Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Processing and Extraction of the Essentials Oils
2.3. Oil Composition Analysis
2.4. Antioxidant Assay
2.5. Cytotoxicity Assay (Against Cancer Cell Lines)
2.6. Cell Membrane Disruption
2.7. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Composition
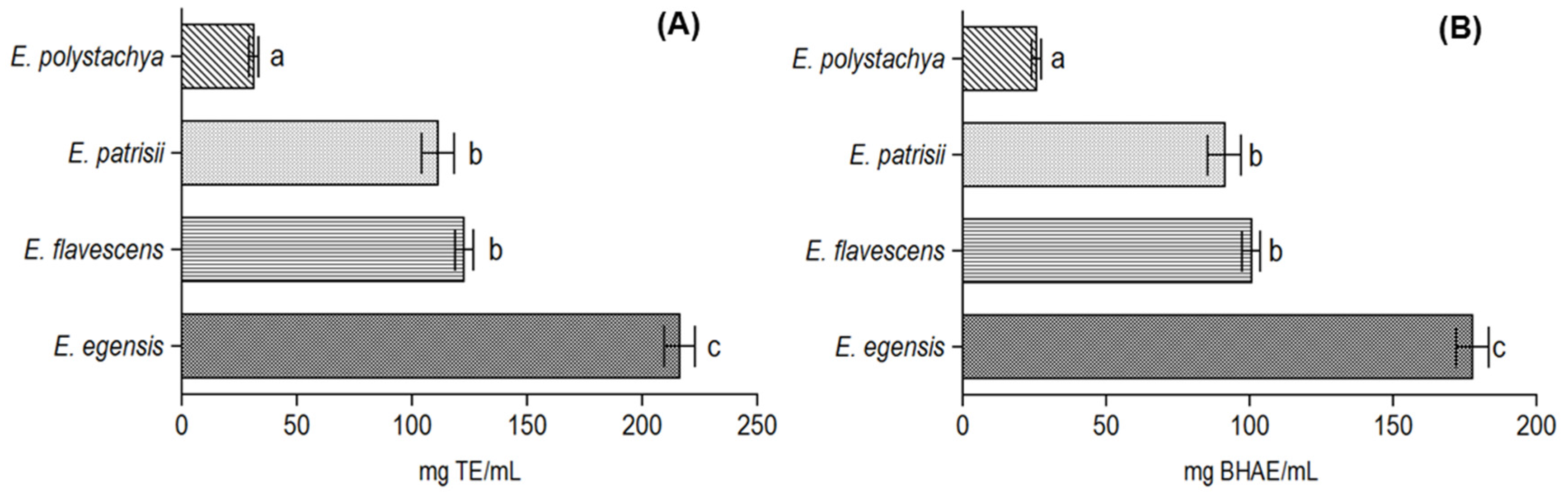
3.2. Antioxidant Activity
3.3. Cytotoxic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMSO | dimethylsulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GC-MS | gas chromatography/mass spectrometry |
| GC-FID | gas chromatography/flame ionization detector |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| TEAC | trolox equivalent antioxidant capacity |
| Triton X-100 | nonionic surfactant, octylphenol ethoxylate |
References
- Wilson, P.G.; O’Brien, M.M.; Gadek, P.A.; Quinn, C.J. Myrtaceae revisited: A reassessment of intrafamilial groups. Am. J. Bot. 2001, 88, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Landrum, L.R.; Kawasaki, M.L. The genera of Myrtaceae in Brazil: An illustrated synoptic treatment and identification keys. Brittonia 1977, 49, 508–536. [Google Scholar] [CrossRef]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae: Lista de Espécies da Flora do Brazil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2010. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB171 (accessed on 15 May 2016).
- Missouri Botanical Garden. 2016. Available online: http://www.tropicos.org (accessed on 11 November 2016).
