Antinociceptive Activity of Macaranga denticulata Muell. Arg. (Family: Euphorbiaceae): In Vivo and In Silico Studies
Abstract
:1. Introduction
2. Methods and Materials
2.1. Plant Sample Collection and Identification
2.2. Extraction Procedure
2.3. Drugs and Chemicals
2.4. Swiss Albino Mice
2.5. Phytochemical Screening
2.6. Acute Toxicity Study
2.7. In Vivo Antinociceptive Activity
2.7.1. Acetic Acid-Induced Writhing Test
2.7.2. Formalin-Induced Licking Test
2.8. In Silico Molecular Docking Study
2.8.1. Ligand and Protein Preparation
2.8.2. Receptor Grid Generation
2.8.3. Glide Standard Precision Ligand Docking
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Screening
3.2. Acute Toxicity Study
3.3. In Vivo Antinociceptive Activity: Acetic Acid Test
3.4. In Vivo Antinociceptive Activity: Formalin Test
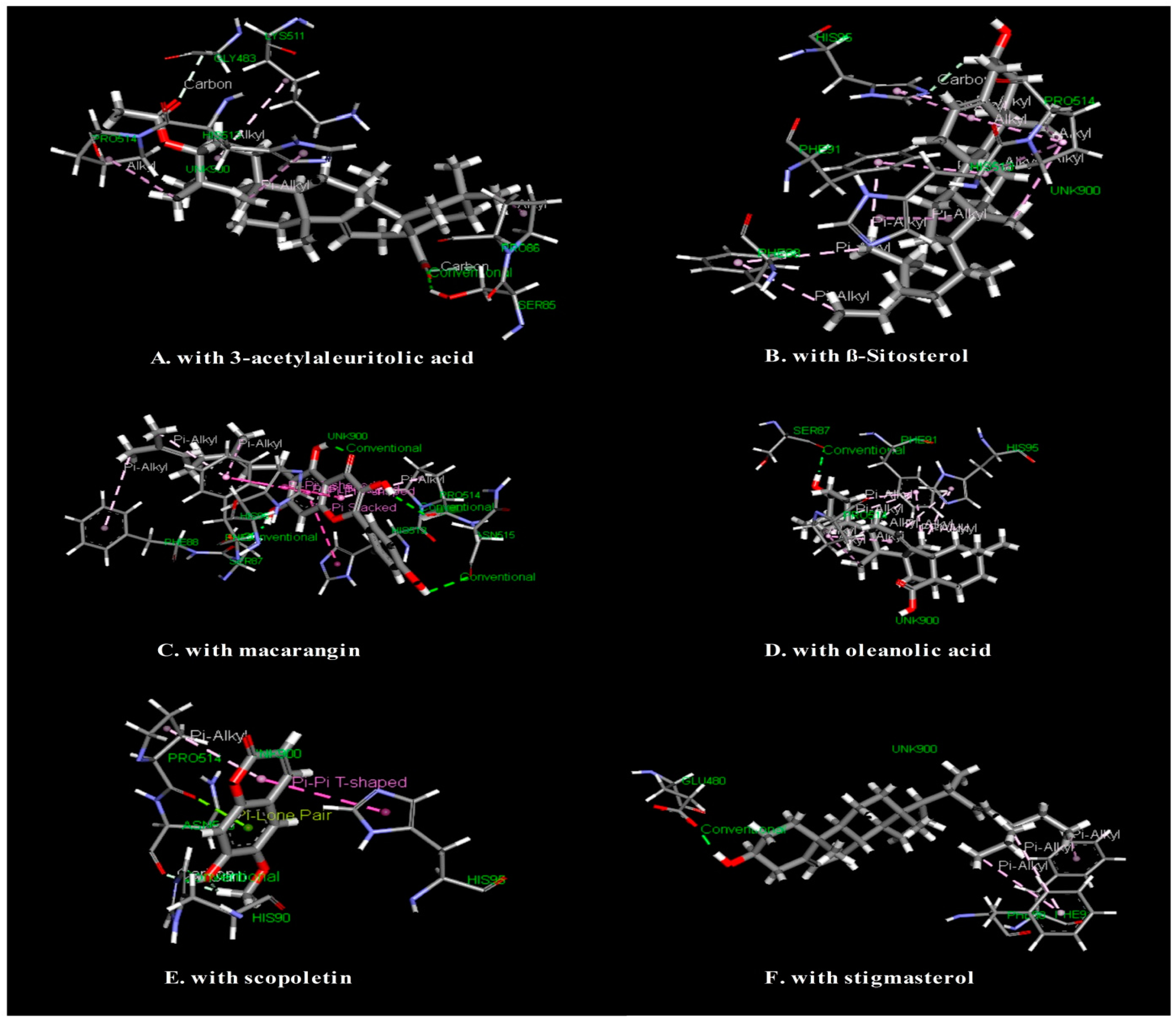
3.5. In Silico Study: Molecular Docking Study for Antinociceptive Activity
4. Discussion
5. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Medicinal Plants of Bangladesh. Available online: http://www.mpbd.info/plants/macaranga-denticulata.php (accessed on 10 October 2017).
- Hasanat, A.; Kabir, M.S.H.; Hossain, M.M.; Hasan, M.; Masum, M.A.A.; Chowdhury, T.A.; Bhuiyan, D.I.; Mamur, A.; Kibria, A. Antibacterial activity of methanol extract of macaranga denticulata leaves and in silico pass prediction for its six secondary metabolites. World J. Pharm. Sci. 2015, 3, 1258–1266. [Google Scholar]
- Somyote, S.; Sasinee, U. Chemical Constituents of Macaranga Denticulata. In Proceedings of the 26th Congress on Science & Technology of Thailand, Bangkok, Thailand, 18 October 2000. [Google Scholar]
- Huang, J.; Lu, W.; Tan, X.; Lu, G.; Huang, Z. Chemical Constituents from Macaranga denticulata Root. Zhong Yao Cai 2015, 38, 1671–1673. [Google Scholar] [PubMed]
- Magadula, J.J. Phytochemistry and pharmacology of the genus Maca ranga: A review. J. Med. Plants Res. 2014, 8, 489–503. [Google Scholar]
