Antioxidant and Anthocyanin Content in Fermented Milks with Sweet Cherry is Affected by the Starter Culture and the Ripening Stage of the Cherry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Starters
2.3. Fermented Milk Preparation and Cherry Addition
2.4. Plant Material Analysis
2.4.1. Total Phenolics
2.4.2. Anthocyanin Determination
2.4.3. Total Antioxidant Activity
2.5. Fermented Beverages Analysis
2.5.1. Physico-Chemical and Microbiology Analysis
2.5.2. Total Phenolics, Anthocyanin Determination and Total Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters and Microbiology of the Fermented Beverages
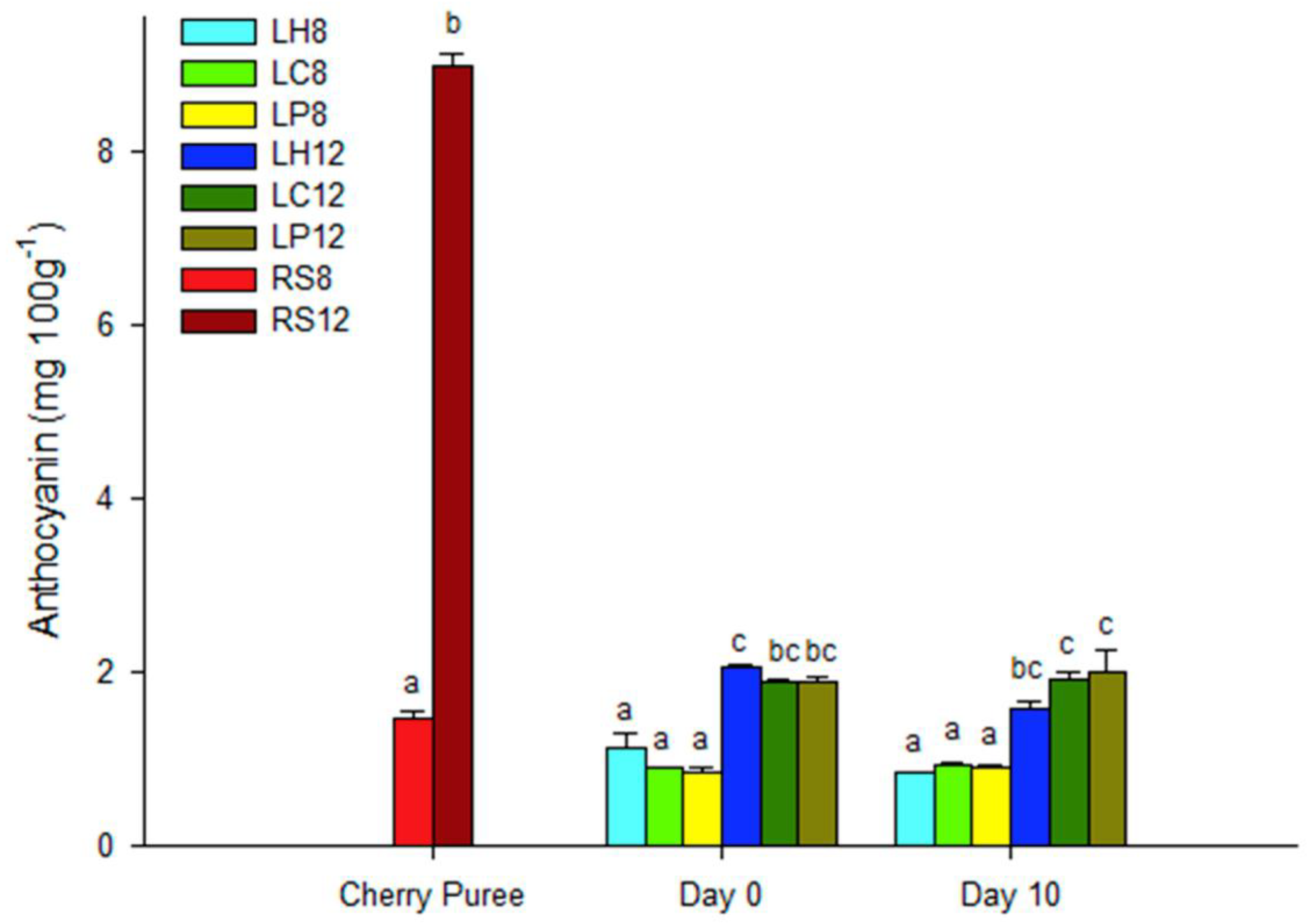
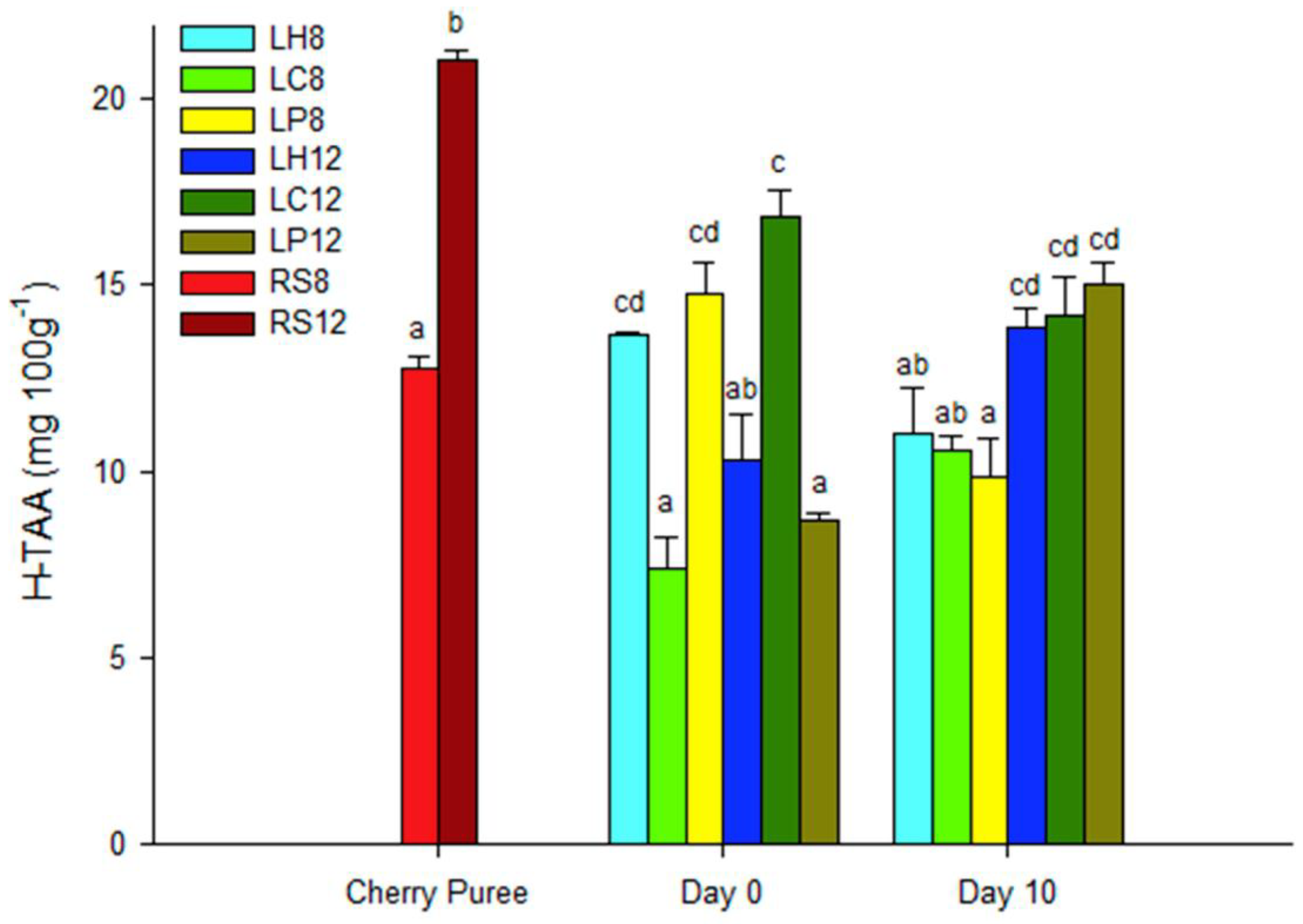