- Keszei, A.; Brubaker, C.L.; Foley, W.J. A molecular perspective on terpene formation in Australian Myrtaceae. Aust. J. Bot. 2008, 56, 197–213. [Google Scholar] [CrossRef]
- Padovan, M.; Keszei, A.; Külheim, C.; Foley, W.J. The evolution of foliar terpene diversity in Myrtaceae. Phytochem. Rev. 2014, 13, 695–716. [Google Scholar] [CrossRef]
- Costa, D.P.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Seasonal variability of essential oils of Eugenia uniflora leaves. J. Braz. Chem. Soc. 2009, 20, 1287–1293. [Google Scholar] [CrossRef]
- Henriques, A.T.; Sobral, M.E.; Cauduro, A.D.; Schapoval, E.E.S.; Bassani, V.L.; Lamaty, G.; Menut, C.; Bessière, J.M. Aromatic plants from Brazil. II. The chemical composition of some Eugenia essential oils. J. Essent. Oil Res. 1993, 5, 501–505. [Google Scholar] [CrossRef]
- Craveiro, A.A.; Andrade, C.H.S.; Matos, F.J.A.; Alencar, J.W.; Machado, M.I.L. Essential oil of Eugenia jambolana. J. Nat. Prod. 1983, 46, 591–592. [Google Scholar] [CrossRef]
- Apel, M.A.; Sobral, M.E.; Schapoval, E.E.S.; Henriques, A.T.; Menut, C.; Bessiére, J.M. Chemical composition of the essential oils of E. beaurepaireana and E. pyriformis: Section Dichotomae. J. Essent. Oil Res. 2004, 16, 191–192. [Google Scholar] [CrossRef]
- Feitosa, C.M.; Barbosa, A.R.; de Melo, C.H.S.; Freitas, R.M.; Fontes, J.E.N.; Costa, E.V.; Rashed, K.N.Z.; da Costa Junior, J.S. Antioxidant and anticholinesterase activities of the essential oil of Eugenia dysenterica DC. Afr. J. Pharm. Pharmacol. 2017, 11, 241–249. [Google Scholar]
- Siani, A.C.; Azevedo, M.B.M.; Ramos, M.F.S.; Trigo, J.R. Monoterpenes and sesquiterpenes of Neotropical Myrtaceae. In Current Trends in Phytochemistry; Research Signpost: Trivandrum, India, 2008; pp. 223–251. [Google Scholar]
- Nakamura, M.J.; Monteiro, S.S.; Bizarri, C.H.B.; Siani, A.C.; Ramos, M.F.S. Essential oils of four Myrtaceae species from the Brazilian Southeast. Biochem. Syst. Ecol. 2010, 38, 1170–1175. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Kamalinejad, M.; Javadi, M.; Majzoob, S.; Sayyah, M. Evaluation of the anticonvulsant activity of the essential oil of Eugenia caryophyllata in male mice. J. Ethnopharmacol. 1999, 64, 167–171. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Olawore, N.O.; Ekundayo, O.; Walker, T.M.; Schmidt, J.M.; Setzer, W.N. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147–152. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, J.; Shin, S.-C.; Lee, S.-G.; Park, I.-K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flav. Fragr. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Santos, K.K.A.; Matias, E.F.F.; Tintino, S.R.; Souza, C.E.S.; Braga, M.F.B.M.; Guedes, G.M.M.; Rolón, M.; Veja, C.; de Arias, A.R.; Costa, J.G.M.; et al. Anti-Trypanosoma cruzi and cytotoxic activities of Eugenia uniflora L. Exp. Parasitol. 2012, 131, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.S.; Lima, B.G.; Oliveira, A.F.R.; Nunes, D.D.; Fernandes, C.P.; Santos, M.G.; Tietbohl, L.A.C.; Mello, C.B.; Rocha, L.; Federa, D. Effects of essential oil from leaves of Eugenia sulcata on the development of agricultural pest insects. Rev. Bras. Farmacogn. 2014, 24, 413–418. [Google Scholar] [CrossRef]
