1. Introduction
Beverages are consumed in Nigeria regardless of age, gender and socio-economic background and play a vital function in refreshing and improving life. Contaminated and or expired beverages have capability to cause disease when they are consumed [
1]. Manufacture of beverages normally involves the use of plant- based product as an active ingredient.
Malt drinks are beverages brewed from barley, hops, sorghum and water. Malt drinks are rich in minerals and multivitamins such as vitamins A, B, B2, B3, B5, B6 and calcium which provide nourishment to the body [
2]. Malt beverages were originally brewed to be used as food for children and the sick due to the high nutritional content. However, it is now a popular beverage consumed by people of all ages [
2].
Beverages are consumed during ceremony, leisure, sport and during or after hard work as refreshment and/or to quench thirst. The intake of these non-alcoholic beverages in Nigeria was rated at 159.85 g/person/day in 2007, 58 kcal/capita/day in 2011, 261 kcal/capita/day in 2012 and 246 kcal/capital/day in 2013 [
3].
Nigerian brewers have set their gaze upon sorghum, one of the main ingredients in the production of malt drinks and an important cereal crop. Cereal such as millet and sorghum are mainly cultivated in the northern part of Nigeria. It is rich in mineral nutrients (such as iron, magnesium, thiamin, phosphorous, potassium, copper, riboflavin and calcium) which have various health benefits; hence, sorghum is economically beneficial. Due to this discovery, Sorghum is now being greatly exploited and used in place of barley for brewing malt drinks [
4].
The World Health Organization (WHO, 2017) [
5] defined a pesticide as a chemical compound that is used to kill pests, including insects, rodents, fungi and unwanted plants (weeds). The Food and Agriculture Organization (FAO) of the United Nations defined a pesticide as any substance or mixture of substances intended for preventing, destroying, or controlling any pest, including vectors of human or animal diseases, unwanted species of plants or animals causing harm during, or otherwise interfering with, the production, processing, storage, or marketing of food, agricultural commodities, wood and wood products, or animal feedstuffs, or which may be administered to animals for the control of insects, arachnids or other pests in or on their bodies [
6]. Pesticides are used in the cultivation of crops to prevent or control pests and other pathogens responsible for losses and low-quality produce. Pesticides, therefore, improve yield as well as quality and appearance of the produce [
7]. While pesticides have positive effects on plants, many of them are harmful to human beings, this is because they are intended to be a poison. Pesticides find their way into the food chain by the direct application of the substance on plants. They have various modes of entry into the human body such as inhalation (respiratory entry), ingestion (oral entry) and dermal entry (penetration through the skin) [
8].
Pesticides possess the ability to bioaccumulate (increase in concentration in an organism’s body) and biomagnify (increase in concentration up the food chain). This ability makes them even more dangerous because they can be retained in an organism’s body for a long period of time. Pesticides can be classified by chemical structure (e.g., organic, inorganic and synthetic) or are grouped into chemical families such as organochlorines, organophosphates and carbamates [
9].
The use of some pesticides and organic matter have been banned due to their toxicity, however, more agricultural aids are being discovered, each with their “pros and cons”. Therefore, as it is impossible to ban the use of all chemicals which threaten human health in the slightest way, regulatory tests and boards have been set up to monitor and control these substances in the best possible way.
Organochlorines, also referred to as chlorinated hydrocarbons, are organic compounds that contain at least one covalently bonded atom of Chlorine. Organochlorines, due to their structural variety, have a broad range of uses and applications, however, some are of great environmental concern [
10]. Organochlorine compounds can be found in nearly every class of biomolecules and can be produced naturally by forest fires, biological decomposition and volcanoes or from organisms such as bacteria and algae [
11].
The biggest application of Organochlorines is as a pesticide. The most infamous organochlorine pesticide is Dichlorodiphenyltrichloroethane, DDT. Organochlorines, like most pesticides, are persistent and toxic to biological organisms. They resist chemical and biological degradation [
12], have high lipophilic affinity [
13] and have the ability to bioaccumulate and biomagnify [
14]. Organochlorine pesticides were used extensively from the 1940s through the 1960s in agriculture and mosquito control, and were later banned in the United States. Short term exposure to organochlorine pesticides may cause headaches, dizziness, convulsions, nausea, and muscle weakness, slurred speech, vomiting and sweating. Long term exposure causes more serious conditions such as damage to the liver, kidney, central nervous system, thyroid, and bladder.
The use of agricultural pesticides for agricultural production has led not only to the increase in yield, but also increase in environmental pollution. The dangerous characteristics of pesticides such as long half-life, bioaccumulation and high lipophilicity enables them to remain in the environment after many years of application. A study by Pimentel [
15] showed that only 0.3% of applied pesticides goes into the target pest while 99.7% goes into the environment. This has adverse effects on the environment, human and animal’s health.
Persistent organic pesticide residues are widely distributed in Nigerian soil, water and cultivated crops especially cereals like barley and sorghum. Dichlorodiphenyltrichloroethane, DDT, and Hexachlorocyclohexane, HCH, have been restricted from use in Nigeria as a result of their adverse effects. However, despite the ban, these chemicals (e.g., y-HCH) are still being used by farmers on a large-scale Nigeria and a few other countries [
16].
Soil is the foremost basin of persistent organic pesticides and plays significant part in the worldwide input and fate of OCPs, in addition to their large retention capacity they also re-emit this organic pollutant into the atmosphere, groundwater and living organisms as secondary source [
17]. The inference of this in agriculture is the entrance of OCPs into growing plants and persistence of their residues [
17]
Trace metals or trace elements are elements that normally occur at very low levels or concentrations in the environment [
18]. Very small amounts of some of these metals are beneficial and essential for human beings, however, high concentrations of these same metals can be toxic.
Trace metals can reach agricultural lands through different routes and affect the soil, and cause serious problems to human health [
19]. The use of organic wastes is the most common and significant way by which agricultural soils can be contaminated by trace metals. Municipal wastewaters are used for agriculture irrigation [
20]. They contain low concentrations of heavy metals, however, long-term use of these wastewaters for irrigation often results in the buildup of metals in soils [
21]. These metals are taken up by the plants via their roots and are usually soluble ions in the form of organic or inorganic complexes. The concentrations, type and chemical nature of the complexes determine the plant’s ability to accumulate the trace metals [
22].
All metals are toxic at high concentrations and excessive levels can be damaging to the organism [
23]. These metals could be built up in different parts of human body such as heart, kidney, liver, blood and spleen whereby they cause diseases capable of causing damage to human body [
24]. In biological systems, heavy metals have been reported to affect cellular organelles and components such as cell membrane, mitochondrial, lysosome, endoplasmic reticulum, nuclei, and some enzymes involved in metabolism, detoxification, and damage repair [
25]. Metal ions have been found to interact with cell components such as DNA and nuclear proteins, causing DNA damage and conformational changes that may lead to cell cycle modulation, carcinogenesis or apoptosis [
26,
27].
Thus, it is necessary to look into these malt drinks to determine whether or not they are safe for consumption. The consumers need to be re-assured that these beverages are free from pesticide residues and other toxic substances.
2. Materials and Methods
2.1. Sampling
Five different brands (MAG, DUB, HIM, MLT and AMS) of locally produced malt drinks were randomly chosen out of about twelve different brands of malt drinks available for sales in Nigerian markets. Three samples of each brand having different batch numbers were purchased from the local supermarkets in Iwo, Osun state, Nigeria. A total of fifteen samples were collected and analyzed. The content of these drinks is water, sucrose, malted barley, malted sorghum, sorghum, caramel, hops, calcium, Vitamins A, B1, B2, B3, B5 and C, natural flavor and foam stabilizer. Others specific ingredients are citric acid (HIM), carbonated water (MAG) and sugar (DUB).
2.2. Reagent Used and Their Sources
Acetone, nitric acid and perchloric acid were purchased from Park Scientific Ltd. (Northamptom, UK); dichoromethane, n-hexane from GFS chemicals, (Inc Colombus, Powell, Ohio, USA); anhydrous sodium sulphate from BDH, (Poole England); and Silical gel (silica gel 60, particle size 0.063–0.200 mm, 7–230 mesh) from Lab Tech Chemicals (Boksburg, Guateng, South Africa).
