Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
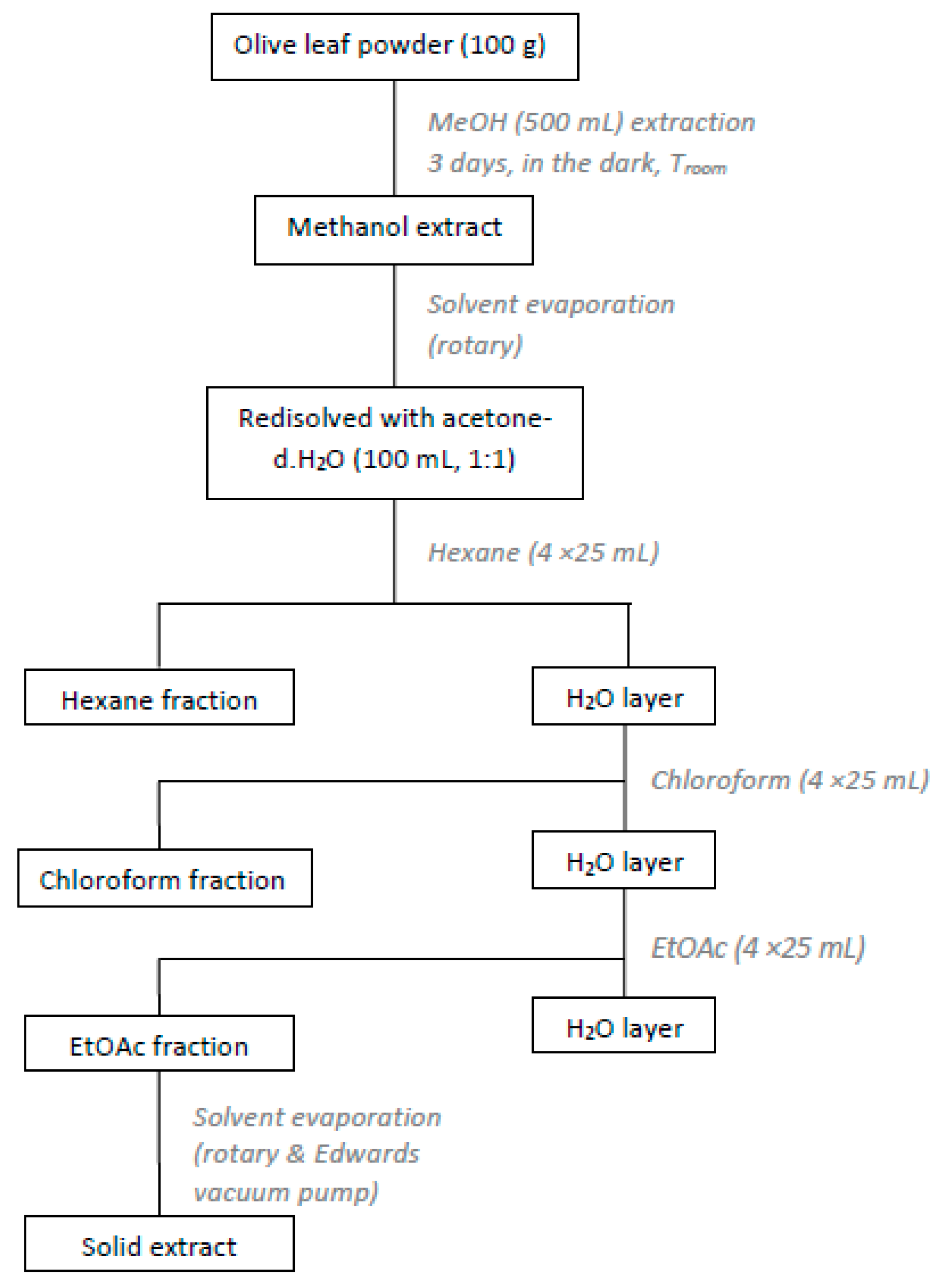
2.2.1. Preparation of Olive Leaves Extract (OLE)
2.2.2. High-Performance Liquid Chromatography Analysis of Olive Leaves Extract
2.2.3. Luminol Chemiluminescence Assay
2.2.4. Preparation of PLA Nanoparticles (PLA NPs)
2.2.5. 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) Radical Scavenging Assay
2.2.6. Total Phenolic Content
2.3. Nanoparticles (NPs) Characterization
2.3.1. Determination of Particle Size, Pdi and ζ-Potential
2.3.2. Morphology
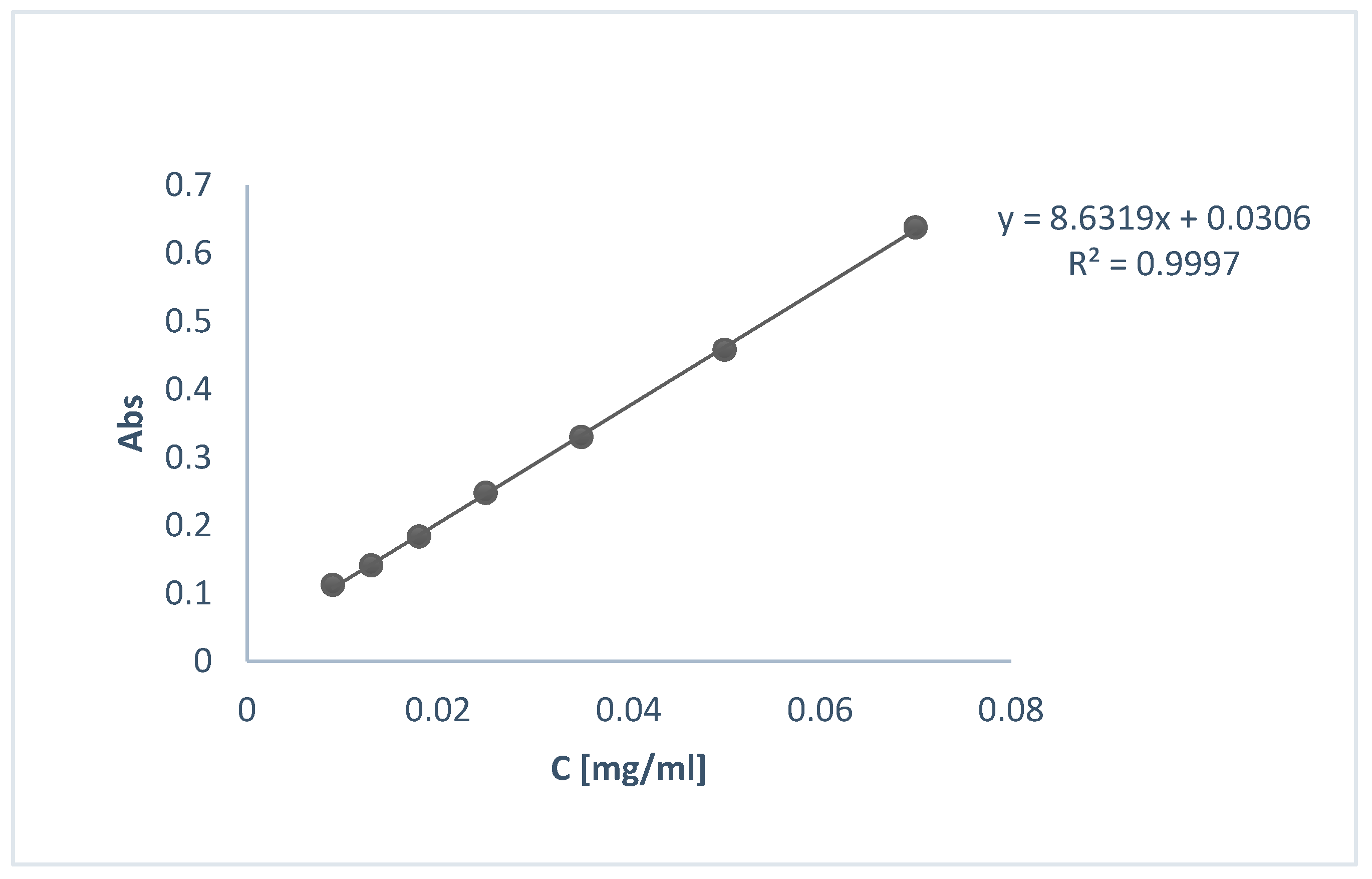
2.3.3. Encapsulation Efficiency Determination
2.3.4. Thermal Properties
2.3.5. FT-IR Spectroscopy
2.3.6. In Vitro Release Study
2.3.7. Incorporation of OLE-NPs in Cosmetic Formulation and Stability Studies
3. Results and Discussion
3.1. Antioxidant Activity of Olive Leaves Extract (OLE), PLA and OLE-NPs
3.2. Phytochemical Profile of Olive Leaves Extract
3.3. Nanoparticles (NPs) Characterization and Encapsulation Efficiency (EE%)
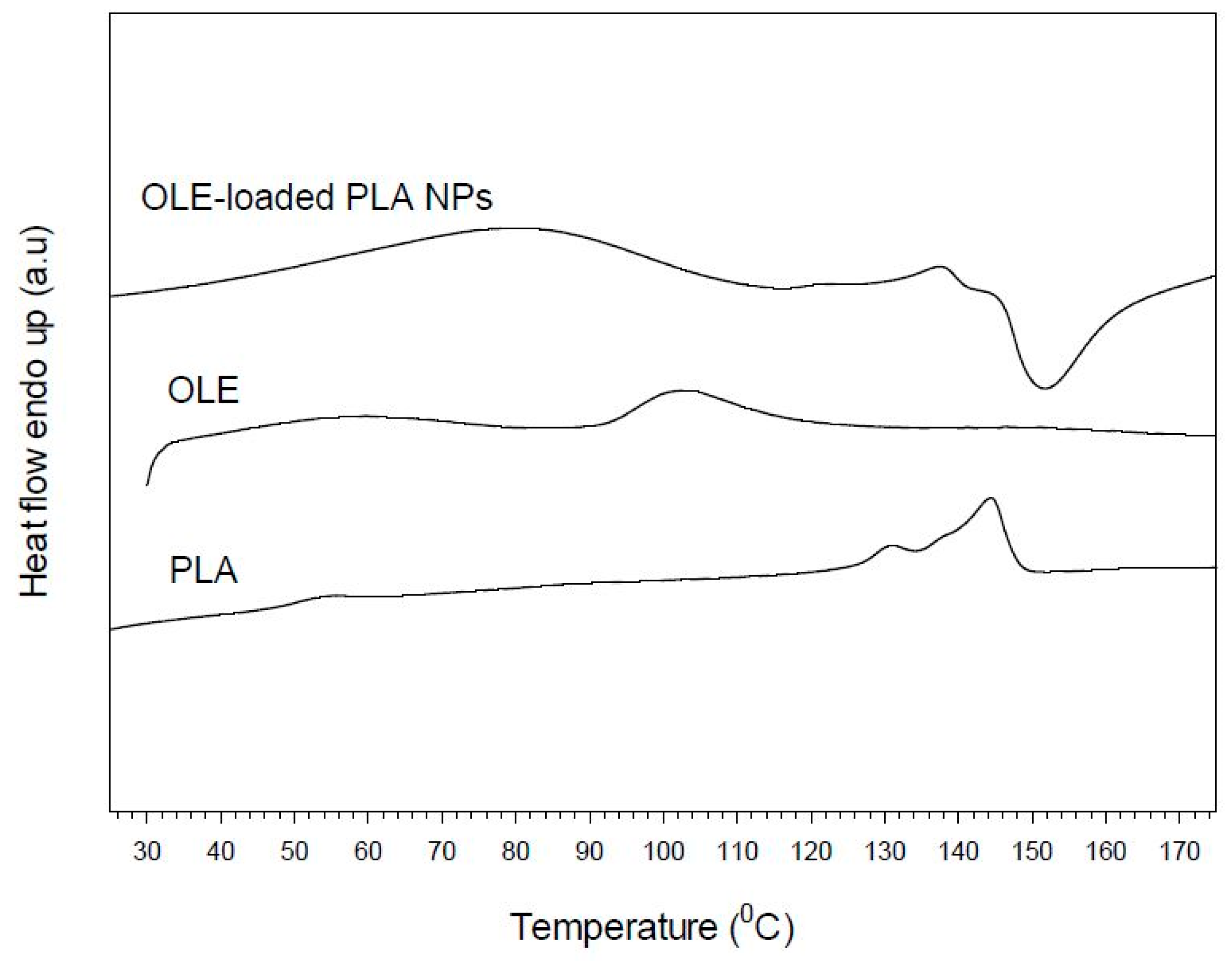
3.4. Thermal Properties
3.5. FT-IR Spectroscopy
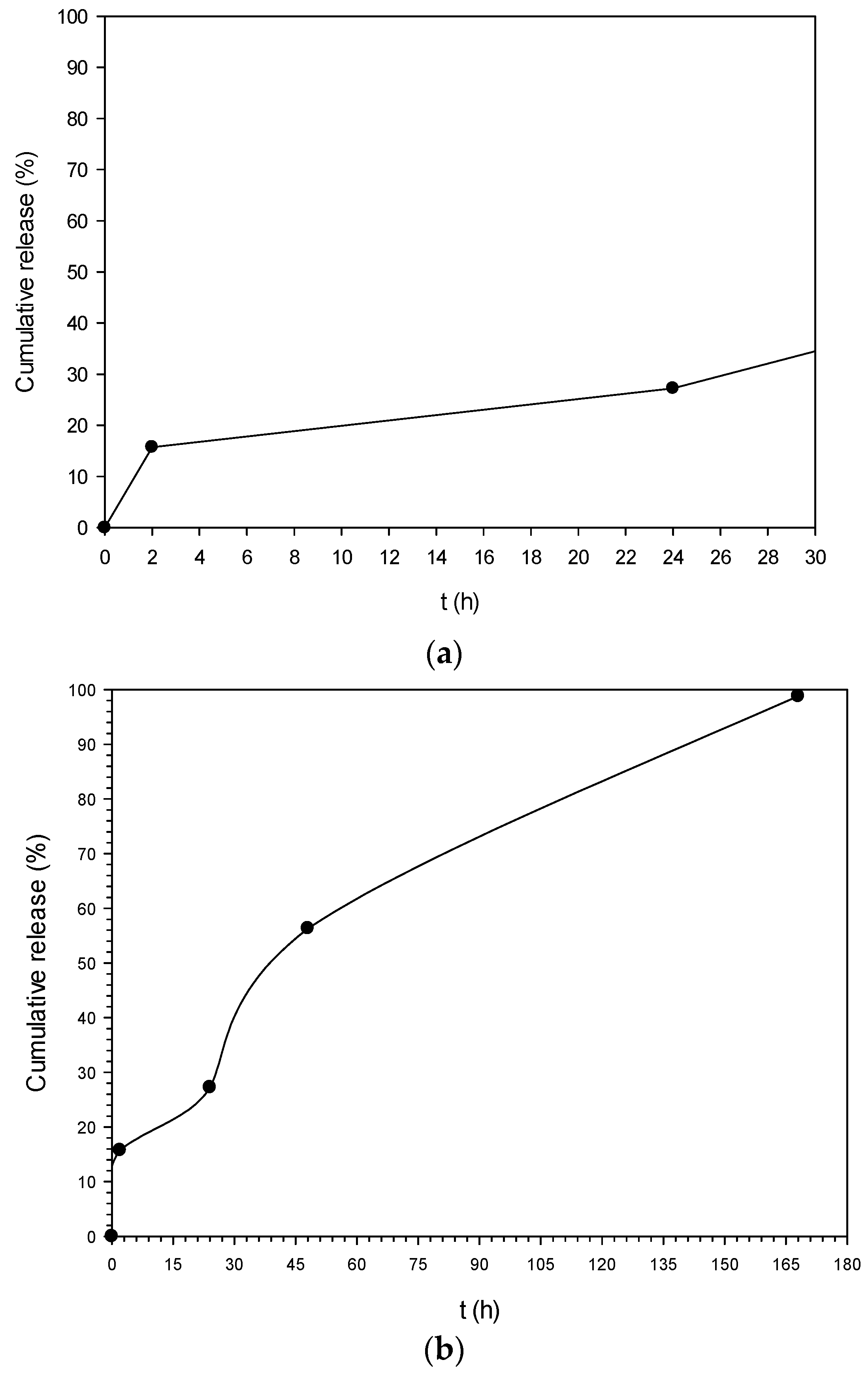
3.6. In Vitro Release Study
3.7. Stability Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Dtojiljkovic, D.; Pavlovic, D.; Arsic, I. Oxidative stress, skin aging and antioxidant therapy. Sci. J. Fac. Med. 2014, 31, 207–217. [Google Scholar]
- Misaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, Y. Photoaging form an oxidative standpoint. J. Dermatol. Sci. 1995, 9, 79–86. [Google Scholar] [CrossRef]
