Characterization of Haptoglobin Isotype in Milk of Mastitis-Affected Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Bacteriological Examination
2.2. Isolation of Total RNA from Milk
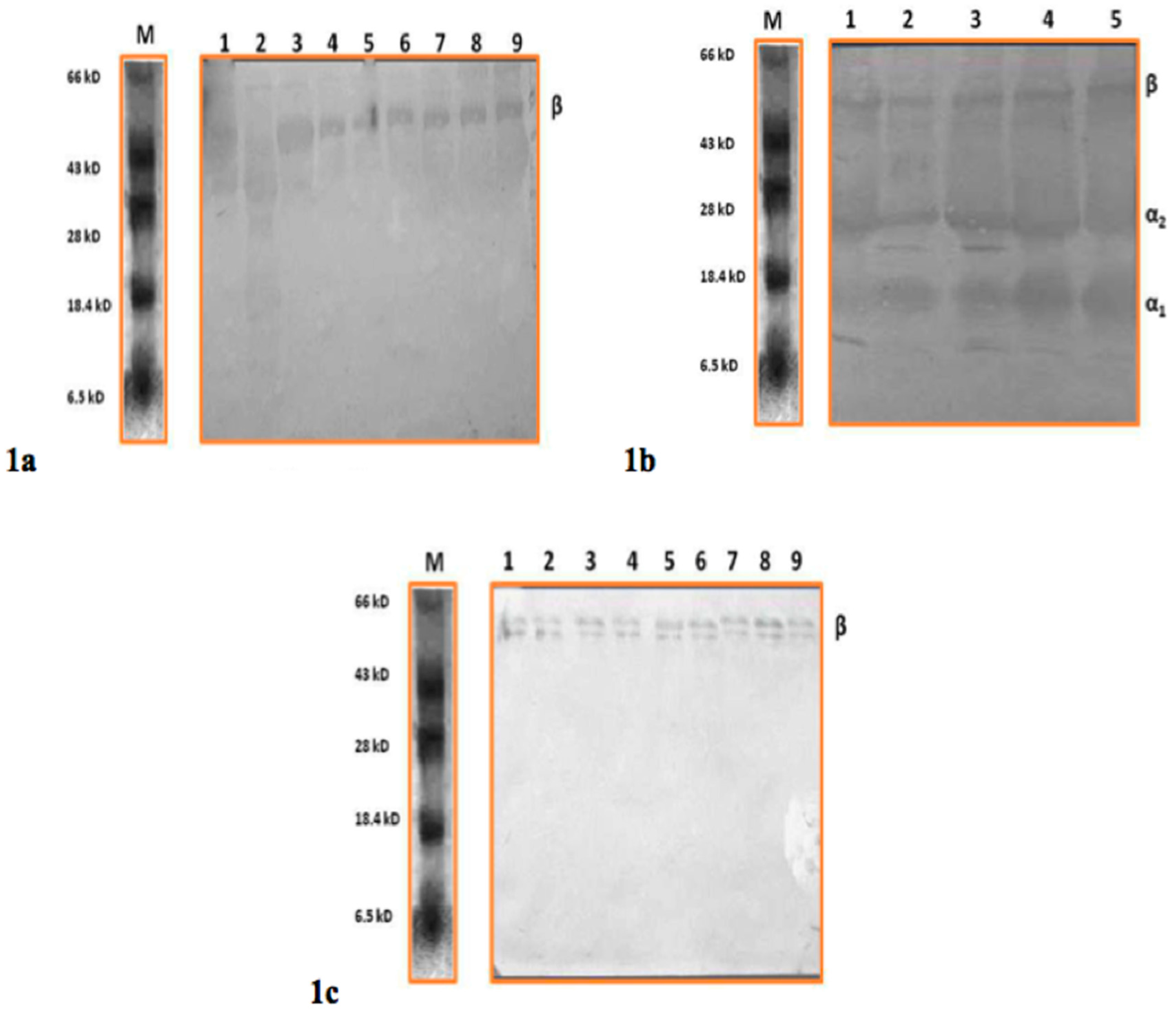
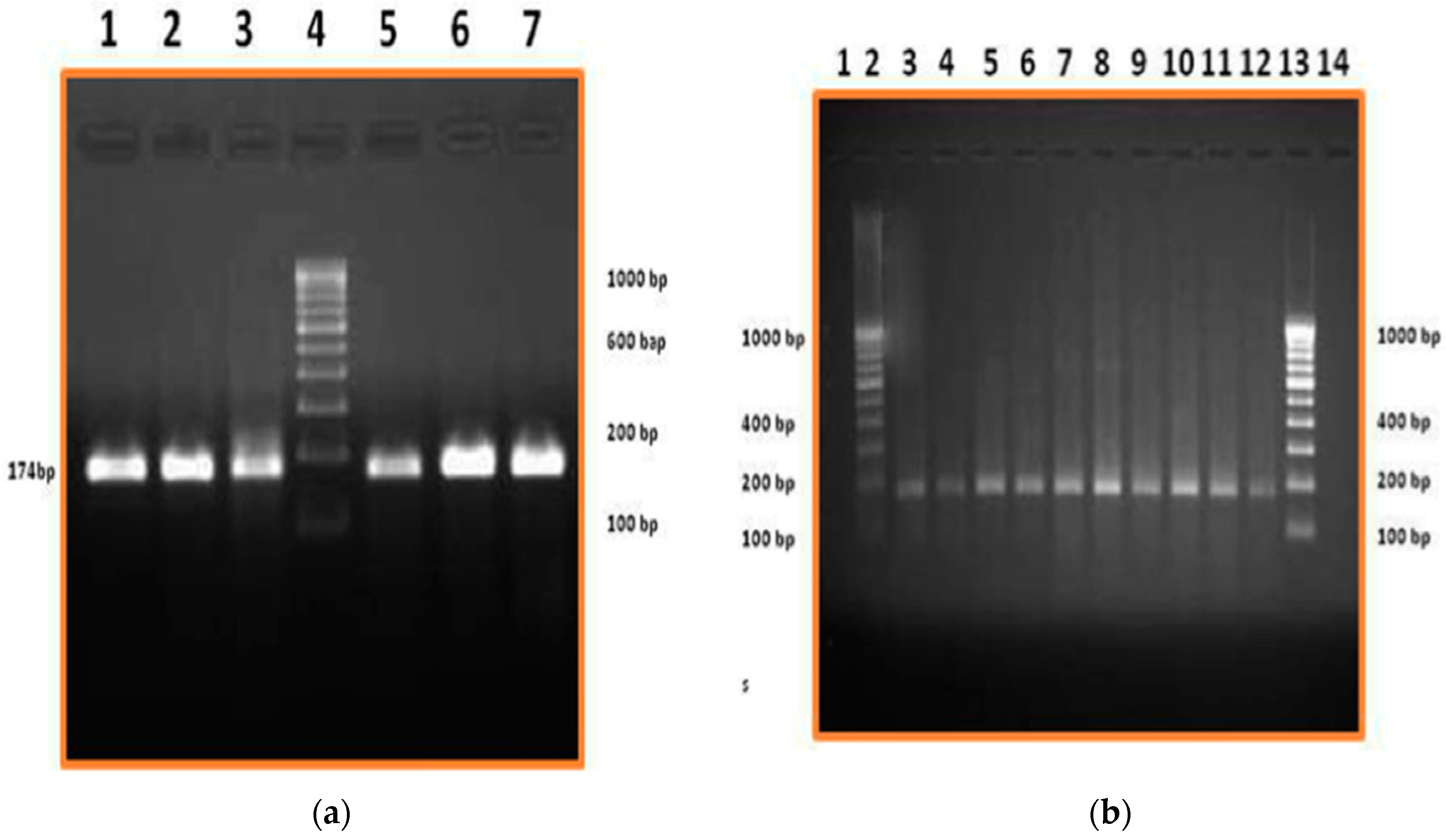
2.3. RT-PCR (Reverse Transcription Polymerase Chain Reaction) and Western Blot for Haptoglobin Characterization
2.4. Statistical Analysis
3. Results and Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kossaibati, M.A.; Esslemont, R.J. The costs of production diseases in dairy herds in England. Vet. J. 1997, 154, 41–51. [Google Scholar] [CrossRef]
- Fourichon, C.; Seegers, H.; Beaudeau, F.; Verfaille, L.; Bareille, N. Health-control costs in dairy farming systems in western France. Livest. Prod. Sci. 2001, 68, 141–156. [Google Scholar] [CrossRef]
- Pyorala, S. Indicators of inflammation in the diagnosis of mastitis. Vet. Res. 2003, 34, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, G.; Bernardini, D.; Elia, C.A.; Ferrari, V.; Iob, L.; Segato, S. Use of serum amyloid A and milk amyloid A in the diagnosis of subclinical mastitis in dairy cows. J. Dairy Res. 2009, 76, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.; Khoshvaghti, A.; Jafarzadeh, S.R.; Bolourchi, M.; Nowrouzian, I. Acute phase proteins in the diagnosis of bovine subclinical mastitis. Vet. Clin. Pathol. 