Lycopene in Beverage Emulsions: Optimizing Formulation Design and Processing Effects for Enhanced Delivery
Abstract
:1. Introduction
2. Lycopene as Functional Ingredient: Structure, Properties and Health Effects
3. Stability of Lycopene in Emulsions: Formulation and Processing Implications
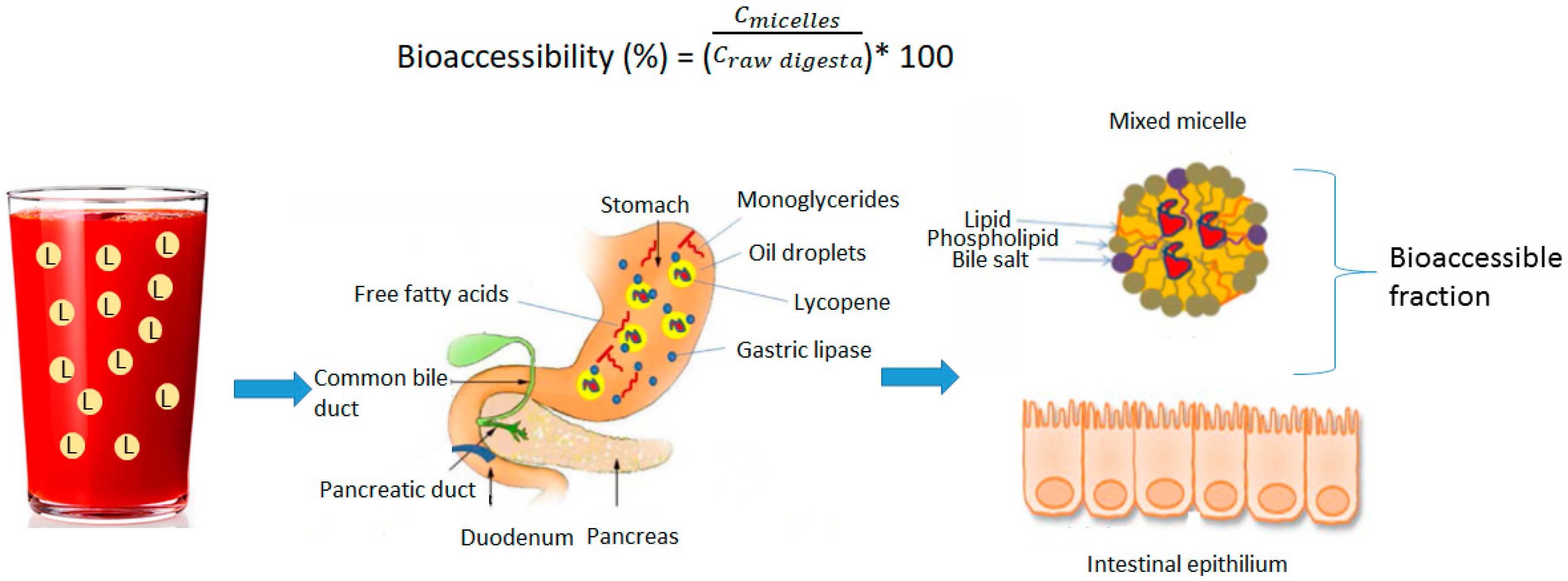
4. Factors Affecting Bioaccessibility of Lycopene in Emulsions
5. Conclusions and Future Trends
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vogele, A.C. Effect of environmental factors upon the color of the tomato and the watermelon. Plant Physiol. 1937, 12, 929–955. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Xianquan, S.; Shi, J.; Kakuda, Y.; Yueming, J. Stability of lycopene during food processing and storage. J. Med. Food 2005, 8, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Schwartz, S.J. Lycopene stability during food processing. Exp. Biol. Med. 1998, 218, 101–105. [Google Scholar] [CrossRef]
- Zumbrunn, A.; Uebelhart, P.; Eugster, C.H. HPLC of carotenes with y-end groups and (Z)-configuration at terminal conjugated double bonds, isolation of (5Z)-lycopene from tomatoes. Helv. Chim. Acta 1985, 68, 1540–1542. [Google Scholar] [CrossRef]
- Raikos, V.; Ranawana, V. Designing emulsion droplets of foods and beverages to enhance delivery of lipophilic bioactive components—A review of recent advances. Int. J. Food Sci. Technol. 2017, 52, 68–80. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Lycopene: A biologically important carotenoid for humans? Arch. Biochem. Biophs. 1996, 336, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Maguer, L.M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.H.; Holden, J.M.; Beecher, G.R.; Khachik, F.; Davis, C.S.; Generose, M.G. Carotenoid content of thermally processed tomato-based food products. J. Agric. Food Chem. 1995, 43, 579–586. [Google Scholar] [CrossRef]
- Boileau, T.W.; Boileau, A.C.; Erdman, J.W. Bioavailability of all-trans and cis-isomers of licopene. Exp. Biol. Med. 2002, 227, 914–919. [Google Scholar] [CrossRef]
- Jenab, M.; Ferrari, P.; Mazuir, M.; Tjonneland, A.; Clavel-Chapelon, F.; Linseisen, J.; Trichopoulou, A.; Tumino, R.; Bueno-de-Mesquita, H.B.; Lund, E.; et al. European prospective investigation into cancer and nutrition (EPIC) study. Variations in lycopene blood levels and tomato consumption across European countries based on the european prospective investigation into cancer and nutrition (EPIC) study. J. Nutr. 2005, 135, 2032S–2036S. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Bioavailability and in vivo antioxidant properties of lycopene from tomato products and their possible role in the prevention of cancer. Nutr. Cancer 1998, 31, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K. Lycopene; chemistry, biosynthesis, metabolism and degradation under various abiotic parameters. J. Food Sci. Technol. 2015, 52, 41–53. [Google Scholar] [CrossRef]
- Bramley, P.M. Is lycopene beneficial to human health? Phytochemistry 2000, 54, 233–236. [Google Scholar] [CrossRef]
- Kun, Y.; Lule, U.S.; Xiao-Lin, D. Lycopene: Its Properties and Relationship to Human Health. Food Rev. Int. 2006, 22, 309–333. [Google Scholar] [CrossRef]
- Sundquist, A.R.; Briviba, K.; Sies, H. Singlet oxygen quenching by carotenoids. Methods Enzymol. 1994, 234, 384–388. [Google Scholar] [PubMed]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. 2016, 56, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, X.; Peng, Y.; Lin, J. Protective effects of licopene against H2O2-induced oxidative injury and apoptosis in human endothelial cells. Cardiovasc. Drugs Ther. 2009, 23, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Huang, T.F.; Chen, B.H.; Shieh, J.M.; Wu, P.H.; Wu, W.B. Lycopene inhibits TNF-alpha-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur. J. Pharmacol. 