- Nishigori, C.; Hattori, Y.; Arima, Y.; Miyachi, Y. Photoaging and oxidative stress. Exp. Dermatol. 2003, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljsak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Peres, P.S.; Terra, V.A.; Guarnier, F.A.; Cecchini, R.; Cecchini, A.L. Photoaging and chronological aging profile: Understanding oxidation of the skin. J. Photochem. Photobiol. B 2011, 103, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Lyko, A.; Arct, J.; Pytkowska, K. Methods for evaluation of cosmetic antioxidant capacity. Skin Res. Technol. 2012, 18, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Akhtar, N.; Mumtaz, A.M.; Khan, M.S.; Iqbal, F.M.; Zaidi, S.S. In vivo skin irritation potential of a cream containing Moringa oleifera leaf extract. Afr. J. Pharm. Pharmacol. 2013, 7, 289–293. [Google Scholar] [CrossRef]
- Almeida, I.F.; Valentao, P.; Andradr, P.B.; Seabra, R.M.; Pereira, T.M.; Amaral, M.H.; Costa, P.C.; Bahia, M.F. In vivo skin irritation potential of a potential of a Castanea sativa (Chestnut) leaf extract, a putative natural antioxidant for topical application. Basic Clin. Pharmacol. Toxicol. 2008, 103, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, M.; Sayadi, S. Isolation and evaluation of antioxidants from leaves of Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar] [CrossRef]