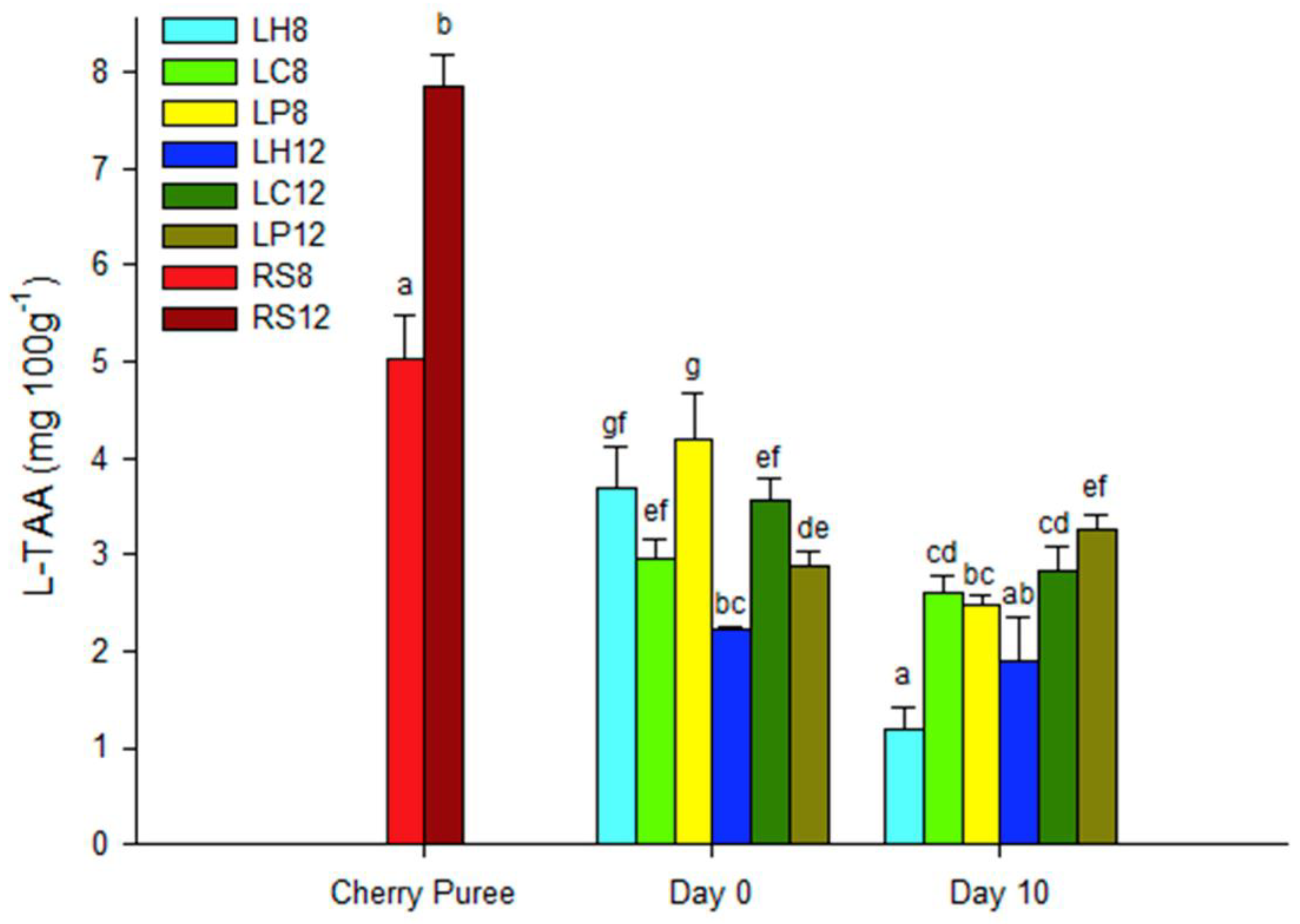
3.2. Total Phenolics, Anthocyanin Determination and Total Antioxidant Activity of the Fermented Beverages
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A. review. Crit. Rev. Food Sci. Nutr. 2011, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; D́iaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valverde, J.M.; Valero, D. Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Mula, H.M.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J.; Guillén, F.; Serrano, M. Sensory, nutritive and functional properties of sweet cherry as affected by cultivar and ripening stage. Food Sci. Technol. Int. 2009, 15, 535–543. [Google Scholar] [CrossRef]