2.3. Trace Metal Analysis
The trace metals determined are zinc, copper, chromium, cadmium, nickel, and lead. This was achieved by digesting 50 mL of each sample using 5 mL of nitric acid and 1ml of perchloric acid. The digested samples were reduced to a volume of about 2 mL and made up to the mark with distilled water in 25 mL standard flask. The digested solutions were analyzed via Atomic Absorption Spectroscopy at the National Institute of Oceanography, Victoria Island, Lagos, Nigeria.
2.4. Extraction Procedure for OCPs
The OCPs were extracted from malt samples by separating funnel method. Composite sample of the three batches of each brand of the malt drinks were made and about 500 mL of the samples were each decanted into a clean separating funnel and 30 mL of Dichloromethane was added to it. CThe separating funnel was then carefully agitated for twenty minutes. After agitation, the sample was left to settle and the OCPs were extracted from malt samples. This process was repeated three times for each sample to ensure maximum extraction of the OCPs.
2.5. Clean-up Procedure for OCPs
The Clean-up method, USEPA Method 3630C, was used in this study. A column of 15 cm × 1 cm (internal diameter) was packed with 5 g of activated silica gel. One (1 g) of anhydrous Sodium Sulphate was placed at the top of the column and Dichloromethane was added to condition it and cause elution to occur. The eluate was collected and evaporated to dryness.
2.6. Validity of Analytical Methods for Trace Metals and OCPs
Calibration curve for trace metal were plotted at various concentrations (0.2, 0.5, 1.0, 2.0, 4.0 mg/L) from stock solution. Stock solution was prepared from standard reference materials (1000 mg/L). The linear calibration obtained were Zn (0.99952), Pb (0.99962), Ni (0.99993), Cd (0.99958), and Cu (0.99986). The precision of the method was proven with an estimation of the percentage residual standard. Limit of detection (LOD) and limit of quantification (LOQ) for trace metals (mg/L) are Cu (0.0024, 0.024), Ni (0.0105, 0.105), Zn (0.0009, 0.009), Cd (0.0028, 0.028), Cr (0.005, 0.05) and Pb (0.012, 0.12).
The limit of detection (LOD) for OCPs were obtained at a signal-to-noise ratio (S/N) of three replicates while that of limit of quantification (LOQ) at S/N ratio of ten replicates for each pesticide, respectively. Standard addition method was employed to validate the analytical method employed in this study. The linearity of the analytical method was appraised using a concentration range of pesticide residues analyzed by GC-ECD. The calibration curve was plotted with the standard solution in n-hexane containing four different concentrations (0.1, 0.25, 0.5, 1.0 ng/µL). In all the several cases, good linearity was attained with correlation coefficient > 0.995. The malt drink samples were spiked with (1, 2, 5 µg/L) mixed OCP standard solution. The spiked samples were permitted to stand for some hours before extraction. This was followed by cleanup and subsequent GC-ECD analysis. Replicate analysis was carried out and the percentage recovery for each compound were determined. Linearity was determined by plotting the calibration curve with the standard solution in n-hexane containing four different concentrations (0.1, 0.25, 0.5, 1.0 ng/µL).