2009, 38, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Eckersall, P.D.; Gow, J.W.; McComb, C.; Bradley, B.; Rodgers, J.; Murray, M.; Kennedy, P.G.E. Cytokines and the acute phase response in post-treatment reactive encephalopathy of Trypanosoma brocei brucei infected mice. Parasitol. Int. 2001, 50, 15–26. [Google Scholar] [CrossRef]
- Pedersen, L.H.; Aalback, B.; Rontved, C.M.; Ingvartsen, K.L.; Sorensen, N.S.; Heegard, P.M.; Jensen, H.E. Early pathogenesis and inflammatory response in experimental bovine mastitis due to Streptococcus uberis. J. Comp. Pathol. 2003, 128, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Gronlund, U.; Hallen, S.C.; Persson, W.K. Haptoglobin and serum amyloid A in milk from dairy cows with chronic sub-clinical mastitis. Vet. Res. 2005, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Ceron, J.J.; Eckersall, P.D.; Martinez-Subiela, S. Acute phase proteins in dogs and cats; current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.H.; Tsao, J.H.; Lu, Y.P.; Lee, J.W.; Zhao, X.; Chien, F.L.; Mao, S.J. Neutrophils as one of the major haptoglobin sources in mastitis affected milk. Vet. Res. 2009, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Hiss, S.; Mielenz, M.; Bruckmaier, R.M.; Sauerwein, H. Haptoglobin concentrations in blood and milk after endotoxin challenge and quantification of mammary Hp mRNA expression. J. Dairy Sci. 2004, 87, 3778–3784. [Google Scholar] [CrossRef]