- Lee, O.; Lee, B.; Lee, J.; Lee, H.; Son, J.; Park, C.; Shetty, K.; Kim, Y. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-Cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Panchal, S.; Vyas, N.; Butani, A.; Kumar, V. Olea europaea: A phyto-pharmacological Review. Pharmacogn. Rev. 2007, 1, 112–116. [Google Scholar]
- Xinos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A. Development of a green extraction procedure with super/subcritical fluid to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Manna, C.; Migliardi, V.; Golino, P.; Scognamiglio, A.; Galleti, P.; Chiariello, M.; Zappia, V. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J. Nutr. Biochem. 2004, 15, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Levy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceuticals 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Ammala, A. Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Duclairoir, C.; Orecchioni, A.M.; Depraetere, P.; Nakache, E. α-Tocopherol encapsulation and in vitro release from wheat gliadin nanoparticles. J. Microencapsul. 2002, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Failloux, N.; Baron, M.H.; Abdul-Malak, N.; Perrier, E. Contribution of encapsulation on the biodisponibility of retinol. Int. J. Cosmet. Sci. 2004, 26, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.; Pakade, Y.; Singh, B.; Yadah, S. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B. Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Roussaki, M.; Gaitanarou, A.; Diamanti, P.Ch.; Vouyiouka, S.; Papaspyrides, C.; Kefalas, P.; Detsi, A. Encapsulation of the natural antioxidant aureusidin in biodegradable PLA nanoparticles. Polym. Degrad. Stab. 2014, 108, 182–187. [Google Scholar] [CrossRef]
