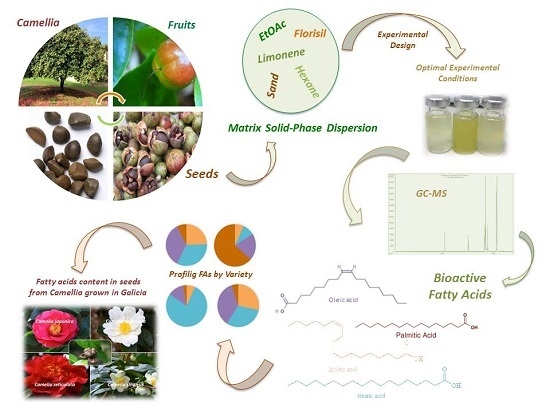
Profiling the Fatty Acids Content of Ornamental Camellia Seeds Cultivated in Galicia by an Optimized Matrix Solid-Phase Dispersion Extraction
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Samples
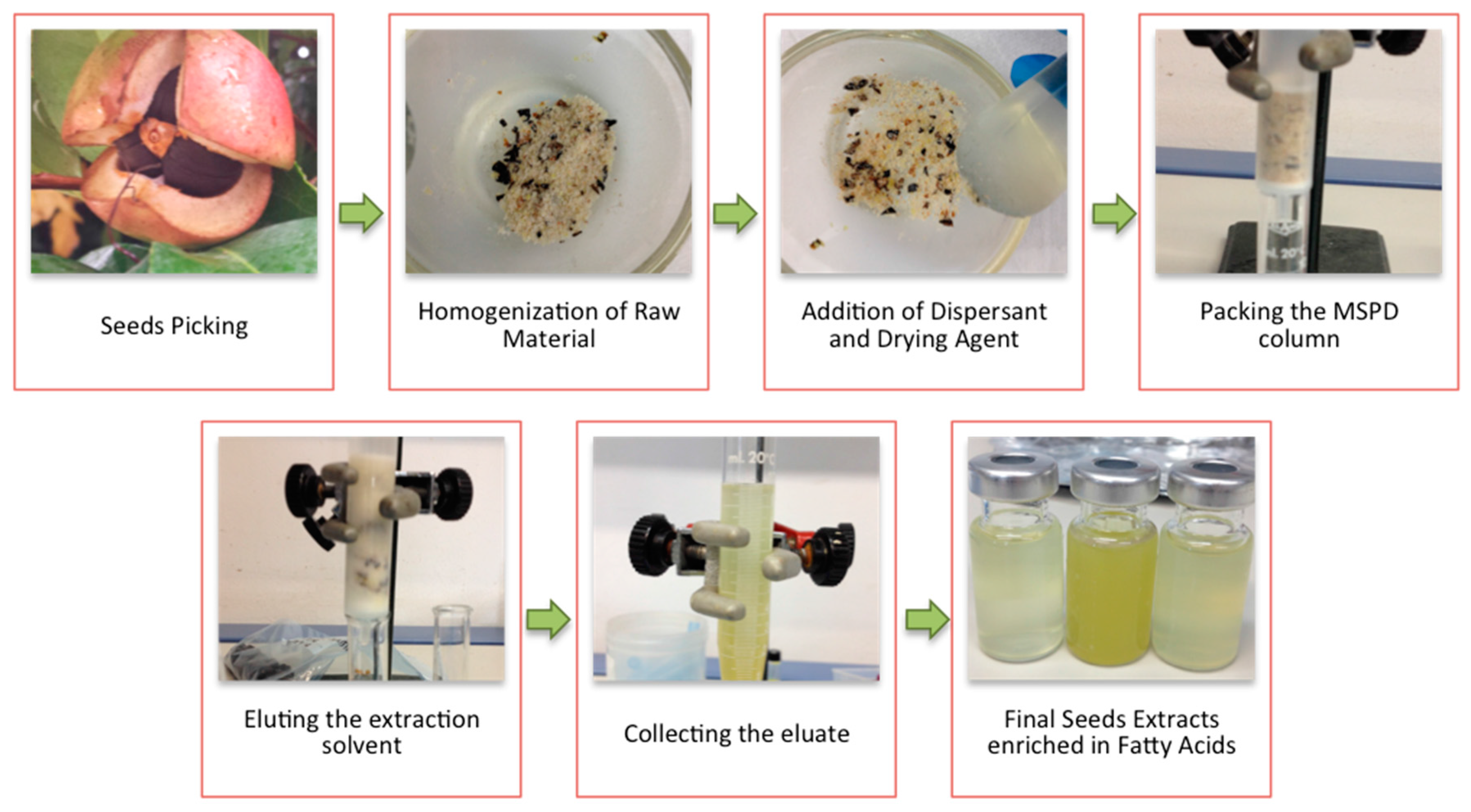
2.3. MSPD
2.4. Gas Chromatography-Mass Spectrometry
2.5. Statistical Analysis
3. Results and Discussion
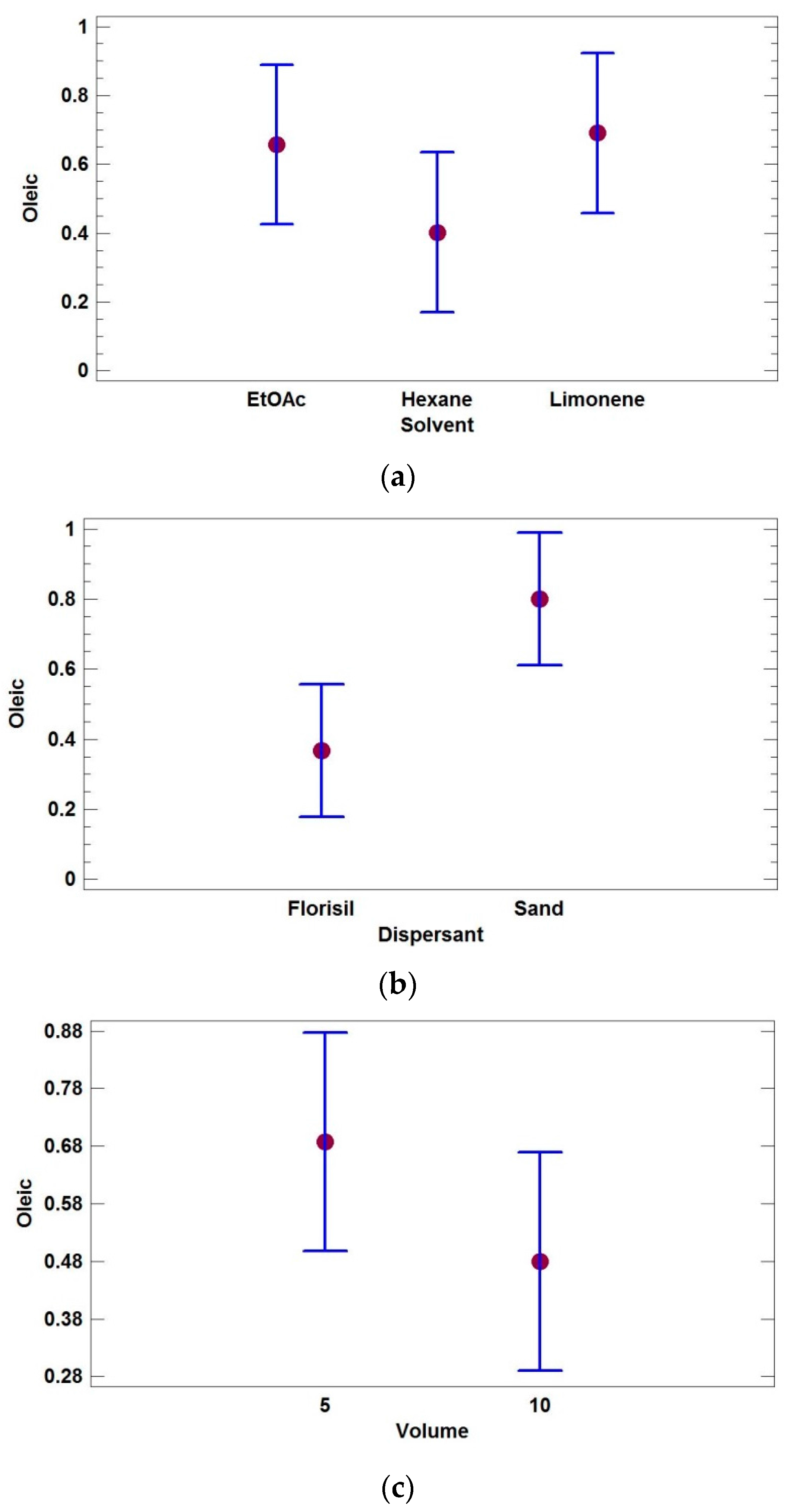
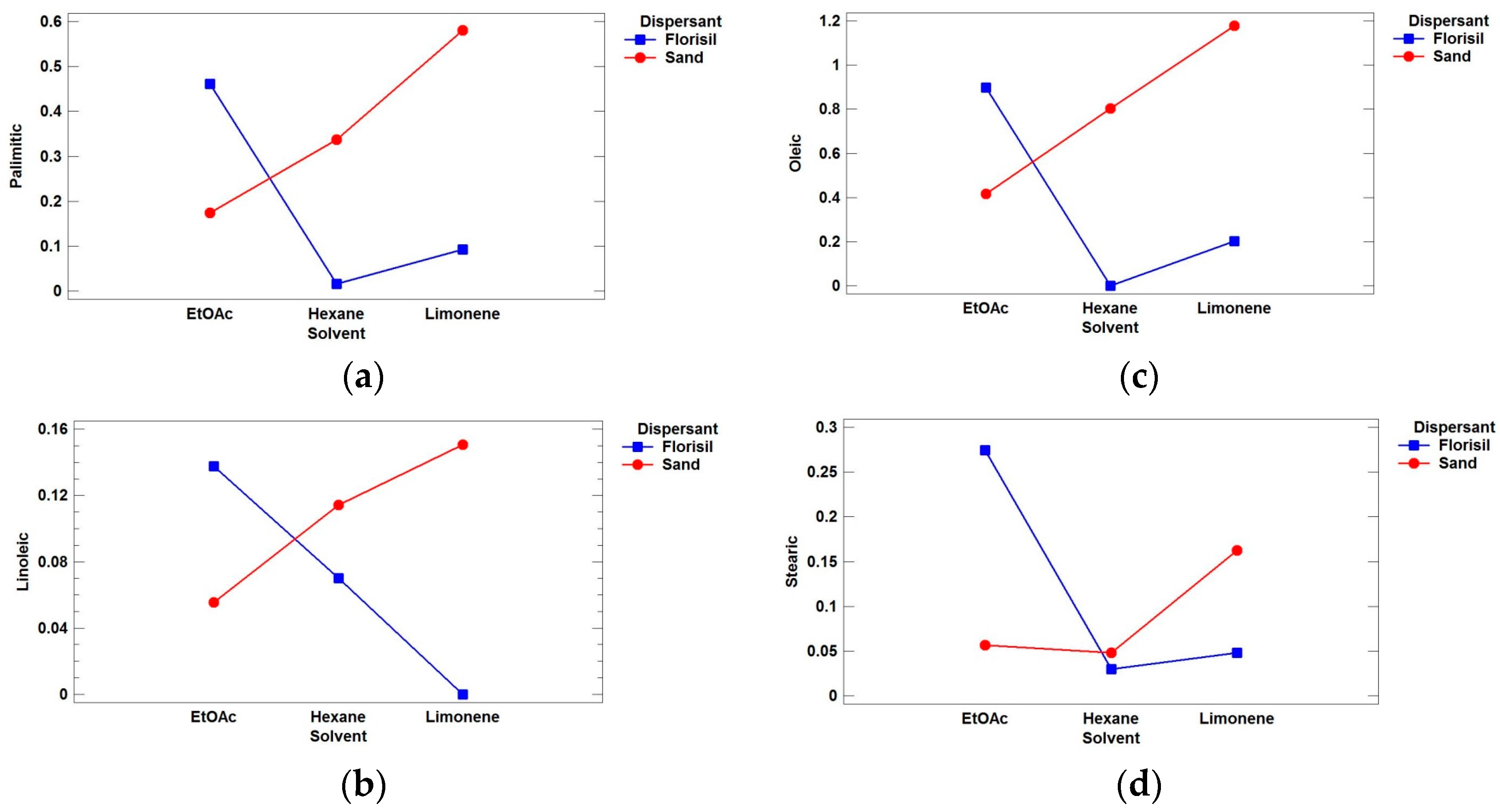
3.1. Optimization of the MSPD Extraction
3.2. Method Performance
3.3. Fatty Acids Profiles of Camellia Seeds Cultivated in Galicia
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vela, P.; Salinero, C.; Sainz, M.J. Phenological growth stages of Camellia japonica. Ann. Appl. Biol. 2013, 162, 182–190. [Google Scholar] [CrossRef]
- Dias, T.R.; Tomás, G.; Teixeira, N.F.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. White Tea (Camellia sinensis (L.)): Antioxidant Properties and Beneficial Health Effects. Int. J. Food Sci. Nutr. Diet. 2013, 2, 19–26. [Google Scholar]
- Feás, X.; Estevinho, L.M.; Salinero, C.; Vela, P.; Sainz, M.J.; Vázquez-Tato, M.P.; Seijas, J.A. Triacylglyceride, Antioxidant and Antimicrobial Features of Virgin Camellia oleifera, C. reticulata and C. sasanqua Oils. Molecules 2013, 18, 4573–4587. [Google Scholar]
- Kim, Y.-S.; Kim, R.; Soon-Na, M.; Choi, D. Effect on the physicochemical characteristics of seed oil of Camellia sinensis. Appl. Chem. Eng. 2010, 21, 148–153. [Google Scholar]