- Aguilera, M.; Reza, M.C.; Gerardo, R.; Madinaveitia, C.; Meza, J.A. Functional properties of anthocyanins. Ciencias biológicas y de la salud de la Universidad de Sonora 2011, 13, 16–22. [Google Scholar]
- Cano-Lamadrid, M.; Trigueros, L.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Sendra, E. Anthocyanins decay in pomegranate enriched fermented milks as a function of bacterial strain and processing conditions. LWT Food Sci. Technol. 2017, 80, 193–199. [Google Scholar] [CrossRef]
- Trigueros, L.; Wojdyło, A.; Sendra, E. Antioxidant activity and protein-polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J. Agric. Food Chem. 2014, 62, 6417–6425. [Google Scholar] [CrossRef] [PubMed]
- Batt, C.A. Lactobacillus, introduction. In Encyclopedia of Food Microbiology; Robinson, R.K., Ed.; Elsevier: Oxford, UK, 1999; pp. 1134–1136. [Google Scholar]
- Sendra, E.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J. Effect of food composition on probiotic bacteria viability. Bioact. Foods Health Promot. 2016, 17, 257–269. [Google Scholar]
- Gobbetti, M. Lactobacillus, lactobacillus casei. In Encyclopedia of Food Microbiology; Robinson, R.K., Ed.; Elsevier: Oxford, UK, 1999; pp. 1157–1164. [Google Scholar]
- MAPAMA. Informe del Consumo de Alimentación en España 2016. Available online: http://www.mapama.gob.es/images/es/informe_del_consumo_de_alimentos_en_espana_2016_web_tcm30-419484.pdf (accessed on 25 May 2018).