- Gülçin, I.; Elmastas, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Yoo, C.-B.; Hana, K.-T.; Cho, K.-S.; Hab, J.; Park, H.-J.; Nam, J.-H.; Kil, U.-H.; Lee, K.-T. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Van den Dool, H.; Kratz, P.D.J.A. Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Choi, H.-S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of Citrus essential oils and their components: Detection using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Hillard, E.A.; Pigeon, P.; Rocha, D.D.; Rodrigues, F.A.R.; Montenegro, R.C.; Costa-Lotufo, L.V.; Goulart, M.O.F.; Jaouen, G. Biological evaluation of twenty-eight ferrocenyltetrasubstitued olefins: Cancer cell growth inhibition, ROS production and hemolytic activity. Eur. J. Med. Chem. 2011, 46, 3778–3787. [Google Scholar] [CrossRef] [PubMed]
- NIST—National Institute of Standards and Technology. Mass Spectral Library (NIST/EPA/NIH, v. 2.0d); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Zoghbi, M.G.B.; Guilhon, G.M.S.P.; Sarges, F.N.; Pereira, R.A.; Oliveira, J. Chemical variability of the volatiles from the leaves of Eugenia protenta McVaugh (Myrtaceae) growing wild in the North of Brazil. Biochem. Syst. Ecol. 2011, 9, 660–665. [Google Scholar] [CrossRef]
- Victoria, F.N.; Lenardão, E.J.L.; Perin, G.; Jacob, R.G.; Alves, D.; da Silva, W.P.; da Motta, A.S.; Nascente, O.S. Essential oil of the leaves of Eugenia uniflora L.: Antioxidant and antimicrobial properties. Food Chem. Toxicol. 2012, 50, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Zoghbi, M.G.B.; Bastos, M.N.C. Essential oils of twelve species of Myrtaceae growing wild in the sandbank of the Resex Maracanã, State of Pará, Brazil. J. Essent. Oil Bear. Plants 2010, 13, 440–450. [Google Scholar] [CrossRef]
- Henriques, A.T.; Sobral, M.; Bridi, R.; Vérin, P.; Menut, C.; Lamaty, G.; Bessière, J.M. Essential oil from five southern Brazilian species of Myrcia (Myrtaceae). J. Essent. Oil Res. 1997, 9, 13–18. [Google Scholar] [CrossRef]
- Limberger, R.P.; Sobral, M.; Henriques, A.T. Óleos voláteis de espécies de Myrcia nativas do Rio Grande do Sul. Quim. Nova 2004, 27, 916–919. [Google Scholar] [CrossRef]
