Methylmercury Exposure and Developmental Outcomes in Tohoku Study of Child Development at 18 Months of Age
Abstract
1. Introduction
2. Materials and Methods
2.1. TSCD Outline
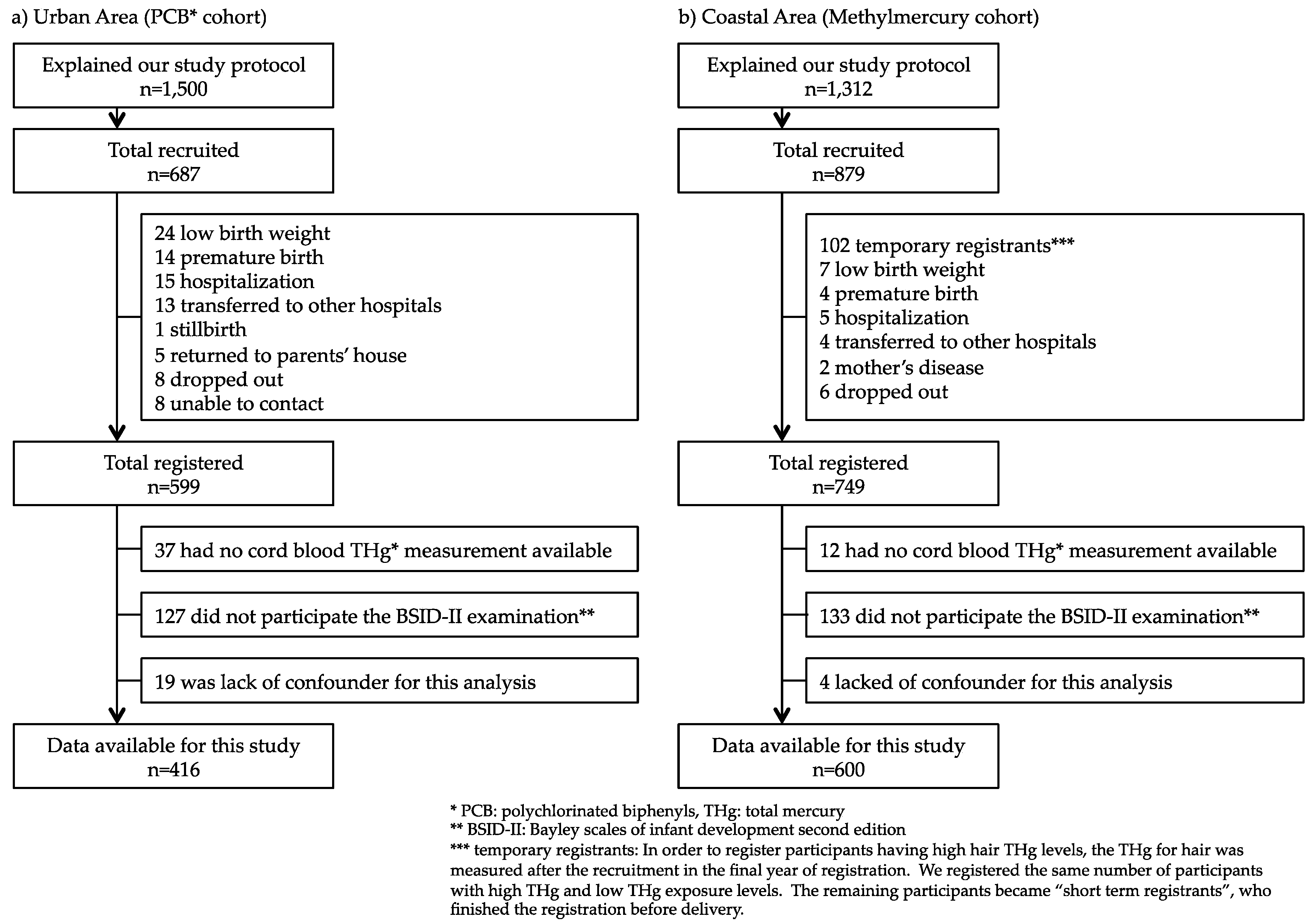
2.1.1. Urban Area Cohort (PCB Cohort)
2.1.2. Coastal Area Cohort (Methylmercury Cohort)
2.2. Exposure Markers
2.3. Outcome
2.4. Confounding Variables
2.5. Statistics
3. Results
4. Discussion
4.1. Outline of the TSCD
4.2. Exposure Levels
4.3. Gender-Specific Analyses
4.4. Regional Difference
4.5. Future Vision
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strain, J.J.; Yeates, A.J.; van Wijngaarden, E.; Thurston, S.W.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Love, T.M.; Smith, T.H.; Yost, K.; et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: Associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am. J. Clin. Nutr. 2015, 101, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Muckle, G.; Jacobson, J.L.; Carter, R.C.; Kaplan-Estrin, M.; Ayotte, P.; Dewailly, E.; Jacobson, S.W. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: Results from the Environmental Contaminants and Child Development Study of Nunavik. Environ. Health Perspect. 2014, 122, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Mendez, M.; Femandez-Barres, S.; Romaguera, D.; Vioque, J.; Liop, S.; Ibarluzea, J.; Cuxens, M.; Avella-Garcia, C.; Tordon, A.; et al. Maternal consumption of seafood in pregnancy and child neuropsychological development: A longitudinal study based on a population with high consumption levels. Am. J. Epidemiol. 2016, 183, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Weihe, P.; Grandjean, P.; Debes, F.; White, R. Health implications for Faroe islanders of heavy metals and PCBs from pilot whales. Sci. Total Environ. 1996, 186, 141–148. [Google Scholar] [CrossRef]
- Marques, R.C.; Doreac, J.G.; Bastosa, R.W.; de Freitas Rebelob, M.; de Freitas Fonsecab, M.; Malm, O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int. J. Hyg. Environ. Health 2007, 210, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.L.; Longnecker, M.P.; Rowland, A.S.; Golding, J.; ALSPAC Study Team. University of Bristol Institute of Child Health. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology 2004, 15, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Yaginuma-Sakurai, K.; Murata, K.; Iwai-Shimada, M.; Nakai, K.; Kurokawa, N.; Tatsuta, N.; Satoh, H. Hair-to-blood ratio and biological half-life of mercury: Experimental study of methylmercury exposure through fish consumption in humans. J. Toxicol. Sci. 2012, 37, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Choi, A.L.; Oken, E.; Horvat, M.; Schoeny, R.; Kamai, E.; Cowell, W.; Grandjean, P.; Korrick, S. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 2012, 120, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; White, R.F.; Debes, F.; Araki, S.; Yokoyama, K.; Murata, K.; Sørensen, N.; Dahl, R.; Jørgensen, P.J. Cognitive deficit in 7-year-old with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997, 19, 417–428. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Jankowski, J.; Flak, E.; Skarupa, A.; Mroz, E.; Sochacka-Tatara, E.; Lisowska-Miszczyk, I.; Szpanowska-Wohn, A.; Rauh, V.; Skolicki, Z.; et al. Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: Epidemiologic cohort study in Poland. Ann. Epidemiol. 2006, 16, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Perera, F.; Jankowski, J.; Rauh, V.; Flak, E.; Caldwell, K.L.; Jones, R.L.; Pac, A.; Lisowska-Miszczyk, I. Fish consumption in pregnancy, cord blood mercury level and cognitive and psychomotor development of infants followed over the first three years of life: Krakow epidemiologic study. Environ. Int. 2007, 33, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Valent, F.; Mariuz, M.; Bin, M.; Little, D.; Mazej, D.; Tognin, V.; Tratnik, J.; McAfee, A.J.; Mulhern, M.S.; Parpinel, M.; et al. Associations of prenatal mercury exposure from maternal fish consumption and polyunsaturated fatty acids with child neurodevelopment: A prospective cohort study in Italy. J. Epidemiol. 