- Augustin, M.; Clarke, P.T.; Craven, H. Powdered milk: Characteristics of milk powders. In Encyclopaedia of Food Science and Nutrition, 2nd ed.; Caballero, B., Ed.; Elsevier Science: London, UK, 2003; pp. 4703–4711. [Google Scholar]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. Hplc-dad-esims analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; García-Viguera, C.; Castillo, S.; Serrano, M. Preharvest application of oxalic acid increased fruit size, bioactive compounds, and antioxidant capacity in sweet cherry cultivars (Prunus avium L.). J. Agric. Food Chem. 2014, 62, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Trigueros, L.; Viuda-Martos, M.; Perez-Alvarez, J.A.; Sendra, E. Low fat set yoghurt rich in pomegranate juice: A new antioxidant dairy product. Milchwissenschaft 2012, 67, 177–180. [Google Scholar]
- Limsowtin, G.K.Y.; Broome, M.C.; Powell, I. Lactic Acid Bacteria, Taxonomy; Encyclopedia of Dairy Sciences, Academic Press: London, UK, 2002; pp. 1470–1478. [Google Scholar]
- Sendra, E.; Fayos, P.; Lario, Y.; Fernández-López, J.; Sayas-Barberá, E.; Pérez-Alvarez, J. Incorporation of citrus fibers in fermented milk containing probiotic bacteria. Food Microbiol. 2008, 25, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.E.; Wheater, D.M. Lactobacillus helveticus. J. Gen. Microbiol. 1957, 16, 676–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, L.; Silva, T.; Perina, N.; Bogsan, C.; Oliveira, M. Addition of strawberry, raspberry and “pitanga” pulps improves the physical properties of symbiotic yoghurts. Chem. Eng. 2014, 38, 499–504. [Google Scholar]
- Espirito-Santo, A.P.; Cartolano, N.S.; Silva, T.F.; Soares, F.; Gioielli, L.; Perego, P.; Converti, A.; Oliveira, M. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase cla content in yoghurts. Int. J. Food Microbiol. 2012, 154, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Rotar, A.; Vodnar, D.; Bunghez, F.; Catunescu, G.; Pop, C.; Jimborean, M.; Semeniuc, C. Effect of goji berries and honey on lactic acid bacteria viability and shelf life stability of yoghurt. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2015, 43. [Google Scholar] [CrossRef]

| Color | Cherry Ripening Stage | Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 1 | 10 | |||
| L. helveticus CECT 541 | 8 | L* | 33.76 ± 0.06 | 33.39 ± 0.06 | 32.52 ± 0.51 |
| a* | 0.16 ± 0.02 | 0.82 ± 0.05 | 1.23 ± 0.12 | ||
| b* | 3.94 ± 0.02 | 6.17 ± 0.09 | 6.60 ± 0.22 | ||
| 12 | L* | 28.24 ± 0.04 | 27.14 ± 0.25 | 27.85 ± 0.35 | |
| a* | 1.96 ± 0.05 | 4.07 ± 0.15 | 3.88 ± 0.06 | ||
| b* | 3.11 ± 0.03 | 5.46 ± 0.10 | 5.99 ± 0.12 | ||
| L. casei CECT 475 | 8 | L* | 34.31 ± 0.04 | 34.95 ± 0.33 | 33.86 ± 0.07 |
| a* | 0.02 ± 0.02 | 0.61 ± 0.05 | 0.67 ± 0.06 | ||
| b* | 4.08 ± 0.03 | 6.83 ± 0.05 | 6.75 ± 0.06 | ||
| 12 | L* | 28.30 ± 0.04 | 29.74 ± 0.28 | 27.34 ± 0.07 | |
| a* | 1.96 ± 0.01 | 3.55 ± 0.04 | 3.60 ± 0.08 | ||
| b* | 2.83 ± 0.04 | 6.14 ± 0.13 | 5.37 ± 0.07 | ||
| L. paracasei CECT 277 | 8 | L* | 33.94 ± 0.25 | 27.85 ± 0.35 | 33.17 ± 1.01 |