- Stefanello, M.E.A.; Cervi, A.C.; Wisniewski, A., Jr.; Simionatto, E.L. Essential oil composition of Myrcia laruotteana Camb. J. Essent. Oil Res. 2007, 19, 466–467. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Andrade, E.H.A.; da Silva, M.H.L.; Carreira, L.M.M.; Maia, J.G.S. Essential oils from three Myrcia species. Flav. Fragr. J. 2003, 18, 421–424. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Azevedo, M.M.B.; Chaves, F.C.M.; Almeida, C.A.; Bizzo, H.R.; Duarte, R.S.; Campos-Takaki, G.M.; Alviano, C.S.; Alviano, D.S. Antioxidant and antimicrobial activities of 7-hydroxycalamenene-rich essential oils from Croton cajucara Benth. Molecules 2013, 8, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Lee, H.J.; Kim, C.Y.; Son, J.-K.; Jung, S. 8-Hydroxycalamenene isolated from the rhizomes of Reynoutria elliptica exerts neuroprotective effects both in vitro and in vivo. Food Chem. Toxicol. 2013, 51, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Rahimi-Nasrabadi, M.; Batooli, H.; Ebrahimabadi, A.H. Chemical composition and antioxidant activities of the essential oil and methanol extracts of Psammogeton canescens. Food Chem. Toxicol. 2009, 48, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, M.C.; Wright, H.L.; Agius, B.R.; Wright, B.S.; Moriarity, D.M.; Haber, W.A.; Setzer, W.N. Chemical compositions and biological activities of leaf essential oils of six species of Annonaceae from Monteverde, Costa Rica. Rec. Nat. Prod. 2009, 3, 153–160. [Google Scholar]
- Da Silva, E.B.P.; Matsuo, A.L.; Figueiredo, C.R.; Chaves, M.H.; Sartorelli, P.; Lago, J.H.G. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae). Nat. Prod. Commun. 2013, 8, 277–279. [Google Scholar] [PubMed]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J. Biol. Chem. 2005, 280, 5812–5819. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.F. The biochemistry of antioxidants revisited. Nutr. Clin. Pract. 2002, 17, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Philpott, M.; Karunasinghe, N. Dietary cancer and prevention using antimutagens. Toxicology 2004, 198, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. Antioxidant intervention as a route to cancer prevention. Eur. J. Cancer 2005, 41, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, M.A.H.; Sathisha, U.V.; Dharmesh, S.M. Cytoprotective and antioxidant activity of free, conjugated and insoluble-bound phenolic acids from swallow root (Decalepis hamiltonii). Food Chem. 2010, 119, 1307–1312. [Google Scholar] [CrossRef]
| Constituents | RICalc. | RILit. | Eugenia egensis | Eugenia flavescens | Eugenia patrisii | Eugenia polystachya |
|---|---|---|---|---|---|---|
| Limonene | 1026 | 1024 | 0.1 | |||
| α-Terpineol | 1187 | 1186 | 0.1 | |||
| Thymol | 1290 | 1289 | 0.1 | |||
| δ-Elemene | 1337 | 1335 | 1.6 | 0.5 | 0.3 | 4.1 |
| α-Cubebene | 1348 | 1345 | 2.2 | 0.1 | ||
| α-Ylangene | 1374 | 1373 | 0.1 | 0.1 | ||
| α-Copaene | 1376 | 1374 | 1.6 | 0.1 | 0.6 | |
| β-Bourbonene | 1388 | 1387 | 0.2 | 0.3 | ||
| β-Elemene | 1390 | 1389 | 2.5 | 0.2 | 0.1 | 2.2 |
| 7-epi-Sesquithujene | 1392 | 1390 | 0.1 | |||
| Sesquithujene | 1407 | 1405 | 0.1 | |||
| α-Gurjunene | 1410 | 1409 | 1.5 | |||
| (Z)-α-Bergamotene | 1412 | 1411 | 0.2 | |||
| β-Caryophyllene | 1418 | 1417 | 8.9 | 2.8 | 0.9 | 2.3 |