- Thielen, M.A.; Mielenz, M.; Hiss, S.; Zerbe, H.; Petzl, W.; Schuberth, H.J.; Seyfert, H.M.L.; Sauerwein, H. Short communication: Cellular localization of haptoglobin mRNA in the experimentally infected bovine mammary gland. J. Dairy Sci. 2007, 90, 1215–1219. [Google Scholar] [CrossRef]
- Kalmovarin, N.; Friedrichs, W.E.; O’Brien, H.V. Extrahepatic expression of plama protein gene during inflammation. Inflammation 1991, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- D’Armiento, J.; Dalal, S.S.; Chada, K. Tissue, temporal and inducible expression pattern of haptoglobin in mice. Gene 1997, 195, 19–27. [Google Scholar] [CrossRef]
- Ervasti, J.M.; Campbell, K.P. Membrane organization of the dystrophin-glycoprotein complex. Cell 1991, 66, 1121–1131. [Google Scholar] [CrossRef]
- Dingwell, R.T.; Leslie, K.E.; Schukken, Y.H.; Sargeant, J.M.; Timms, L.L. Evaluation of the California Mastitis Test to detect an intra-mammary infection with a major pathogen in early lactation dairy cows. Can. Vet. J. 2003, 44, 413–415. [Google Scholar] [PubMed]
- Leslie, K.E.; Jansen, J.T.; Lim, G.H. Opportunities and implications for improved on-farm cowside diagnostics. In Proceedings of the International De Laval Hygiene Symposium, Tumba County, Sweden, 15–16 May 2002; pp. 147–160.
