Chemical Composition, Antimicrobial and Antioxidant Activities of the Volatile Oil of Ganoderma pfeifferi Bres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungus (Mushroom) Material
2.2.Volatile Oil Extraction
2.3. Gas Chromatographic-Mass Spectral Analysis
2.4. Determination of Antimicrobial Activities
2.4.1. Microorganisms
2.4.2. Antimicrobial Assays
Agar Diffusion Method
Broth Micro-Dilution Assay for Minimum Inhibitory Concentrations (MIC)
2.5. Determination of Radical Scavenging Activity
3. Results
3.1. Chemical Composition of the Volatile Oil
3.2. Antimicrobial Activities of the Volatile Oil
3.3. Antioxidant Activity of the Volatile Oil
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay |
| VO | Volatile oil |
| MIC | Minimal Inhibitory Concentration |
References
- Lindequist, U. Ganoderma. In Hagers Handbuch der Pharmazeutischen Praxis/Hrsg F. von Bruchhausen, 5. Vollständige Neubearbeitete Auflage; Springer-Verlag Berlin-Heidelberg: New York, NY, USA, 1998; pp. 750–761, Folgeband 2 Drogen A–K (Hrsg W. Blaschek). [Google Scholar]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [PubMed]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, H. Zur Lebensdauer des Kupferroten Lackporlings, Ganoderma pfeifferi. Mykol. Mitt. Bl. 1985, 28, 63. [Google Scholar]
- Mothana, R.; Jansen, R.; Jülich, W.D.; Lindequist, U. Ganomycin A and B, new antimicrobial farnesyl hydroquinones from the basidiomycete Ganoderma pfeifferi. J. Nat. Prod. 2000, 63, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M. Isolierung und Charakterisierung antibiotisch wirksamer Verbindungen aus Ganoderma pfeifferi Bres. und aus Podaxis pistillaris (L.: Pers.) Morse. Ph.D. Thesis, University of Greifswald, Greifswald, Germany, 8 October 2001. [Google Scholar]
- Niedermeyer, T.H.; Lindequist, U.; Mentel, R.; Gördes, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral Terpenoid Constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Jülich, W.D.; Witt, S. Ganoderma pfeifferi—A European relative of Ganoderma lucidum. Phytochemistry 2015, 114, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Misharina, T.A.; Mukhutdinova, S.M.; Zharikova, G.G.; Terenina, M.B.; Krikunova, N.I. The composition of volatile components of cepe (Boletus edulis) and oyster mushrooms (Pleurotus ostreatus). Appl. Biochem. Microbiol. 2009, 45, 187–193. [Google Scholar] [CrossRef]
- Ayoub, N.; Lass, D.; Schultze, W. Volatile constituents of the medicinal fungus Inonotus obliquus (Pers.:Fr.) Pillát (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2009, 11, 55–60. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.S.; Cirić, A.; Nikolić, M.; Bukvički, D.; Guerzoni, M.E.; Soković, M.D. Laetiporus sulphureus, edible mushroom from Serbia: Investigation on volatile compounds, in vitro antimicrobial activity and in situ control of Aspergillus flavus in tomato paste. Food Chem. Toxicol. 2013, 59, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Mata, G.; Valdez, K.; Mendoza, R.; Trigos, A. HS/GC-MS analyzed chemical composition of the aroma of fruiting bodies of two species of genus Lentinus (Higher Basidiomycetes). Int. J. Med. Mushrooms 2014, 16, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Characteristic volatiles from young and aged fruiting bodies of wild Polyporus sulfureus (Bull.:Fr.) Fr. J. Agric. Food. Chem. 2005, 53, 4524–4528. [Google Scholar] [CrossRef] [PubMed]
- Thakeow, P.; Angeli, S.; Weissbecker, B.; Schütz, S. Antennal and behavioral responses of Cis boleti to fungal odor of Trametes gibbosa. Chem. Senses 2008, 33, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Kim, S.Y.; Choi, H.K.; Kim, Y.S. Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef] [PubMed]
- Ziegenbein, F.C.; Hanssen, H.P.; König, W.A. Secondary metabolites from Ganoderma lucidum and Spongiporus leucomallelius. Phytochemistry 2006, 67, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, Z.; Liu, Z.; Yang, B.; Tu, W.; Li, L. Chemical composition and antimicrobial activity of essential oil isolated from the cultured mycelia of Ganoderma japonicum. J. Nanjing Med. Univ. 2009, 23, 168–172. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sheriss, J.C.; Turck, M. Antibiotic susceptibility testing by standardised single method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Mann, C.M.; Markham, J.L. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Sievers, A.; Oshinowo, L.; Schultze, W.; Koch, A.; Richter, R. Einfache dueunnschicht-chromatographische Pruefung auf antioxidative Verbindungen mit dem DPPH-test. C35. Camag. Bibliogr. Serv. 2000, 88, 14–15. [Google Scholar]
- Brand, W.W.; Cuvelier, H.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- De Pinho, P.G.; Ribeiro, B.; Gonçalves, R.F.; Baptista, P.; Valentão, P.; Seabra, R.M.; Andrade, P.B. Correlation between the pattern volatiles and the overall aroma of wild edible mushrooms. J. Agric. Food Chem. 2008, 56, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.; Dobrovin-Pennington, A.; Hobbs, P.J.; Pederby, J.; Rodger, A. Volatile C8 compounds and pseudomonads influence primordium formation of Agaricus bisporus. Mycologia 2009, 101, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Kalkhove, S.I.; Lugones, L.G.; Baars, J.J.; Wösten, H.A.; Bakker, P.A. Effects of the mushroom-volatile 1-octen-3-ol on dry bubble disease. Appl. Microbiol. Biotechnol. 2013, 97, 5535–5543. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, S.; Miyazawa, M. Characterization of volatile components and odor-active compounds in the oil of edible mushroom Boletopsis leucomelas. J. Oleo Sci. 2014, 63, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Garcia, M.J.; Estarron-Espinosa, M.; Ogura, T. Volatile compounds secreted by the oyster mushroom (Pleurotus ostreatus) and their antibacterial activities. J. Agric. Food Chem. 1997, 45, 4049–4052. [Google Scholar] [CrossRef]
- Usami, A.; Motooka, R.; Nakahashi, H.; Okuno, Y.; Miyazawa, M. Characteristic odorants from bailingu oyster mushroom (Pleurotus eryngii var. tuoliensis) and summer oyster mushroom (Pleurotus cystidiosus). J. Oleo Sci. 2014, 63, 731–739. [Google Scholar] [PubMed]
- Usami, A.; Nakaya, S.; Nakahashi, H.; Miyazawa, M. Chemical composition and aroma evaluation of volatile oils from edible mushrooms (Pleurotus salmoneostramineus and Pleurotus sajor-caju). J. Oleo Sci. 2014, 63, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.; Racioppi, R.; Rana, G.L.; Laurita, A. Studies on volatile organic compounds of some truffles and false truffles. Nat. Prod. Res. 2014, 28, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Usami, A.; Ono, T.; Kashima, Y.; Nakahashi, H.; Marumoto, S.; Nosaka, S.; Watanabe, S.; Miyazawa, M. Comparison of agitake (Pleurotuseryngii var. ferulae) volatile components with characteristic odors extracted by hydrodistillation and solvent-assisted flavor evaporation. J. Oleo Sci. 2014, 63, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cecotti, R.; Carpana, E.; Bergomi, P.; Tava, A. Volatile constituents of Trifolium pratense spp. nivale quantified at different growth stages, and evaluation of their antimicrobial activity. Nat. Prod. Commun. 2013, 8, 1625–1628. [Google Scholar] [PubMed]
- Glamočlija, J.; Soković, M.; Tešević, V.; Linde, G.A.; Colauto, N.B. Chemical characterization of Lippia alba essential oil: An alternative to control green molds. Braz. J. Microbiol. 2011, 42, 1537–1546. [Google Scholar] [PubMed]
- Kim, Y.S.; Shin, D.H. Volatile constituents from the leaves of Callicarpa japonica Thunb. and their antibacterial activities. J. Agric. Food Chem. 2004, 52, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Luo, W.; Yang, B.; Jiang, Y. Identification of volatile components in Phyllanthus emblica L. and their antimicrobial activity. J. Med. Food. 2009, 12, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Pant, C.C.; Melkani, A.B.; Mohan, L.; Dev, V. Composition and antibacterial activity of essential oil from Scutellaria grossa Wall ex Benth. Nat. Prod. Res. 2012, 26, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Shafaghat, A. Antibacterial activity and GC/MS analysis of the essential oils from flower, leaf and stem of Origanum vulgare ssp. viride growing wild in north-west Iran. Nat. Prod. Commun. 2011, 6, 1351–1352. [Google Scholar] [PubMed]
- Singab, A.N.; Mostafa, N.M.; Eldahshan, O.A.; Ashour, M.L.; Wink, M. Profile of volatile components of hydrodistilled and extracted leaves of Jacaranda acutifolia and their antimicrobial activity against foodborne pathogens. Nat. Prod. Commun. 2014, 7, 1007–1010. [Google Scholar]
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry 2004, 65, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Wu, W.W.; Li, G.K. A GC–MS Study of the volatile organic composition of straw and oyster mushrooms during Maturity and its relation to antioxidant activity. J. Chromatogr. Sci. 2008, 46, 690–696. [Google Scholar] [CrossRef] [PubMed]
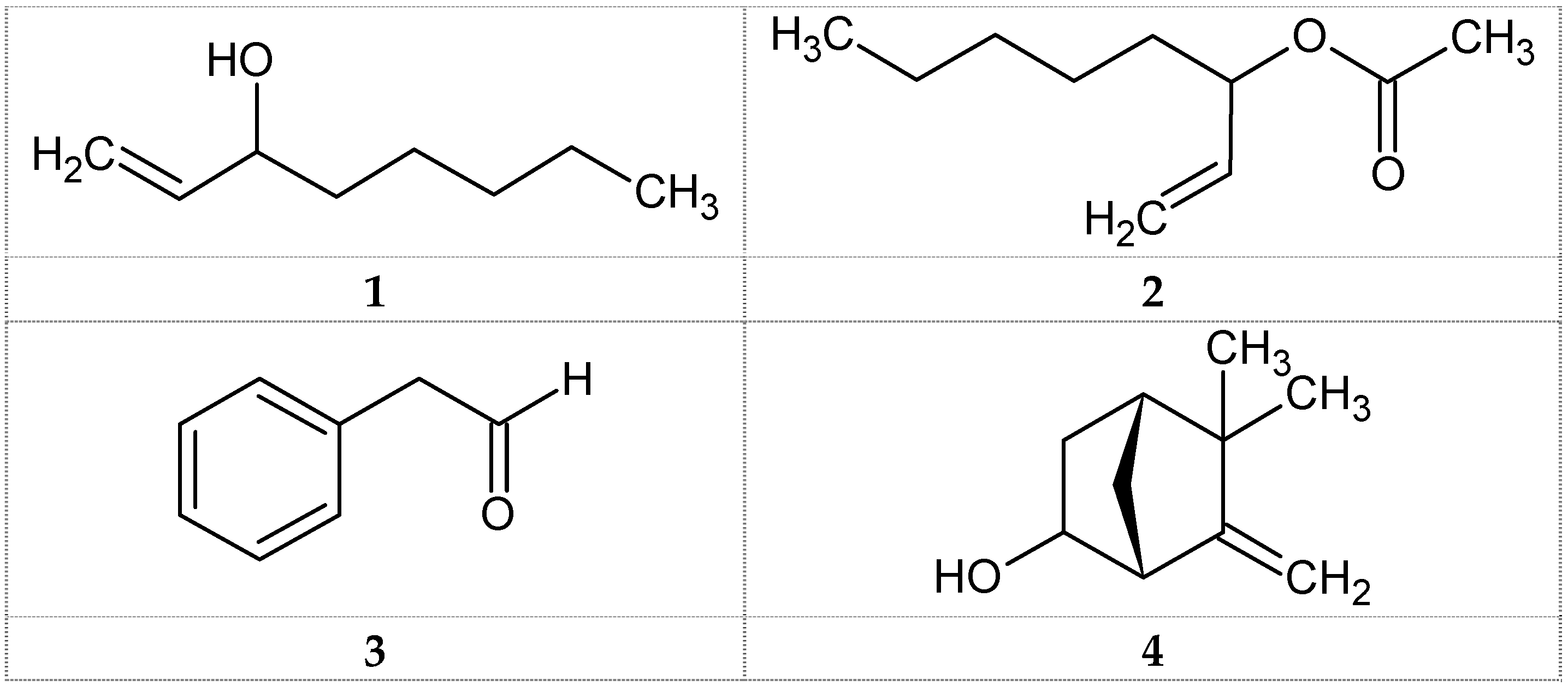
| No. | RI | Compounds a | Content % |
|---|---|---|---|
| 1 | 973 | unidentified | 0.8 |
| 2 | 976 | 1-Octen-3-ol (Amyl vinyl carbinol, 1) | 73.6 |
| 3 | 980 | unidentified | 2.2 |
| 4 | 988 | unidentified | 2.2 |
| 5 | 1027 | Phenylacetaldehyde (Hyacinthin, 3) | 3.0 |
| 6 | 1044 | unidentified | 4.3 |
| 7 | 1081 | 1-Octen-3-ol, acetate (Amyl vinyl carbinol acetate, 2) | 12.4 |
| 8 | 1447 | 6-Camphenol, (4) | 1.5 |
| Total identified | 90.5 |
| Microbial Strains | Volatile Oil | Reference Antibiotics IZ (mm) | |||
|---|---|---|---|---|---|
| IZ (mm) 10 μL/disc | MIC (mg/mL) | Ampicillin 10 μg/disc | Gentamicin 10 μg/disc | Nystatin 100 μg/disc | |
| Gram-Positive Bacteria | |||||
| B. subtilis | 20 | 0.6 | 29 | n.t. | n.t. |
| M. flavus | 10 | 4.5 | 31 | n.t. | n.t. |
| S. aureus | 30 | 0.3 | 28 | 23 | n.t. |
| Gram-Negative Bacteria | |||||
| E. coli | 15 | 1.2 | n.t. | 15 | n.t. |
| P. aeruginosa | 8 | n.t. | n.t. | 18 | n.t. |
| Fungi Strains | |||||
| C. albicans | 20 | 0.6 | n.t. | n.t. | 25 |
| C. maltosa | 15 | n.t. | n.t. | n.t. | 25 |
| Test Probe | Radical Scavenging Activity (%) | ||||
|---|---|---|---|---|---|
| Concentration | 10 μg/mL | 50 μg/mL | 100 μg/mL | 500 μg/mL | 1000 μg/mL |
| Volatile oil | 28.9 | 56.89 | 70.43 | 85.6 | 90.94 |
| Ascorbic acid | 48.5 | 89.5 | 95.8 | 96.1 | 96.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fatimi, M.; Wurster, M.; Lindequist, U. Chemical Composition, Antimicrobial and Antioxidant Activities of the Volatile Oil of Ganoderma pfeifferi Bres. Medicines 2016, 3, 10. https://doi.org/10.3390/medicines3020010
Al-Fatimi M, Wurster M, Lindequist U. Chemical Composition, Antimicrobial and Antioxidant Activities of the Volatile Oil of Ganoderma pfeifferi Bres. Medicines. 2016; 3(2):10. https://doi.org/10.3390/medicines3020010
Chicago/Turabian StyleAl-Fatimi, Mohamed, Martina Wurster, and Ulrike Lindequist. 2016. "Chemical Composition, Antimicrobial and Antioxidant Activities of the Volatile Oil of Ganoderma pfeifferi Bres" Medicines 3, no. 2: 10. https://doi.org/10.3390/medicines3020010