- Moin, M.; Mazumdar, U.; Islam, A.; Tanvir, M. Evaluation of anti-arthritic, thrombolytic and cytotoxic activities of methanolic and ethanolic extract of Macaranga denticulata leaves. J. Med. Plants 2016, 4, 8–12. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Hosseinzadeh, H.; Ramezani, M.; Salmani, G. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J. Ethnopharmacol. 2000, 73, 379–385. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic acid for analgesic screening. Proc. Soc. Exp. Biol. Med. 1959, 18, 412–415. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank, 1999. In International Tables for Crystallography Volume F: Crystallography of Biological Macromolecules; Springer: Berlin, Germany, 2006; pp. 675–684. [Google Scholar]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [PubMed]
- Xu, J.; Zhao, Q.; Wei, L.; Yang, Y.; Xu, R.; Yu, N.; Zhao, Y. Phytochemical composition and antinociceptive activity of Bauhinia glauca subsp. hupehana in rats. PLoS ONE 2015, 10, e0117801. [Google Scholar] [CrossRef]
- Trongsakul, S.; Panthong, A.; Kanjanapothi, D.; Taesotikul, T. The analgesic, antipyretic and anti-inflammatory activity of Diospyros variegata Kruz. J. Ethnopharmacol. 2003, 85, 221–225. [Google Scholar] [CrossRef]
- Khatun, A.; Imam, M.Z.; Rana, M.S. Antinociceptive effect of methanol extract of leaves of Persicaria hydropiper in mice. BMC Complement. Altern. Med. 2015, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Rahman, M.M.; Abdullah-Al-Mamun, M.; Sadik, G. Vanda roxburghii: An experimental evaluation of antinociceptive properties of a traditional epiphytic medicinal orchid in animal models. BMC Complement. Altern. Med. 2015, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- De Sá, P.G.S.; Nunes, X.P.; de Lima, J.T.; de Siqueira Filho, J.A.; Fontana, A.P.; de Siqueira, J.; Quintans-Júnior, L.J.; Damasceno, P.K.F.; Branco, C.R.C.; Branco, A.; et al. Antinociceptive effect of ethanolic extract of Selaginella convoluta in mice. BMC Complement. Altern. Med. 2012, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.Z.; Moniruzzaman, M. Antinociceptive effect of ethanol extract of leaves of Lannea coromandelica. J. Ethnopharmacol. 2014, 154, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Sitzmann, M.; Pugliese, A.; Nicklaus, M.C. Software and resources for computational medicinal chemistry. Future Med. Chem. 2011, 3, 1057–1085. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods Mol. Biol. 2008, 443, 365–382. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Writhing | % Inhibition |