- Sanna, V.; Lubinu, G.; Madau, P.; Pala, N.; Nurra, S.; Mariani, A.; Sechi, M. Polymeric nanoparticles encapsulating white tea extract for nutraceutical application. J. Agric. Food Chem. 2015, 63, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Vettor, M.; Perugini, P.; Scalia, S.; Conti, B.; Genta, I.; Modena, T.; Pavanetto, F. Poly(d,l-lacticide) nanoencapsulation to reduce photoinactivation of a sunscreen agent. Int. J. Cosmet. Sci. 2008, 30, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Blum-Peytavi, U.; Vogt, A. Utilization of biodegradable polymeric materials as delivery agents in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E. Nanoparticles and the skin—Applications and limitations. J. Microencapsul. 2011, 28, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Morganti, P. Use and potential of nanotechnology in cosmetic dermatology. Clin. Cosmet. Investig. Dermatol. 2010, 3, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Grabnar, P.A.; Kristl, J. The manufacturing techniques of drug-loaded polymeric nanoparticles from preformed polymers. J. Microencapsul. 2011, 28, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Van der Walle, C.F. Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J. Pharm. Sci. 2008, 279, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Vouyiouka, S.; Theodoulou, P.; Symeonidou, A.; Papaspyrides, C.D.; Pfaendner, P. Solid state polymerization of poly(lactic acid): Some fundamental parameters. Polym. Degrad. Stab. 2013, 98, 2473–2481. [Google Scholar] [CrossRef]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Evaluation of extracts prepared from olive oil by-products using microwave-assisted enzymatic extraction: Effect of encapsulation on the stability of final products. Waste Biomass Valoriz. 2016, 7, 831–842. [Google Scholar] [CrossRef]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Wiechers, J.; Musee, N. Engineered inorganic nanoparticles and cosmetics: Facts, issues, knowledge gaps and challenges. J. Biomed. Nanotechnol. 2010, 6, 408–431. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.; Aminabhavi, T.M.; Kulkarni, A.; Rudzinski, W. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Izumikawa, S.; Yoshioka, S.; Aso, Y.; Takeda, Y. Preparation of poly(l-lactide) microspheres of different crystalline morphology and effect of crystalline morphology on drug release rate. J. Control. Release 1991, 15, 133–140. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yannakopoulou, K.; Gioxari, A.; Chiou, A.; Makris, D. Polyphenol characterization and encapsulation in β-cyclodextrin of flavonoid-rich Hypericum perforatum (St John’s wort) extract. LWT-Food Sci. Technol. 2010, 43, 882–889. [Google Scholar] [CrossRef]
- Musumeci, T.; Ventura, C.A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int. J. Pharm. 2006, 325, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Cortez Tornello, P.; Feresin, G.; Tapia, A.; Veiga, I.G.; Moraes, Â.M.; Abraham, G.A.; Cuadrado, T.R. Dispersion and release of embelin from electrospun biodegradable, polymeric, membranes. Polym. J. 2012, 44, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]