2013, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Hsi, H.C.; Jiang, C.B.; Yang, T.H.; Chien, L.C. The neurological effects of prenatal and postnatal mercury/methylmercury exposure on three-year-old children in Taiwan. Chemosphere 2014, 100, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Abreu, L.; Bernardi, J.V.; Dorea, J.G. Neurodevelopment of Amazonian children exposed to ethylmercury (from Thimerosal in vaccines) and methylmercury (from fish). Environ. Res. 2016, 149, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden, E.; Thurston, S.W.; Myers, G.J.; Harrington, D.; Cory-Slechta, D.A.; Strain, J.J.; Watson, G.E.; Zareba, G.; Love, T.; Henderson, J.; et al. Methylmercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24years. Neurotoxicol. Teratol. 2017, 59, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Prpić, I.; Milardović, A.; Vlašić-Cicvarić, I.; Špiric, Z.; Radić Nišević, J.; Vukelić, P.; Snoj Tratnik, J.; Mazej, D.; Horvat, M. Prenatal exposure to low-level methylmercury alters the child’s fine motor skills at the age of 18 months. Environ. Res. 2017, 152, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Suzuki, K.; Oka, T.; Murata, K.; Sakamoto, M.; Okamura, K.; Hosokawa, T.; Sakai, T.; Nakamura, T.; Saito, Y.; et al. The Tohoku Study of Child Development: A cohort study of effects of perinatal exposures to methylmercury and environmentally persistent organic pollutants on neurobehavioral development in Japanese children. Tohoku J. Exp. Med. 2004, 202, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakai, K.; Sugawara, T.; Nakamura, T.; Ohba, T.; Shimada, M.; Hosokawa, T.; Okamura, K.; Sakai, T.; Kurokawa, N.; et al. Neurobehavioral effects of prenatal exposure to methylmercury and PCBs, and seafood intake: Neonatal behavioral assessment scale results of Tohoku study of child development. Environ. Res. 2010, 110, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Shimada, M.; Satoh, H.; Nakai, K.; Tatsuta, N.; Murata, K.; Akagi, H. Methylmercury in the breast milk of Japanese mothers and lactational exposure of their infants. Chemosphere 2015, 126, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Nakai, K.; Satoh, H.; Murata, K. Impacts of the Great East Japan earthquake on child’s IQ. J. Pediatr. 2015, 167, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Murata, K.; Iwai-Shimada, M.; Yaginuma-Sakurai, K.; Satoh, H.; Nakai, K. Psychomotor ability in children prenatally exposed to methylmercury: The 18-month follow-up of Tohoku study of child development. Tohoku J. Exp. Med. 2017, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Kurokawa, N.; Nakai, K.; Suzuki, K.; Iwai-Shimada, M.; Murata, K.; Satoh, H. Effects of intrauterine exposures to polychlorinated biphenyls, methylmercury, and lead on birth weight in Japanese male and female newborns. Environ. Health Prev. Med. 2017, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Nakai, K.; Iwai-Shimada, M.; Suzuki, T.; Satoh, H.; Murata, K. Total mercury levels in hair of children aged 7 years before and after the Great East Japan Earthquake. Sci. Total Environ. 2017, 596–597, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.W.; Myers, G.J.; Cox, C.; Shamlaye, C.F.; Marsh, D.O.; Tanner, M.A.; Berlin, M.; Sloane-Reeves, J.; Cemichiari, E.; Choisy, O.; et al. Longitudinal neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from maternal fish ingestion: Outcomes at 19 and 29 months. Neurotoxicology 1995, 16, 677–688. [Google Scholar] [PubMed]
