; Van Ranst, M.; Oldfield, T.; Slaven, M.; O’Hara, A.J.; Warren, K.S.; Nicholls, P.K. Genomic characterization of a novel virus found in papillomatous lesions from a southern brown bandicoot (Isoodon. obesulus) in Western Australia. Virology 2008, 376, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Kiupel, M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet. Pathol. 2010, 47, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Woolford, L.; O’Hara, A.J.; Bennett, M.D.; Slaven, M.; Swan, R.; Friend, J.A.; Ducki, A.; Sims, C.; Hill, S.; Nicholls, P.K.; et al. Cutaneous papillomatosis and carcinomatosis in the western barred bandicoot (Perameles. bougainville). Vet. Pathol. 2008, 45, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Woolford, L.; Rector, A.; Van Ranst, M.; Ducki, A.; Bennett, M.D.; Nicholls, P.K.; Warren, K.S.; Swan, R.A.; Wilcox, G.E.; O’Hara, A.J. A novel virus detected in papillomas and carcinomas of the endangered western barred bandicoot (Perameles. bougainville) exhibits genomic features of both the Papillomaviridae. and Polyomaviridae. J. Virol. 2007, 81, 13280–13290. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.D. Western barred bandicoots in health and disease. Ph.D. Thesis, Murdoch University, Perth, Western Australia, Australia, 2009. [Google Scholar]
- Pardon, L.G.; Brook, B.W.; Griffiths, A.D.; Braithwaite, R.W. Determinants of survival for the northern brown bandicoot under a landscape-scale fire experiment. J. Anim. Ecol. 2003, 72, 106–115. [Google Scholar] [CrossRef]
- Loh, R.; Hayes, D.; Mahjoor, A.; O’Hara, A.; Pyecroft, S.; Raidal, S. The immunohistochemical characterization of Devil Facial Tumor Disease (DFTD) in the Tasmanian Devil (Sarcophilus harrisii). Vet. Pathol. 2006, 43, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Rev. Mol. Cell. Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell. 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012, 22, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Hui, K.M. MicroRNAs involved in regulating epithelial-mesenchymal transition and cancer stem cells as molecular targets for cancer therapeutics. Cancer Gene Ther. 2012, 19, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Thway, K. Synovial sarcoma: Defining features and diagnostic evolution. Ann. Diagn. Pathol. 2014, 18, 369–380. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, A.P.; Shima, A.L.; Bennett, M.D.; Johnson, L.K. Metastatic Squamous Cell Carcinoma in a Northern Brown Bandicoot (Isoodon macrourus). Vet. Sci. 2017, 4, 10. https://doi.org/10.3390/vetsci4010010
Beck AP, Shima AL, Bennett MD, Johnson LK. Metastatic Squamous Cell Carcinoma in a Northern Brown Bandicoot (Isoodon macrourus). Veterinary Sciences. 2017; 4(1):10. https://doi.org/10.3390/vetsci4010010
Chicago/Turabian StyleBeck, Amanda P., Amy L. Shima, Mark D. Bennett, and Linda K. Johnson. 2017. "Metastatic Squamous Cell Carcinoma in a Northern Brown Bandicoot (Isoodon macrourus)" Veterinary Sciences 4, no. 1: 10. https://doi.org/10.3390/vetsci4010010