- Anitha, T.; Thanislass, J.; Antony, P.X.; Sharanabasav, B. Detection of Antibodies in Milk for the Pyruvate formate lyase associated with S. aureus isolated from mastitis affected milk by serological proteome analysis. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 459–467. [Google Scholar]
- Bhuvana, M. Effect of CpG Islands of DNA Isolated from Staphylococcus aureus on Cytokine Gene Expression. Master’s Thesis, Rajiv Gandhi Institute of Veterinary Education and Research, Puducherry, India, September 2008. [Google Scholar]
- Glibetic, M.D.; Baumann, H. Influence of chronic inflammation on the level of mRNA for acute-phase reactants in the mouse liver. J. Immunol. 1986, 137, 1611–1622. [Google Scholar]
- Stahl, W.M. Acute phase protein response to tissue injury. Crit. Care Med. 1987, 15, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.A. Haptoglobin: A potential reporter molecule for glycosylation changes in disease. Adv. Exp. Med. Biol. 1995, 376, 231–238. [Google Scholar] [PubMed]
- Petersen, H.H.; Neilsen, J.P.; Heegaard, P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Eckersall, P.D.; Conner, J.G. Plasma haptoglobin in cattle (Bos taurus) exists as polymers in association with albumin. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 96, 309–314. [Google Scholar] [CrossRef]
- Cooray, R.; Waller, K.P.; Venge, P. Haptoglobin comprises about 10% of granule protein extracted from bovine granulocytes isolated from healthy cattle. Vet. Immunol. Immunop. 2007, 119, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Smeets, M.B.; Sluijter, J.P.; Donners, M.M.; Velema, E.; Heeneman, S.; Pasterkamp, G.; de Kleijn, D.P. Increased arterial expression of a glycosylated haptoglobin isoform after balloon dilation. Cardiovasc. Res. 2003, 58, 689–695. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Young, F.J.; Nolan, A.M.; Knight, C.H.; McComb, C.; Waterston, M.M.; Hogarth, C.J.; Scott, E.M.; Fitzpatrick, J.L. Acute phase proteins in bovine milk in an experimental model of Staphylococcus aureus subclinical mastitis. J. Dairy Sci. 2006, 89, 1488–1501. [Google Scholar] [CrossRef]
- Thomas, F.C.; Waterston, M.; Hastie, P.; Parkin, T.; Haining, H.; Eckersall, P.D. The major acute phase proteins of bovine milk in a commercial dairy herd. BMC Vet. Res. 2015, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Kalmus, P.; Simojoki, H.; Pyörälä, S.; Taponen, S.; Holopainen, J.; Orro, T. Milk haptoglobin, milk amyloid A and N-acetyl-β-d-glucosaminidase activity in bovines with naturally occurring clinical mastitis diagnosed with a quantitative PCR test. J. Dairy Sci. 2013, 96, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyaya, I.; Thanislass, J.; Veerapandyan, A.; Badami, S.; Antony, P.X. Characterization of Haptoglobin Isotype in Milk of Mastitis-Affected Cows. Vet. Sci. 2016, 3, 29. https://doi.org/10.3390/vetsci3040029
Upadhyaya I, Thanislass J, Veerapandyan A, Badami S, Antony PX. Characterization of Haptoglobin Isotype in Milk of Mastitis-Affected Cows. Veterinary Sciences. 2016; 3(4):29. https://doi.org/10.3390/vetsci3040029
Chicago/Turabian StyleUpadhyaya, Indu, Jacob Thanislass, Anitha Veerapandyan, Sharanabasav Badami, and Prabhakar X. Antony. 2016. "Characterization of Haptoglobin Isotype in Milk of Mastitis-Affected Cows" Veterinary Sciences 3, no. 4: 29. https://doi.org/10.3390/vetsci3040029