- Sakamoto, M.; Nakano, A.; Akagi, H. Declining Minamata male birth ratio associated with increased male fetal death due to heavy methylmercury pollution. Environ. Res. 2001, 87, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Bernardi, J.V.; Abreu, L.; Dorea, J.G. Neurodevelopment outcomes in children exposed to organic mercury from multiple sources in a tin-ore mine environment in Brazil. Arch. Environ. Contam. Toxicol. 2015, 68, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Lopez-Espinosa, M.J.; Rebagliato, M.; Ballester, F. Gender differences in the neurotoxicity of metals in children. Toxicology 2013, 311, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M. Framework for gender differences in human and animal toxicology. Environ. Res. 2007, 104, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Bayley, N. Bayley Scales of Infant Development, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 1993. [Google Scholar]
- Yasutake, A.; Matsumoto, M.; Yamaguchi, M.; Hachiya, N. Current hair mercury levels in Japanese: Survey in five districts. Tohoku J. Exp. Med. 2003, 199, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Koopman-Esseboom, C.; Weisglas-Kuperus, N.; de Ridder, M.A.; Van der Paauw, C.G.; Tuinstra, L.G.; Sauer, P.J. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics 1996, 97, 700–706. [Google Scholar] [PubMed]
- Ministry of the Environment, Japan. Mercury Analysis Manual. 2004. Available online: http://www.nimd.go.jp/kenkyu/docs/march_mercury_analysis_manual%28e%29.pdf (accessed on 2 July 2018).
- Nakamura, T.; Nakai, K.; Matsumura, T.; Suzuki, S.; Saito, Y.; Satoh, H. Determination of dioxins and polychlorinated biphenyls in breast milk, maternal blood and cord blood from residents of Tohoku, Japan. Sci. Total Environ. 2008, 394, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Suzuki, K.; Sugawara, T.; Nakai, K.; Hosokawa, T.; Satoh, H. Comparison of Kyoto Scale of Psychological Development and Bayley Scales of Infant Development second edition among Japanese infants. J. Spec. Educ. Res. 2013, 2, 17–24. [Google Scholar] [CrossRef]
- Yaginuma-Sakurai, K.; Shimada, M.; Ohba, T.; Nakai, K.; Suzuki, K.; Kurokawa, N.; Kameo, S.; Satoh, H. Assessment of exposure to methylmercury in pregnant Japanese women by FFQ. Public Health Nutr. 2009, 12, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.C. Standard Progressive Matrices: Sets A, B, C, D and E; Lewis: London, UK, 1985. [Google Scholar]
- Anme, T.; Ueda, R.; Hirayama, M. Evaluation of home stimulation using HSQ (HOME screening questionnaire). J. Child Health 1986, 45, 556–560. [Google Scholar]
- Caldwell, B.M.; Bradley, R.H. Home Observation for Measurement of the Environment; University of Arkansas at Little Rock: Little Rock, AK, USA, 1984. [Google Scholar]
- Japan Food Safety Commission Secretariat. Food Safety Risk Assessment Related to Methylmercury in Seafood. 2005. Available online: http://www.fsc.go.jp/english/topics/methylmercury_risk_assessment.pdf (accessed on 2 July 2018).
- Grandjean, P.; Budtz-Jørgensen, E.; White, R.F.; Jørgensen, P.J.; Weihe, P.; Debes, F.; Keiding, N. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am. J. Epidemiol. 1999, 150, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; Debes, F.; Choi, A.L.; Budtz-Jørgensen, E. Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol. Teratol. 2014, 43, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.W.; Myers, G.J.; Cox, C.; Axtell, C.; Shamlaye, C.; Sloane-Reeves, J.; Cernichiari, E.; Needham, L.; Choi, A.; Wang, Y.; et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: Outcomes at 66 months of age in the Seychelles Child Development Study. JAMA 1998, 280, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Muckle, G.; