Extraction of Mangiferin and Chemical Characterization and Sensorial Analysis of Teas from Mangifera indica L. Leaves of the Ubá Variety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant
2.3. Tea Development through Different Methods of Preparation
2.3.1. Preparation of Teas
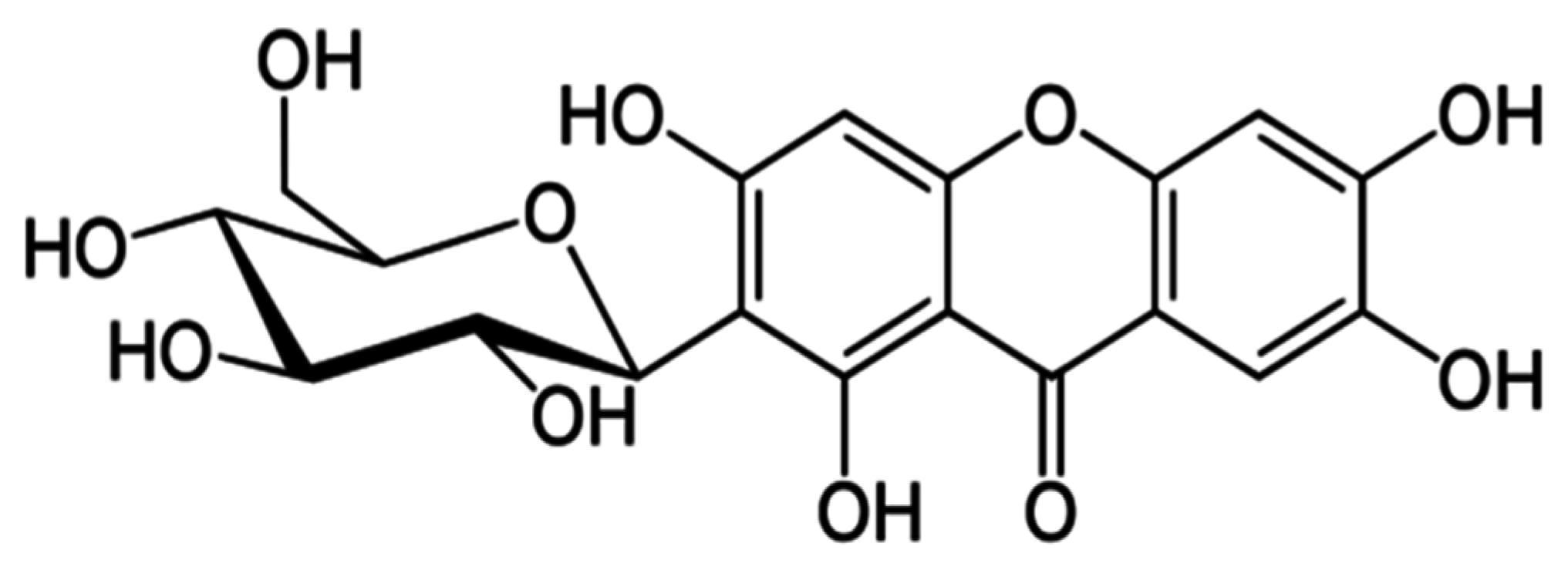
2.3.2. Mangiferin Quantification
2.3.3. Physical and Chemical Characterization of Tea
Total Phenolic (TP) Content
Antioxidant Activity (RSA)
2.4. Oxidative Stability of the Tea
2.5. Sensory Analysis
2.5.1. Preparation of the Tea
2.5.2. Sensorial Test
2.6. Statistical Analysis
2.7. Ethical Statements
3. Results and Discussion
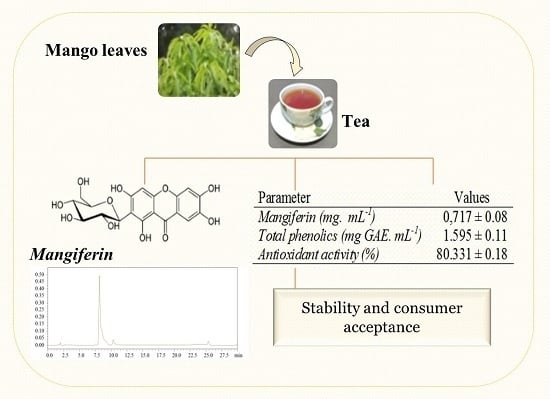
3.1. Mangiferin Concentration in Teas
3.2. Physicochemical Characterization of the Tea
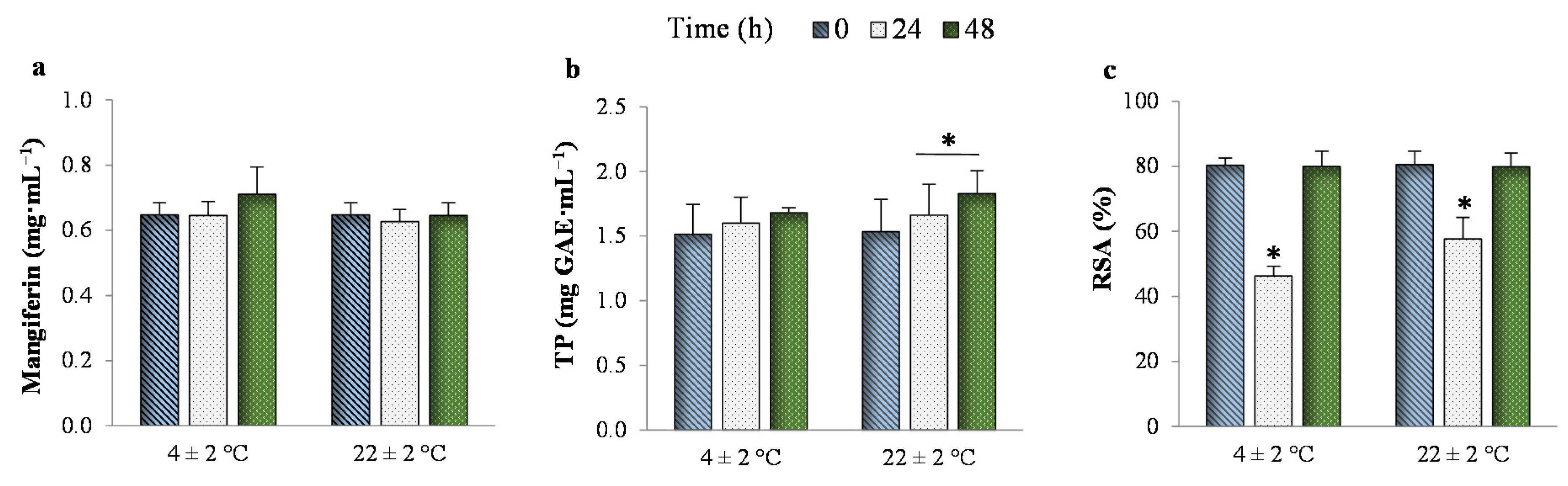
3.3. Stability
3.4. Sensory Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Han, C. Studies on tea and health. J. Hyg. Res. 2011, 40, 802–805. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, X.-B.; Tian, D.-Q.; Fang, X.-P.; Yu, Y.-M.; Zhu, H.-Q.; Ge, Y.-Y.; Ma, G.-Y.; Wang, W.-Y.; Xiao, W.-F.; et al. Antioxidant properties and color parameters of herbal teas in china. Ind. Crops Prod. 2016, 87, 198–209. [Google Scholar] [CrossRef]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-D.; He, F.; Ye, X.-J.; Shen, W.; Wu, Y.-P.; Zhai, Y.-J.; Wang, X.-Y.; Lin, J.-F. Tea consumption is inversely associated with depressive symptoms in the elderly: A cross-sectional study in eastern china. J. Affect. Disord. 2016, 199, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wauthoz, N.; Balde, A.; Balde, E.S.; Damme, M.V.; Duez, P. Ethnopharmacology of Mangifera indica L. Bark and pharmacological studies of its main c glucosylxanthone, mangiferin. Int. J. Biomed. Pharm. Sci. 2007, 1, 112–119. [Google Scholar]
- Saleh, I.G.; Ali, Z.; Abe, N.; Wilson, F.D.; Hamada, F.M.; Abd-Ellah, M.F.; Walker, L.; Khan, I.A.; Ashfaq, M.K. Effect of green tea and its polyphenols on mouse liver. Fitoterapia 2013, 90, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Journal Valor Economico Consumo de Chá Aumenta e se Sofistica No Brasil. Available online: http://www.valor.com.br/empresas/2737452/consumo-de-cha-aumenta-e-se-sofistica-no-brasil (accessed on 16 July 2014).