|---|---|---|
| Control | 59.33 ± 1.84 | - |
| Diclofenac-Na (10 mg/kg) | 18.00 ± 0.82 ** | 69.66 |
| Met.MD (200 mg/kg) | 33.67 ± 1.18 * | 43.26 |
| Met.MD (400 mg/kg) | 25.33 ± 0.82 ** | 57.30 |
| Treatment | Early Phase (s) | % Inhibition of Early Phase | Late Phase (s) | % Inhibition of Late Phase |
|---|---|---|---|---|
| Control | 57.31 ± 1.06 | - | 41.74 ± 1.46 | - |
| Diclofenac-Na (10 mg/kg) | 14.95 ± 0.60 ** | 73.91 | 12.26 ± 0.52 ** | 70.62 |
| Met.MD (200 mg/kg) | 30.47 ± 1.82 ** | 46.83 | 25.58 ± 1.56 * | 38.72 |
| Met.MD (400 mg/kg) | 21.70 ± 1.16 ** | 62.14 | 21.36 ± 0.92 ** | 48.83 |
| Compound Name | Molecular Docking Score | Glide E Model | Glide G Score |
|---|---|---|---|
| 3-acetylaleuritolic acid | −2.226 | −32.351 | −2.232 |
| β-Sitosterol | −3.024 | −21.251 | −3.024 |
| macarangin | −5.816 | −51.977 | −5.816 |
| oleanolic acid | −0.023 | −24.329 | −2.700 |
| scopoletin | −5.381 | −31.695 | −5.386 |
| stigmasterol | −2.397 | −19.423 | −2.397 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanat, A.; Chowdhury, T.A.; Kabir, M.S.H.; Chowdhury, M.S.; Chy, M.N.U.; Barua, J.; Chakrabarty, N.; Paul, A. Antinociceptive Activity of Macaranga denticulata Muell. Arg. (Family: Euphorbiaceae): In Vivo and In Silico Studies. Medicines 2017, 4, 88. https://doi.org/10.3390/medicines4040088
Hasanat A, Chowdhury TA, Kabir MSH, Chowdhury MS, Chy MNU, Barua J, Chakrabarty N, Paul A. Antinociceptive Activity of Macaranga denticulata Muell. Arg. (Family: Euphorbiaceae): In Vivo and In Silico Studies. Medicines. 2017; 4(4):88. https://doi.org/10.3390/medicines4040088
Chicago/Turabian StyleHasanat, Abul, Tanvir Ahmad Chowdhury, Mohammad Shah Hafez Kabir, Mohammed Sohel Chowdhury, Md. Nazim Uddin Chy, Jackie Barua, Nishan Chakrabarty, and Arkajyoti Paul. 2017. "Antinociceptive Activity of Macaranga denticulata Muell. Arg. (Family: Euphorbiaceae): In Vivo and In Silico Studies" Medicines 4, no. 4: 88. https://doi.org/10.3390/medicines4040088