| β-Ylangene | 1420 | 1419 | 5.0 | |||
| β-Gurjunene | 1432 | 1431 | 0.7 | 0.2 | 0.1 | 3.5 |
| (E)-α-Bergamotene | 1433 | 1432 | 0.4 | |||
| α-Guaiene | 1439 | 1437 | 0.1 | 0.2 | ||
| Aromadendrene | 1440 | 1439 | 0.1 | 0.1 | ||
| (Z)-β-Farnesene | 1442 | 1440 | 0.4 | |||
| 6,9-Guaiadiene | 1444 | 1442 | 1.2 | |||
| (E)-Muurola-3,5-diene | 1454 | 1451 | 5.9 | |||
| α-Humulene | 1455 | 1452 | 2.0 | 0.4 | 1.3 | |
| Geranyl acetone | 1456 | 1453 | 0.6 | |||
| (E)-β-Farnesene | 1457 | 1454 | 4.7 | |||
| β-Santalene | 1460 | 1457 | 0.1 | |||
| allo-Aromadendrene | 1462 | 1458 | 0.9 | 0.2 | ||
| (Z)-Cadina-1(6),4-diene | 1464 | 1461 | 0.1 | 0.6 | ||
| Ishwarane | 1467 | 1465 | 15.7 | |||
| β-Acoradiene | 1470 | 1469 | 0.2 | |||
| Dauca-5,8-diene | 1473 | 1471 | 1.2 | |||
| (E)-Cadina-1(6),4-diene | 1476 | 1475 | 1.6 | |||
| γ-Gurjunene | 1477 | 1475 | 0.7 | |||
| γ-Muurolene | 1480 | 1478 | 0.3 | 0.1 | 1.0 | |
| Germacrene D | 1486 | 1484 | 2.2 | 0.8 | 18.4 | |
| Aristolochene | 1488 | 1487 | 2.1 | |||
| β-Selinene | 1490 | 1489 | 0.4 | 0.1 | 0.9 | |
| (Z)-β-Guaiene | 1494 | 1492 | 3.8 | 1.4 | ||
| α-Zingiberene | 1496 | 1493 | 1.6 | |||
| Viridiflorene | 1497 | 1496 | 0.3 | |||
| Bicyclogermacrene | 1501 | 1500 | 1.2 | 0.5 | 5.1 | |
| α-Muurolene | 1502 | 1500 | 0.5 | 0.2 | 1.7 | |
| β-Bisabolene | 1506 | 1505 | 34.7 | 0.3 | ||
| (Z)-α-Bisabolene | 1508 | 1506 | 0.4 | |||
| δ-Amorphene | 1512 | 1511 | 0.3 | |||
| γ-Cadinene | 1515 | 1513 | 0.2 | 0.1 | 0.8 | |
| Cubebol | 1516 | 1514 | 0.1 | 0.1 | 0.5 | |
| 7-epi-α-Selinene | 1521 | 1520 | 7.5 | |||
| β-Sesquiphellandrene | 1523 | 1521 | 3.4 | 0.1 | ||
| (E)-Calamenene | 1523 | 1521 | 6.1 | 0.1 | 0.3 | |
| δ-Cadinene | 1524 | 1522 | 2.3 | 0.6 | ||
| (E)-iso-γ-Bisabolene | 1530 | 1529 | 5.1 | |||
| (E)-γ-Bisabolene | 1531 | 1530 | 35.0 | |||
| (E)-Cadina-1,4-diene | 1534 | 1533 | 6.3 | 0.2 | ||
| 10-epi-Cubebol | 1535 | 1533 | 0.1 | |||
| α-Cadinene | 1539 | 1537 | 0.1 | 0.2 | ||
| α-Calacorene | 1546 | 1544 | 0.3 | |||
| Elemol | 1549 | 1548 | 0.1 | 0.7 | ||
| 1-nor-Bourbonanone | 1562 | 1561 | 0.1 | |||
| (E)-Nerolidol | 1563 | 1561 | 0.2 | |||
| β-Calacorene | 1566 | 1564 | 0.2 | |||
| Palustrol | 1568 | 1567 | 0.3 | 0.3 | 0.2 | |
| Spathulenol | 1578 | 1577 | 0.3 | 0.1 | 4.4 | 3.0 |
| Caryophyllene oxide | 1582 | 1582 | 0.1 | 0.2 | 0.8 | |
| Globulol | 1591 | 1590 | 0.7 | 0.2 | 0.7 | |
| Viridiflorol | 1592 | 1592 | 0.2 | 0.1 | 0.7 | 0.3 |
| Cubeban-11-ol | 1596 | 1595 | 0.2 | |||
| Rosifoliol | 1601 | 1600 | 0.2 | 2.0 | ||
| Guaiol | 1602 | 1600 | 0.8 | 0.1 | ||
| Ledol | 1603 | 1602 | 5.0 | 0.3 | 0.3 | |
| Humulene epoxide II | 1609 | 1608 | 0.2 | 0.6 | ||
| Junenol | 1620 | 1618 | 0.4 | |||
| β-Cedrene epoxide | 1621 | 1620 | 0.4 | |||
| α-Corocalene | 1623 | 1622 | 0.3 | |||
| 1-epi-Cubenol | 1629 | 1627 | 0.7 | 0.5 | ||
| α-Acorenol | 1633 | 1632 | 0.1 | |||