- Fang, X.; Fei, X.; Sun, H.; Jin, Y. Aqueous enzymatic extraction and demulsification of Camellia seed oil (Camellia oleifera Abel.) and the oil’s physicochemical properties. Eur. J. Lipid Sci. Technol. 2016, 118, 244–251. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Cara, P.D.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, D.; Chen, H.; Qian, L.; Xu, P. Fatty Acid Composition and Antioxidant Activity of Tea (Camellia sinensis L.) Seed Oil Extracted by Optimized Supercritical Carbon Dioxide. Int. J. Mol. Sci. 2011, 12, 7708–7719. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Chen, Z.; Lin, Y.; Wang, S. Comparison of Oil Content and Fatty Acid Profile of Ten New Camellia oleifera Cultivars. J. Lipids 2016, 2016, 3982486. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, Q.; Verardo, V.; Contreras, M.M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “FancyTiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, A.; Barzegar, M.; Yamini, Y. Supercritical fluid extraction of tea seed oil and its comparison with solvent extraction. Eur. Food Res. Technol. 2004, 220, 401–405. [Google Scholar] [CrossRef]
- Demirbas, A. Oil from tea seed by supercritical fluid extraction. Energy Sources Part A 2009, 31, 217–222. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, D.; Chen, X. A novel process for extraction of tea oil from Camellia oleifera seed kernels by combination of microwave puffing and aqueous enzymatic oil extraction. Eur. J. Lipid Sci. Technol. 2012, 114, 352–356. [Google Scholar] [CrossRef]
- Yu, X.; Li, Q.; Du, S.; Zhang, R.; Xu, C. A novel process for the aqueous extraction of oil from Camellia oleifera seeds and its antioxidant activity. Grasas y Aceites 2013, 64, 407–414. [Google Scholar] [CrossRef]
- Barker, S.A.; Long, A.R.; Short, C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. 1989, 475, 353–361. [Google Scholar] [CrossRef]
- Barker, S.A. Matrix solid phase dispersion (MSPD). J. Biochem. Biophys. Methods 2007, 70, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Haiyan, Z.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2007, 100, 1544–1551. [Google Scholar] [CrossRef]
- Su, M.H.; Shih, M.C.; Lin, K.-H. Chemical composition of seed oils in native Taiwanese Camellia species. Food Chem. 2014, 156, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hu, C.; Qian, J.; Wu, D. Determination of underivatized long chain fatty acids using HPLC with an evaporative light scattering detector. JAOCS 2012, 89, 183–187. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Cgen, H.; Zhou, H.; Ye, J. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandón, M.; Lores, M.; Domínguez, J. Comparison of extraction and derivatization methods for fatty acid analysis in solid environmental matrixes. Anal. Bioanal. Chem. 2008, 392, 505–514. [Google Scholar] [CrossRef] [PubMed]

| Splitless Pulse Injection | 30 psi for 1.25 min |
| Injection volume | 1 µL |
| Injector temperature | 260 °C |
| Column | ZB-Semivolatiles (30 m × 0.25 mm × 0.25 μm) |
| Temperature Program | 60 °C (1 min), 20 °C min−1 to 150 °C; |
| 10 °C min−1 to 290 °C (10.5 min) | |
| 10 °C min−1 to 310 °C (3 min) | |
| Mass spectrometer | Transfer line at 310 °C |
| Ion source at 230 °C | |
| Quadrupole at 150 °C | |
| Mode | Electronic Ionization (EI) |
| Full Scan m/z: 45–450 amu |
| Factor | Code | Low Level | Central Level | High Level | Continuous |
| Extraction Solvent | A | Ethyl acetate | Hexane | Limonene | No |
| Dispersive Phase | B | Sand | Florisil | No | |
| Eluting Volume (mL) | C | 5 | 10 | Yes | |
| Fixed factors | Value | ||||
| Sample Size | 1 g | ||||
| Ratio Sample: Dispersant | 1:4 | ||||
| Drying agent | 2 g |
| Acid | A: Solvent | B: Dispersant | C: Volume | D: Interaction AB | ||||
|---|---|---|---|---|---|---|---|---|
| F Ratio | p Value | F Ratio | p Value | F Ratio | p Value | F Ratio | p Value | |
| Palmitic (C16:0) | 9.13 | 0.0987 | 27.01 | 0.0351 | 5.88 | 0.1362 | 49.75 | 0.0197 |
| Stearic (C18:0) | 2.97 | 0.2518 | 0.44 | 0.5742 | 0.09 | 0.7938 | 5.39 | 0.1564 |
| Oleic (C18:1) | 4.28 | 0.1896 | 24.20 | 0.0389 | 5.57 | 0.1422 | 27.20 | 0.0355 |
| Linoleic (C18:2) | 1.07 | 0.4836 | 8.84 | 0.0969 | 3.90 | 0.1868 | 28.51 | 0.0339 |
| Acid | Linearity | IDL (µg mL−1) | Precision (RSD%) | |||||
|---|---|---|---|---|---|---|---|---|
| Range (µg mL−1) | R2 | Intra-Day (n = 3) | Inter-Day (n = 9) | |||||
| 5 | 10 | 50 | 5 | 35 | ||||
| C16:0 | 1–50 | 0.9929 | 0.198 | 5.09 | 2.45 | 2.55 | 3.05 | 3.13 |
| C18:0 | 1–50 | 0.9916 | 0.056 | 3.23 | 7.63 | 3.75 | 7.12 | 3.15 |
| Cis-C18:1 | 1–50 | 0.9922 | 0.051 | 10.4 | 3.00 | 2.14 | 5.40 | 9.99 |
| Trans-C18:1 | 1–50 | 0.9921 | 0.100 | 6.19 | 1.15 | 1.06 | 7.14 | 2.88 |
| C18:2 | 1–50 | 0.9960 | 0.135 | 23.0 | 4.39 | 1.15 | 14.0 | 8.67 |
| C18:3 | 1–50 | 0.9926 | 0.123 | 2.98 | 0.77 | 2.83 | 2.61 | 1.89 |
| C. sasanqua | C. reticulata | C. sinensis | C. japonica | |
|---|---|---|---|---|
| C16:0 | 5.8 ± 0.9 | 6.7 ± 0.6 | 9.4 ± 0.0 | 7.5 ± 0.6 |
| C18:0 | 1.8 ± 0.4 | 2.8 ± 0.1 | 7.5 ± 1.2 | 1.4 ± 0.4 |
| C18:1 | 84 ± 2 | 78 ± 1 | 64 ± 0 | 82 ± 1 |
| C18:2 | 8.7 ± 0.5 | 13 ± 0 | 19 ± 1 | 9.5 ± 0.4 |
| SUA | 92 ± 1 | 91 ± 1 | 83 ± 1 | 91 ± 1 |
| SSA | 7.6 ± 1.3 | 9.4 ± 0.7 | 17 ± 1 | 8.9 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Jares, C.; Sanchez-Nande, M.; Lamas, J.P.; Lores, M. Profiling the Fatty Acids Content of Ornamental Camellia Seeds Cultivated in Galicia by an Optimized Matrix Solid-Phase Dispersion Extraction. Bioengineering 2017, 4, 87. https://doi.org/10.3390/bioengineering4040087
Garcia-Jares C, Sanchez-Nande M, Lamas JP, Lores M. Profiling the Fatty Acids Content of Ornamental Camellia Seeds Cultivated in Galicia by an Optimized Matrix Solid-Phase Dispersion Extraction. Bioengineering. 2017; 4(4):87. https://doi.org/10.3390/bioengineering4040087
Chicago/Turabian StyleGarcia-Jares, Carmen, Marta Sanchez-Nande, Juan Pablo Lamas, and Marta Lores. 2017. "Profiling the Fatty Acids Content of Ornamental Camellia Seeds Cultivated in Galicia by an Optimized Matrix Solid-Phase Dispersion Extraction" Bioengineering 4, no. 4: 87. https://doi.org/10.3390/bioengineering4040087