| a* | 0.05 ± 0.05 | 0.63 ± 0.09 | 0.89 ± 0.13 | ||
| b* | 4.22 ± 0.05 | 6.85 ± 0.14 | 6.67 ± 0.13 | ||
| 12 | L* | 26.89 ± 0.47 | 28.04 ± 0.55 | 28.64 ± 0.16 | |
| a* | 1.84 ± 0.13 | 3.81 ± 0.15 | 3.55 ± 0.06 | ||
| b* | 2.84 ± 0.02 | 5.23 ± 0.10 | 5.80 ± 0.09 | ||
| Starter | Cherry Ripening Stage | Time | ||||||
|---|---|---|---|---|---|---|---|---|
| L. helveticus | L. casei | L. paracasei | 8 | 12 | 0 | 1 | 10 | |
| L* | a | b | a | b | a | a | b | a |
| a* | b | a | b | a | b | a | b | b |
| b* | a | c | b | b | a | a | b | c |
| Starters | Cherry Ripening Stage | Time (Days) | ||
|---|---|---|---|---|
| 0 | 1 | 10 | ||
| pH | ||||
| L. helveticus CECT 541 | Control | 6.64 ± 0.08 | 3.52 ± 0.02 | 3.75 ± 0.05 |
| 8 | 6.01 ± 0.01 | 3.59 ± 0.05 | 3.72 ± 0.07 | |
| 12 | 6.16 ± 0.01 | 3.55 ± 0.02 | 3.71 ± 0.08 | |
| L. casei CECT 475 | Control | 6.66 ± 0.02 | 4.98 ± 0.21 | 4.54 ± 0.03 |
| 8 | 6.05 ± 0.03 | 4.15 ± 0.01 | 4.03 ± 0.02 | |
| 12 | 6.20 ± 0.01 | 4.14 ± 0.04 | 3.96 ± 0.05 | |
| L. paracasei CECT 277 | Control | 6.68 ± 0.01 | 5.27 ± 0.12 | 4.24 ± 0.06 |
| 8 | 6.05 ± 0.02 | 3.91 ± 0.04 | 3.92 ± 0.04 | |
| 12 | 6.15 ± 0.01 | 3.90 ± 0.03 | 3.93 ± 0.01 | |
| Microbial counts (log cfu mL−1) | ||||
| L. helveticus CECT 541 | Control | 6.92 ± 0.01 | 9.14 ± 0.09 | 8.60 ± 0.03 |
| 8 | 6.92 ± 0.01 | 9.28 ± 0.02 | 8.61 ± 0.05 | |
| 12 | 6.92 ± 0.01 | 9.59 ± 0.07 | 8.91 ± 0.20 | |
| L. casei CECT 475 | Control | 6.83 ± 0.04 | 9.14 ± 0.05 | 8.67 ± 0.08 |
| 8 | 6.83 ± 0.04 | 9.66 ± 0.54 | 9.22 ± 0.04 | |
| 12 | 6.83 ± 0.04 | 9.48 ± 0.12 | 9.28 ± 0.10 | |
| L. paracasei CECT 277 | Control | 6.61 ± 0.03 | 9.35 ± 0.04 | 9.42 ± 0.17 |
| 8 | 6.61 ± 0.03 | 9.48 ± 0.02 | 9.29 ± 0.08 | |
| 12 | 6.61 ± 0.03 | 9.77 ± 0.31 | 9.31 ± 0.08 | |
| Starters | Cherry Ripening Stage | Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L. helveticus | L. casei | L. paracasei | Control | 8 | 12 | 0 | 1 | 10 | |
| pH | a | c | b | c | a | b | c | b | a |
| Microbial counts | a | ab | b | a | ab | b | a | c | b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bravo, P.; Zapata, P.J.; Martínez-Esplá, A.; Carbonell-Barrachina, Á.A.; Sendra, E. Antioxidant and Anthocyanin Content in Fermented Milks with Sweet Cherry is Affected by the Starter Culture and the Ripening Stage of the Cherry. Beverages 2018, 4, 57. https://doi.org/10.3390/beverages4030057
Sánchez-Bravo P, Zapata PJ, Martínez-Esplá A, Carbonell-Barrachina ÁA, Sendra E. Antioxidant and Anthocyanin Content in Fermented Milks with Sweet Cherry is Affected by the Starter Culture and the Ripening Stage of the Cherry. Beverages. 2018; 4(3):57. https://doi.org/10.3390/beverages4030057
Chicago/Turabian StyleSánchez-Bravo, Paola, Pedro Javier Zapata, Alejandra Martínez-Esplá, Ángel A. Carbonell-Barrachina, and Esther Sendra. 2018. "Antioxidant and Anthocyanin Content in Fermented Milks with Sweet Cherry is Affected by the Starter Culture and the Ripening Stage of the Cherry" Beverages 4, no. 3: 57. https://doi.org/10.3390/beverages4030057