| DPPH Radical Scavenging Ability IC50 (mg/mL) | Total Phenolic Content GAE (mggallic acid/gof dry extract) | H2O2 Scavenging Ability IC50 (mg/mL) | |
|---|---|---|---|
| OLE | 0.283 | 391.7 | 0.254 ± 0.007 |
| PLA | n.t. * | n.t. | 36.45 ± 2.03 |
| OLE-NPs | n.t. | n.t. | 4.37 ± 0.12 |
| Quercetin | 0.073 | n.t. | 0.049 ± 0.003 |
| Phenolic Compound | Retention Time (min) | Variation Coefficient (%) for Retention Time (n = 10) | Calibration Equation | Variation Coefficient (%) for Concentration (n = 10) | C (mg/mL) | % in OLE |
|---|---|---|---|---|---|---|
| Oleuropein | 18.4 | 0.46 | y = 5730.3x − 98.3 R2 = 0.9962 | 0.80 | 0.347 ± 0.035 | 69.5 |
| Vanillin | 15.0 | 0.19 | y = 1755.5x + 3.51 R2 = 1 | 0.15 | 0.005 ± 0.001 | 1.06 |
| Rutin | 17.1 | 0.44 | y = 824.9x − 1.53 R2 = 1 | 0.31 | 0.020 ± 0.005 | 4.03 |
| Size (nm) | Pdi | ζ-Potential (mV) | EE% | |
|---|---|---|---|---|
| OLE-loaded NPs | 246.3 ± 5.3 | 0.21 ± 0.01 | −27.5 ± 0.12 | 49.2 |
| Blank-NPs | 220.6 ± 4.0 | 0.08 ± 0.00 | −19.3 ± 0.74 | - |
| pH Results | Freeze Cycles | Storage at 5 °C | Storage at 25 °C | Storage at 40 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Initial Results | Day 7 | Day 15 | Day 21 | Day 29 | Month 3 | Month 3 | Month 1 | Month 2 | Month 3 |
| o/w Base Cream | 5.47 | 5.49 | 5.5 | 5.52 | 5.48 | 5.6 | 5.49 | 5.47 | 5.37 | 5.48 |