- Moriyama, H.; Takeda, N. Catechin contents in microbial fermented tea, goichi tea. Rep. Kochi Prefect. Ind. Technol. Center 2008, 39, 7–9. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar]
- Da Silva, J.K.; Batista, Â.G.; Cazarin, C.B.B.; Dionísio, A.P.; de Brito, E.S.; Marques, A.T.B.; Maróstica Junior, M.R. Functional tea from a brazilian berry: Overview of the bioactives compounds. LWT Food Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Ponce, M.T.F.; Casas, L.; Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E. Extraction of antioxidant compounds from different varieties of Mangifera indica leaves using green technologies. J. Supercrit. Fluids 2012, 72, 168–175. [Google Scholar] [CrossRef]
- Rodeiro, I.; Donato, M.T.; Jiménez, N.; Garrido, G.; Delgado, R.; Lechón, M.J.G. Effects of Mangifera indica L. Aqueous extract (vimang) on primary culture of rat hepatocytes. Food Chem. Toxicol. 2007, 45, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A.; Kuś, P.; Góralska, E.; Woźniak, D. Mangiferin—A bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev. Med. Chem. 2013, 13, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.G.D.; Serrano, T.; Calderón, O.; Núñez, F.; Tápanes, R.D.; Pérez, J. Effect of vimang in HIV/AIDS patients. Revis. Cuba. Med. Trop. 2010, 62, 200–206. [Google Scholar]
- Milenkovic, D.; Jude, B.; Morand, C. Mirna as molecular target of polyphenols underlying their biological effects. Free Radic. Biol. Med. 2013, 64, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014, 13, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Resolução da Diretoria Colegiada—RDC N° 26; Ministério da Saúde, Agência Nacional de Vigilância Sanitária (ANVISA): Brasilia, Brasil, 2014.
- Vidigal, M.; Minim, V.; Carvalho, N.; Milagres, M.; Gonçalves, A. Effect of a health claim on consumer acceptance of exotic brazilian fruit juices: Açaí (Euterpe oleracea Mart.), Camu-camu (Myrciaria dubia), Cajá (Spondias lutea L.) and Umbu (Spondias tuberosa Arruda). Food Res. Int. 2011, 44, 1988–1996. [Google Scholar] [CrossRef]
- Pineli, L.D.; Rodrigues, J.D.; Costa, A.M.; de Lima, H.C.; Chiarello, M.D.; Melo, L. Antioxidants and sensory properties of the infusions of wild passiflora from brazilian savannah: Potential as functional beverages. J. Sci. Food Agric. 2015, 95, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- De Bouillé, A.G.; Beeren, C.J.M. 7—Sensory evaluation methods for food and beverage shelf life assessment. In The Stability and Shelf Life of Food, 2nd ed.; Subramaniam, D.K.A.P., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2016; pp. 199–228. [Google Scholar]
- Ibrahim, F.Y.; El-Khateeb, A.Y. Effect of herbal beverages of foeniculum vulgare and cymbopogon proximus on inhibition of calcium oxalate renal crystals formation in rats. Ann. Agric. Sci. 2013, 58, 221–229. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Nunes, M.A.; Almeida, I.M.C.; Carvalho, M.R.; Barroso, M.F.; Alves, R.C.; Oliveira, M.B.P.P. Teas, dietary supplements and fruit juices: A comparative study regarding antioxidant activity and bioactive compounds. LWT Food Sci. Technol. 2012, 49, 324–328. [Google Scholar] [CrossRef]
- Ling, L.T.; Yap, S.-A.; Radhakrishnan, A.K.; Subramaniam, T.; Cheng, H.M.; Palanisamy, U.D. Standardised mangifera indica extract is an ideal antioxidant. Food Chem. 2009, 113, 1154–1159. [Google Scholar] [CrossRef]