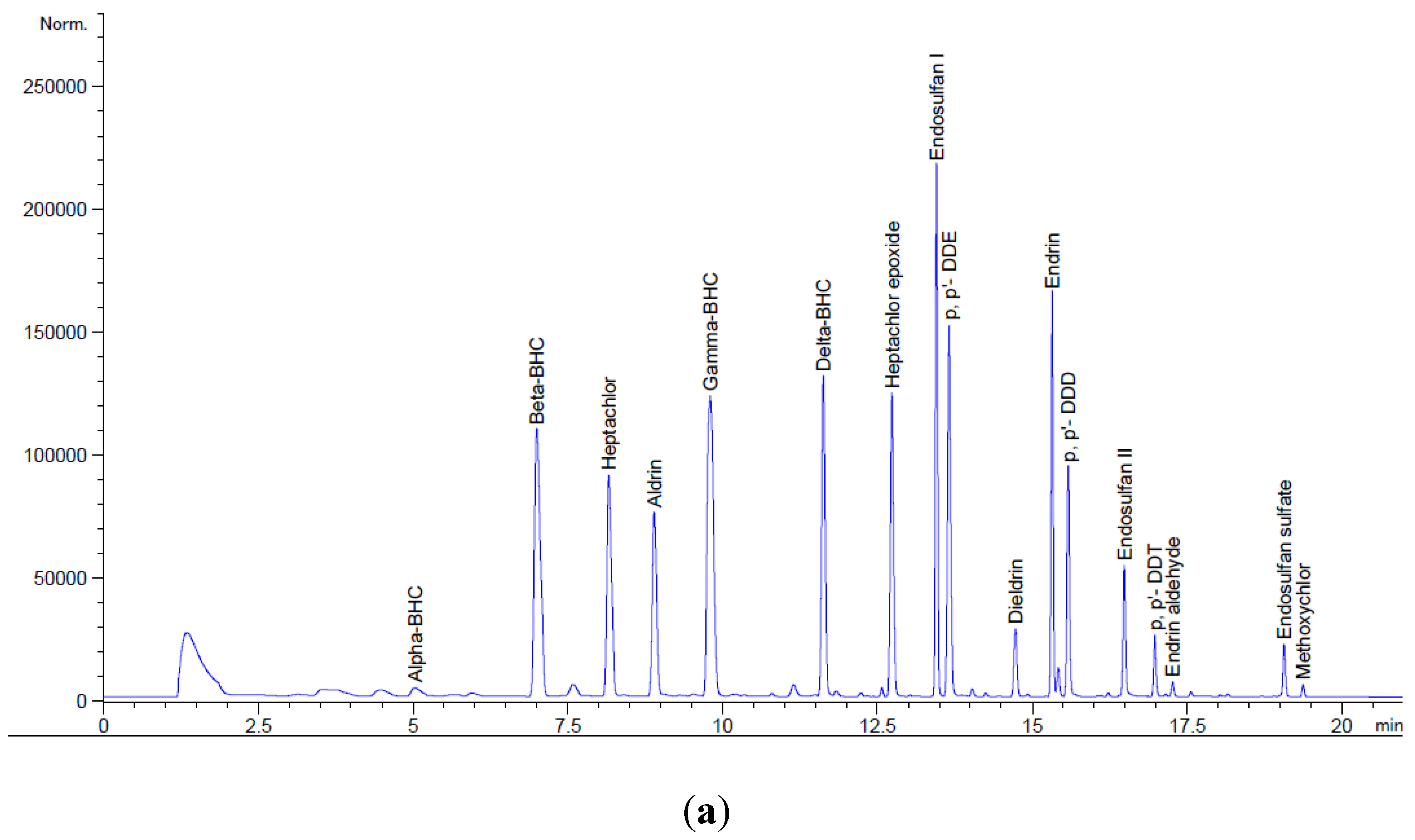
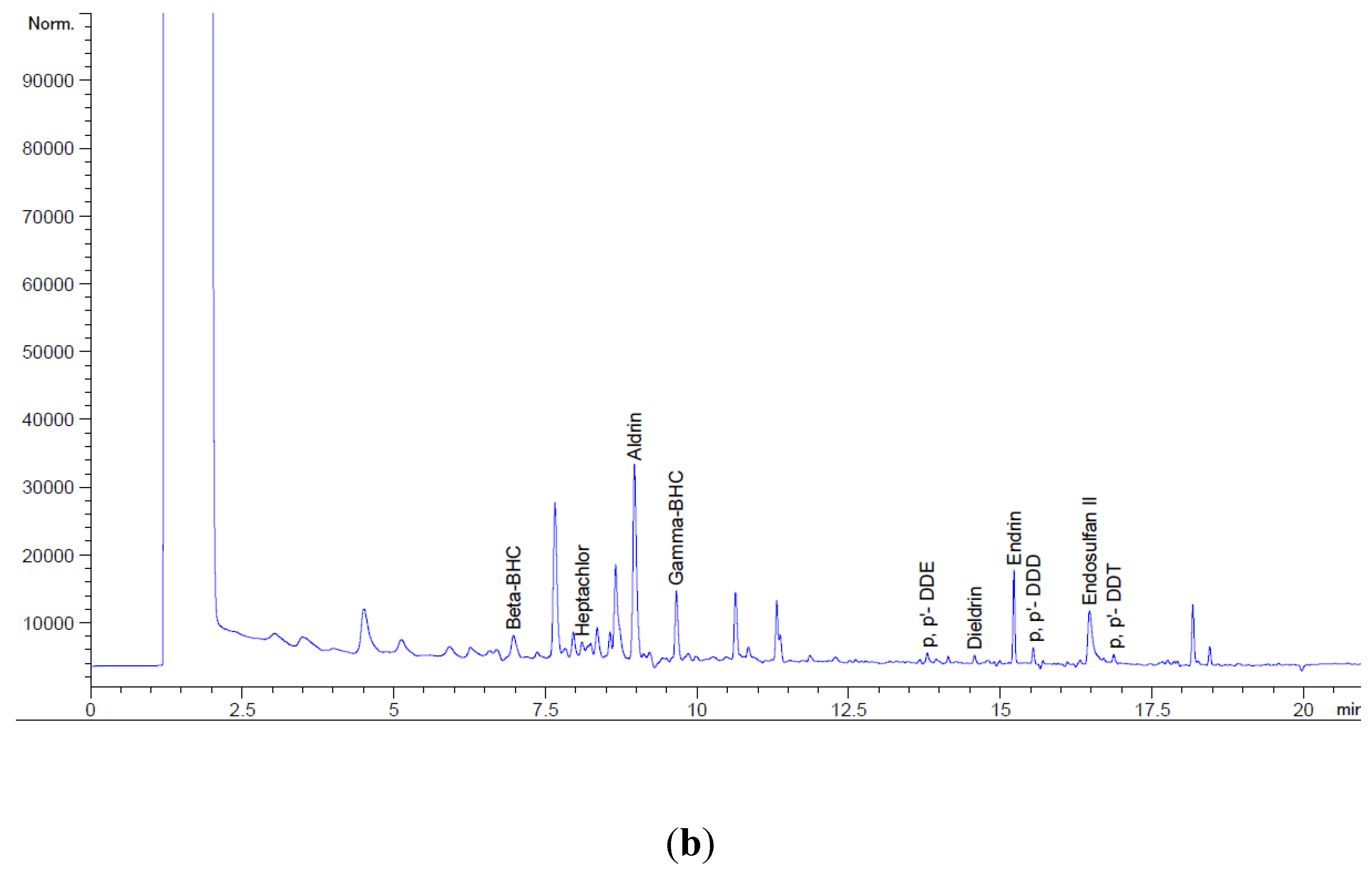
2.7. Chromatography-Electron Capture Detector condition
Gas Chromatography from the Central Laboratory, National Institute of Oceanography and Marine Research (NIOMR), Victoria Island, Lagos, Nigeria, was used to determine the concentrations of organochlorine pesticide residues in the samples. The dried eluate was reconstituted with 0.5 mL hexane and 0.5 mL of 20 ppm of the internal standard. The analysis of the OCPs was carried out with the aid Agilent 7890A GC-ECD. The levels of OCP was obtained from the relationship given below.
The system was fitted with DB 17 (30 m × 250 μm × 0.25 μm) Agilent column. A 1 μL aliquot of measured sample extract was injected into the column in splitless mode at an injector and interface temperature of 250 °C. A flow rate of 2 mL/min was obtained and the initial temperature of 150 °C was ramped to 280 °C at 6 °C/min in the oven. The detector temperature was 290 °C and the total run time was 21.67 min.
2.8. Data Analysis
The raw data were subjected to descriptive analysis and one-way analysis of variance using Statistical Package for Social Science (SPSS) (IBM Corporation, Armonk, United Kingdom)