2008, 586, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.; Catalano, A.; Boninsegna, A.; Bohm, V.; Frohlich, K.; Mele, M.C.; Monego, G.; Ranelletti, F.O. Lycopene prevents 7-ketocholesterol-induced oxidative stress, cell cycle arrest and apoptosis in human macrophages. J. Nutr. Biochem. 2010, 21, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, H.; Inakuma, T. Protective effect of dietary tomato against endothelial dysfunction in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 1999, 63, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Perez-Cornago, A.; Navas-Carretero, S.; Bondia-Pons, I.; Zulet, M.A.; Martinez, J.A. A regular lycopene enriched tomato sauce consumption influences antioxidant status of healthy young-subjects: A crossover study. J. Funct. Foods 2013, 5, 28–35. [Google Scholar] [CrossRef]
- Xaplanteris, P.; Vlachopoulos, C.; Pietri, P.; Terentes-Printzios, D.; Kardara, D.; Alexopoulos, N.; Aznaouridis, K.; Miliou, A.; Stefanadis, C. Tomato paste supplementation improves endothelial dynamics and reduces plasma total oxidative status in healthy subjects. Nutr. Res. 2012, 32, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; von Laar, J.; Martin, H.D.; Emmerich, T.; Sies, H. Stimulation of gap junctional communication: Comparison of acyclo-retinoic acid and lycopene. Arch Biochem. Biophys. 2000, 373, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp. Biol. Med. 2002, 227, 908–913. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willet, W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Countryman, C.; Bankson, D.; Collins, S.; Mar, B.; Lin, W. Lycopene inhibits the growth of the HL-60 promyelocytic leukemia cell line. Clin. Chem. 1991, 37, 1056. [Google Scholar]
- Nagasawa, H.; Mitamura, T.; Sakamoto, S.; Yamamoto, K. Effects of lycopene on spontaneous mammary tumour development in SHN virgin mice. Anticancer Res. 1995, 15, 1173–1178. [Google Scholar] [PubMed]
- Kobayashi, T.; Iijima, K.; Mitamura, T.; Toriizuka, K.; Cyong, J.C.; Nagasawa, H. Effects of lycopene, a carotenoid, on intrathymic T cell differentiation and peripheral CD4/CD8 ratio in a high mammary tumor strain of SHN retired mice. Anticancer Drugs 1996, 7, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Bosin, E.; Feldmen, B.; Giat, Y.; Miinster, A.; Danilenko, M.; Sharoni, Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either α-carotene or β-carotene. Nutr. Cancer 1995, 24, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Helzlsouer, K.J.; Comstock, G.W.; Morris, J.S. Selenium, lycopene, α-tocopherol, β-carotene, retinol and subsequent bladder cancer. Cancer Res. 1989, 49, 6144–6148. [Google Scholar] [PubMed]
- Potischman, N.; McCulloch, C.E.; Byers, T.; Nemoto, T.; Stubbe, N.; Milch, R. Breast cancer and dietary and plasma concentrations of carotenoids and vitamin A. Am. J. Clin. Nutr. 1990, 52, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Andic, F.; Garipagaoglu, M.; Yurdakonar, E.; Tuncel, N.; Kucuk, O. Lycopene in the prevention of gastrointestinal toxicity of radiotherapy. Nutr. Cancer 2009, 61, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, K.; Yalcin, E. Radioprotective effect of lycopene on chromosomal aberrations (CAs) induced by gamma radiation in human lymphocytes. J. Environ. Biol. 2009, 30, 113–117. [Google Scholar] [PubMed]