- Araújo, B.M.; Gonçalves, R.V.; Peluzio, M.D.; Leite, J.P.; dos Santos Chaves, G.; Lopes, S.O.; do Carmo Miranda, C.; de Queiroz, J.H. Use of Mangifera indica L. Leaves extract and mangiferin on the atherosclerotic lesion in ApoE-/- mice. Biosci. J. 2014, 30, 1873–1881. [Google Scholar]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices; Food Science and Technology; Academic Press: New York, NY, USA, 1993; Volume 2, p. 338. [Google Scholar]
- Kulkarni, V.M.; Rathod, V.K. Extraction of mangiferin from Mangifera indica leaves using threephase partitioning coupled with ultrasound. Ind. Crops Prod. 2014, 52, 292–297. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Chew, Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007, 102, 1214–1222. [Google Scholar] [CrossRef]
- Zaleta, B.; Silva, M.T.; Gutiérrez, A.; Vergara, E.; Rodríguez, M.; Hernández, A. UV/Vis, 1H, and 13C NMR spectroscopic studies to determine mangiferin pKa values. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Jin, F.; Liu, H.; Wang, Y.; Jiang, Y. Metabonomic study on the antitumor effect of flavonoid derivative 3d in HepG2 cells and its action mechanism. Talanta 2014, 118, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhang, L.; Jiang, R.; Ye, M.; Yin, X.; Wan, J. Anti-inflammatory effects of mangiferin on sepsis-induced lung injury in mice via up-regulation of heme oxygenase-1. J. Nutr. Biochem. 2013, 24, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Jainu, M.; Sabitha, K.E.; Devi, C.S.S. Role of mangiferin on biochemical alterations and antioxidant status in isoproterenol-induced myocardial infarction in rats. J. Ethnopharmacol. 2006, 107, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Roach, P.D. Effects of aqueous brewing solution ph on the extraction of the major green tea constituents. Food Res. Int. 2013, 53, 713–719. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.B.; Andrade, S.I.E.; Harding, D.P.; Pistonesi, M.F.; Band, B.S.F.; Araújo, M.C.U. Turbidimetric and photometric determination of total tannins in tea using a micro-flow-batch analyzer. Talanta 2012, 88, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Fu, Y.; Xiang, Y.; Yan, S.; Hu, G.; Huang, X.; Huang, G.; Sun, C.; Li, X.; Chen, K. Identification and quantification of gallotannins in mango (Mangifera indica L.) kernel and peel and their antiproliferative activities. J. Funct. Foods 2014, 8, 282–291. [Google Scholar] [CrossRef]
- Jiménez, J.P.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in french adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Caia, Y.; Luob, Q.; Sunc, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Port’s, P.D.S.; Chisté, R.C.; Godoy, H.T.; Prado, M.A. The phenolic compounds and the antioxidant potential of infusion of herbs from the brazilian amazonian region. Food Res. Int. 2013, 53, 875–881. [Google Scholar]
- Jobu, K.; Yokota, J.; Yoshioka, S.; Moriyama, H.; Murata, S.; Ohishi, M.; Ukeda, H.; Miyamura, M. Effects of goishi tea on diet-induced obesity in mice. Food Res. Int. 2013, 54, 324–329. [Google Scholar] [CrossRef]