| Gossonorol | 1638 | 1636 | 0.3 | |||
| epi-α-Cadinol | 1639 | 1638 | 0.6 | 0.3 | 0.8 | |
| Caryophylla-4(12),8(13)-dien-5α-ol | ||||||
| and | ||||||
| Caryophylla-4(12)-8(13)-dien-5β-ol | 1640 | 1639 | 15.6 | |||
| epi-α-Murrolol | 1643 | 1640 | 0.5 | 0.2 | 0.8 | |
| α-Muurolol | 1645 | 1644 | 0.3 | 0.8 | ||
| α-Cadinol | 1654 | 1652 | 0.8 | 0.3 | 2.5 | 3.3 |
| Selin-11-en-4-α-ol | 1661 | 1658 | 0.2 | 0.8 | ||
| epi-β-Bisabolol | 1670 | 1670 | 0.4 | |||
| Bulnesol | 1672 | 1670 | 0.7 | |||
| Cadalene | 1676 | 1675 | 0.3 | |||
| epi-α-Bisabolol | 1684 | 1683 | 0.2 | |||
| Germacra-4(15),5,10(14)-trien-1-α-ol | 1685 | 1685 | 0.1 | 0.6 | ||
| α-Bisabolol | 1686 | 1685 | 1.0 | |||
| Eudesma-4(15),7-dien-1-β-ol | 1687 | 1687 | 0.3 | |||
| 2,3-dihydro-Farnesol | 1688 | 1688 | 1.3 | |||
| cis-Thujopsenal | 1708 | 1708 | 0.1 | |||
| (2E,6Z)-Farnesal | 1714 | 1713 | 0.1 | |||
| 5-hydroxy-(Z)-Calamenene | 1715 | 1713 | 35.8 | |||
| (2E,6Z)-Farnesol | 1716 | 1714 | 23.2 | |||
| (2E,6E)-Farnesal | 1742 | 1740 | 1.8 | |||
| (2E,6E)-Farnesol | 1744 | 1742 | 34.5 | |||
| (2E,6E)-Methyl farnesoate | 1784 | 1783 | 0.7 | |||
| (2Z,6E)-Farnesyl acetate | 1821 | 1821 | 0.3 | |||
| Monoterpene hydrocarbons | 0.1 | |||||
| Oxygenated monoterpenes | 0.2 | |||||
| Sesquiterpenes hydrocarbons | 53.3 | 91.6 | 6.3 | 77.9 | ||
| Oxygenated sesquiterpenes | 45.9 | 4.7 | 88.9 | 16.4 | ||
| Total | 99.2 | 96.3 | 95.4 | 94.4 | ||
| Eugenia Species | IC50 (µg/mL) * | Hemolysis | |||
|---|---|---|---|---|---|
| AGP-01 | HCT-116 | SKMEL19 | MRC5 | (µg/mL) | |
| (Gastric) | (Colon) | (Melanoma) | (Human Fibroblast) | ||
| E. egensis | > 25 | > 25 | > 25 | ND | > 200 |
| E. flavescens | > 25 | 13.9 a (12.0–15.9) | > 25 | 14.0 a (10.4–18.6) | > 200 |
| E. patrisii | > 25 | 16.4 b (14.6–18.3) | > 25 | 18.1 b (13.9–23.4) | > 200 |
| E. polystachya | > 25 | 10.3 c (8.3–12.8) | > 25 | >25 | > 200 |
| Doxorubicin | 0.254 µM (0.19–0.33) | 0.10 µM d (0.047–0.28) | 0.045 µM (0.013–0.15) | 0.20 µM (0.16–0.25) | >2 00 µM |
| HCT-15/HT-29 | Sbc-12/WM3211 | ||||
| Eugenol | 500.0 µM/300 µM | 0.5 µM | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, J.K.R.; Andrade, E.H.A.; Barreto, L.H.; Da Silva, N.C.F.; Ribeiro, A.F.; Montenegro, R.C.; Maia, J.G.S. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines 2017, 4, 51. https://doi.org/10.3390/medicines4030051
Da Silva JKR, Andrade EHA, Barreto LH, Da Silva NCF, Ribeiro AF, Montenegro RC, Maia JGS. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines. 2017; 4(3):51. https://doi.org/10.3390/medicines4030051
Chicago/Turabian StyleDa Silva, Joyce Kelly R., Eloisa Helena A. Andrade, Leilane H. Barreto, Nádia Carolina F. Da Silva, Alcy F. Ribeiro, Raquel C. Montenegro, and José Guilherme S. Maia. 2017. "Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity" Medicines 4, no. 3: 51. https://doi.org/10.3390/medicines4030051