- Gajowik, A.; Dobrzyńska, M.M. Lycopene-antioxidant with radioprotective and anticancer properties. A review. Roczniki Państwowego Zakładu Higieny 2014, 65, 263–271. [Google Scholar] [PubMed]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Strctural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Ax, K.; Mayer-Miebach, E.; Link, B.; Schuchmann, H.; Schubert, H. Stability of lycopene in oil-in-water emulsions. Eng. Life Sci. 2003, 4, 199–201. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Ax, K.; Shubert, H. Stability of lycopene emulsions in food systems. J. Food Sci. 2003, 68, 2730–2734. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Boon, C.S.; Xu, Z.; Yue, X.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors affecting lycopene oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Sakata, M.; Murata, Y.; Nakamura, Y. Effects of emulsifiers on the photostability of lycopene. Food Sci. Technol. Res. 2013, 19, 983–987. [Google Scholar] [CrossRef]
- Shariffa, Y.N.; Tan, T.B.; Uthumporn, U.; Abas, F.; Mirhosseini, H.; Nehdi, I.A.; Wang, Y.H.; Tan, C.P. Producing a lycopene nanodispersion: Formulation development and the effects of high pressure homogenization. Food Res. Int. 2017, 101, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.H.Y.; Schroën, K.; Berton-Carabin, C.C.; San Martín-González, M.F.; Kacie, K.H.Y. Physicochemical stability of lycopene-loaded emulsions stabilized by plant or dairy proteins. Food Struct. 2017, 12, 34–42. [Google Scholar] [CrossRef]
- Kim, S.O.; Ha, T.V.; Choi, Y.J.; Ko, S. Optimization of homogenization-evaporation process for lycopene nanoemulsion production and its beverage applications. J. Food Sci. 2014, 79, N1604-10. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xue, S.J.; Wang, B.; Wang, W.L.; Ye, X.Q.; Quek, S.Y. Optimization of formulation and influence of environmental stresses on stability of lycopene-microemulsion. Food Sci. Technol. 2015, 60, 999–1008. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Neilson, A.P.; Ferruzzi, M.G. Influence of formulation and processing on absorption and metabolism of flavan-3-ols from tea and cocoa. Annu. Rev. Food Sci. Technol. 2011, 2, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J. Nutr. 1992, 122, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Svelander, C.A.; Lopez-Sanchez, P.; Pudney, P.D.A.; Schumm, S.; Alminger, M.A.G. High pressure homogenisation increases the in vitro bioaccessibility of α- and β-carotene but not of lycopene in tomato emulsions. J. Food Sci. 2011, 76, H215–H225. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Colle, I.J.P.; Van Buggenhout, S.; Lemmens, L.; Van Loey, A.M.; Hendrickx, M.E. The type and quantity of lipids present during digestion influence the in vitro bioaccessibility of lycopene from raw tomato pulp. Food Res. Int. 2012, 45, 250–255. [Google Scholar] [CrossRef]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.-S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, S.; McClements, D.J. Enhancement of lycopene bioaccessibility from tomato juice using excipient emulsions: Influence of lipid droplet size. Food Chem. 2016, 201, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Yao, L.; She, L.; Furr, H.C.A. Comparison of lycopene and astaxanthin absorption from corn oil and olive oil emulsions. Lipids 2000, 35, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.; Ruble, P.E., Jr. Beta-Carotene Absorption: Bile, Fatty Acid, pH, and Flow Rate Effects on Transport. Am. J. Physiol. 1978, 235, E686–E691. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meroni, E.; Raikos, V. Lycopene in Beverage Emulsions: Optimizing Formulation Design and Processing Effects for Enhanced Delivery. Beverages 2018, 4, 14. https://doi.org/10.3390/beverages4010014
Meroni E, Raikos V. Lycopene in Beverage Emulsions: Optimizing Formulation Design and Processing Effects for Enhanced Delivery. Beverages. 2018; 4(1):14. https://doi.org/10.3390/beverages4010014
Chicago/Turabian StyleMeroni, Erika, and Vassilios Raikos. 2018. "Lycopene in Beverage Emulsions: Optimizing Formulation Design and Processing Effects for Enhanced Delivery" Beverages 4, no. 1: 14. https://doi.org/10.3390/beverages4010014