- Snoussi, C.; Ducroc, R.; Hamdaoui, M.H.; Dhaouadi, K.; Abaidi, H.; Cluzeaud, F.; Nazaret, C.; Le Gall, M.; Bado, A. Green tea decoction improves glucose tolerance and reduces weight gain of rats fed normal and high-fat diet. J. Nutr. Biochem. 2014, 25, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Taniguchi, Y.; Saka, A.; Yoshida, A.; Yajima, H. Prevention of diet-induced obesity by dietary black tea polyphenols extract in vitro and in vivo. Nutrition 2011, 27, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Luximon-Ramma, A.; Neergheen-Bhujun, V.S.; Gunness, T.K.; Googoolye, K.; Auger, C.; Crozier, A.; Aruom, O.I. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev. Med. 2012, 54, S98–S102. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant capacity and polyphenolic components of teas: Implications for altering in vivo antioxidant status. Proc. Soc. Exp. Biol. Med. 1999, 220, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Bassani, D.C.; Nunes, D.S.; Granato, D. Optimization of phenolics and flavonoids extraction conditions and antioxidant activity of roasted Yerba-Mate leaves (Ilex paraguariensis A. St.-Hil., Aquifoliaceae) using response surface methodology. An. Acad. Bras. Ciênc. 2014, 86, 923–933. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Antiradical and tea polyphenol-stabilizing ability of functional fermented soymilk–tea beverage. Food Chem. 2014, 158, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Vidal-Guevara, M.L.; Zafrilla, P.; Morillas-Ruiz, J.M. A new antioxidant beverage produced with green tea and apple. Int. J. Food Sci. Nutr. 2014, 65, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Lee, Y.-C.; Kyung Rhee, Y.; Lee, S.-Y. Consumer acceptance of ginseng food products. J. Food Sci. 2011, 76, S516–S522. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.J. Effects of sugar and fat consumption on sweet and fat taste. Curr. Opin. Behav. Sci. 2016, 9, 55–60. [Google Scholar] [CrossRef]
- Bray, G.A.; Popkin, B.M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes?: Health be damned! Pour on the sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Montagnese, C.; Mathers, J.C.; Soroka, K.R.; Stephan, B.C.; Wells, J.C. Sugar consumption and global prevalence of obesity and hypertension: An ecological analysis. Public Health Nutr. 2014, 17, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Chung, S.-J.; Kim, H.-S.; Kim, K.-O.K. Effect of sensory characteristics and non-sensory factors on consumer liking of various canned tea products. J. Food Sci. 2005, 70, S532–S538. [Google Scholar] [CrossRef]
- De Godoy, R.C.; Deliza, R.; Gheno, L.B.; Licodiedoff, S.; Frizon, C.N.; Ribani, R.H.; dos Santos, G.G. Consumer perceptions, attitudes and acceptance of new and traditional mate tea products. Food Res. Int. 2013, 53, 801–807. [Google Scholar] [CrossRef]
- Lee, J.; CHAMBERS IV, E.; Chambers, D.; Chun, S.; Oupadissakoon, C.; Johnson, D. Consumer acceptance for green tea by consumers in the united states, korea and thailand. J. Sens. Stud. 2010, 25, 109–132. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Maia, J.G.S.; Maria das Graças, B.Z. Aroma volatile constituents of brazilian varieties of mango fruit. J. Food Compos. Anal. 2000, 13, 27–33. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Uddin, J.; Siddiqui, A.J.; Akram, M.I. Quantification of aroma constituents of mango sap from different pakistan mango cultivars using gas chromatography triple quadrupole mass spectrometry. Food Chem. 2016, 196, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Sook Chung, H.; Lee, S.Y. Modification of ginseng flavors by bitter compounds found in chocolate and coffee. J. Food Sci. 2012, 77, S202–S210. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J. Chemosensory learning and flavour: Perception, preference and intake. Physiol. Behav. 2012, 107, 553–559. [Google Scholar] [CrossRef] [PubMed]


| Leaf Type | Preparation Techniques | Medicinal Plant:Solvent Ratio (g of M. indica Leaves·mL−1 of Water) |
|---|---|---|
| Young | Decoction | 0.0125 |
| 0.0250 | ||
| 0.0500 | ||
| Infusion | 0.0125 | |
| 0.0250 | ||
| 0.0500 | ||
| Ultrasound | 0.0125 | |
| 0.0250 | ||
| 0.0500 | ||
| Mature | Decoction | 0.0125 |
| 0.0250 | ||
| 0.0500 | ||
| Infusion | 0.0125 | |
| 0.0250 | ||
| 0.0500 | ||
| Ultrasound | 0.0125 | |
| 0.0250 | ||
| 0.0500 |
| Preparation Technique | (Medicinal Plant:Solvent) Ratio (g of M. indica Leaves·mL−1 of water) | Mangiferin (mg·mL−1) | |
|---|---|---|---|
| Young Leaves | Mature Leaves | ||
| Decoction | 0.0500 | 0.717 ± 0.08 A | 0.573 ± 0.01 B |
| 0.0250 | 0.419 ± 0.04 a | 0.292 ± 0.06 b | |
| 0.0125 | 0.184 ± 0.01 I | 0.140 ± 0.01 II | |
| Infusion | 0.0500 | 0.285 ± 0.01 C | 0.572 ± 0.01 B |
| 0.0250 | 0.140 ± 0.01 c | 0.243 ± 0.02 b | |
| 0.0125 | 0.074 ± 0.01 III | 0.135 ± 0.01 II | |
| Ultrasound | 0.0500 | 0.177 ± 0.02 D | 0.114 ± 0.01 D |
| 0.0250 | 0.137 ± 0.02 c | 0.078 ± 0.01 c | |
| 0.0125 | 0.073 ± 0.01 III | 0.052 ± 0.01 III | |
| Parameter | Values |
|---|---|
| pH | 5.140 ± 0.04 |
| Total phenolic (mg GAE·mL−1) | 1.595 ± 0.11 |
| Antioxidant activity * (RSA%) | 80.331 ± 0.18 |
| Teas | Attribute | ||
|---|---|---|---|
| Aroma | Flavor | Overall Impression | |
| Natural | 6.5 ± 1.8 b | 5.3 ± 2.1 b | 5.7 ± 1.9 b |
| Orange * | 7.7 ± 1.3 a | 6.2 ± 1.7 a | 6.5 ± 1.6 a |
| Fennel * | 7.7 ± 1.1 a | 6.3 ± 2.3 a | 6.7 ± 1.7 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina Ramírez, N.; Monteiro Farias, L.; Apolonio Santana, F.; Viana Leite, J.P.; De Souza Dantas, M.I.; Lopes Toledo, R.C.; De Queiroz, J.H.; Stampini Duarte Martino, H.; Machado Rocha Ribeiro, S. Extraction of Mangiferin and Chemical Characterization and Sensorial Analysis of Teas from Mangifera indica L. Leaves of the Ubá Variety. Beverages 2016, 2, 33. https://doi.org/10.3390/beverages2040033
Medina Ramírez N, Monteiro Farias L, Apolonio Santana F, Viana Leite JP, De Souza Dantas MI, Lopes Toledo RC, De Queiroz JH, Stampini Duarte Martino H, Machado Rocha Ribeiro S. Extraction of Mangiferin and Chemical Characterization and Sensorial Analysis of Teas from Mangifera indica L. Leaves of the Ubá Variety. Beverages. 2016; 2(4):33. https://doi.org/10.3390/beverages2040033
Chicago/Turabian StyleMedina Ramírez, Natalia, Leticia Monteiro Farias, Francine Apolonio Santana, João Paulo Viana Leite, Maria Inês De Souza Dantas, Renata Celi Lopes Toledo, José Humberto De Queiroz, Hércia Stampini Duarte Martino, and Sônia Machado Rocha Ribeiro. 2016. "Extraction of Mangiferin and Chemical Characterization and Sensorial Analysis of Teas from Mangifera indica L. Leaves of the Ubá Variety" Beverages 2, no. 4: 33. https://doi.org/10.3390/beverages2040033