| Base Cream with OLE-NPs | 5.49 | 5.59 | 5.54 | 5.56 | 5.52 | 5.62 | 5.59 | 5.48 | 5.35 | 5.43 |
| Base Cream with OLE | 5.45 | 5.5 | 5.5 | 5.46 | 5.42 | 5.55 | 5.5 | 5.41 | 5.3 | 5.26 |
| Viscosity Results [cSt] | Freeze Cycles | Storage at 5 °C | Storage at 25 °C | Storage at 40 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Initial Results | Day 7 | Day 15 | Day 21 | Day 29 | Month 3 | Month 3 | Month 1 | Month 2 | Month 3 |
| o/w Base Cream | 20,450 | 47,600 | 48,532 | 38,501 | 39,231 | 30,598 | 33,032 | 40,922 | 39,163 | 34,870 |
| Base Cream with OLE-NPs | 20,219 | 46,813 | 47,408 | 38,612 | 39,688 | 30,703 | 32,760 | 41,224 | 37,817 | 35,268 |
| Base Cream with OLE | 17,319 | 45,314 | 45,347 | 38,614 | 40,302 | 29,623 | 32,814 | 39,602 | 39,084 | 34,286 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.; et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering 2017, 4, 75. https://doi.org/10.3390/bioengineering4030075
Kesente M, Kavetsou E, Roussaki M, Blidi S, Loupassaki S, Chanioti S, Siamandoura P, Stamatogianni C, Philippou E, Papaspyrides C, et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering. 2017; 4(3):75. https://doi.org/10.3390/bioengineering4030075
Chicago/Turabian StyleKesente, Maritina, Eleni Kavetsou, Marina Roussaki, Slim Blidi, Sofia Loupassaki, Sofia Chanioti, Paraskevi Siamandoura, Chrisoula Stamatogianni, Eleni Philippou, Constantine Papaspyrides, and et al. 2017. "Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation" Bioengineering 4, no. 3: 75. https://doi.org/10.3390/bioengineering4030075








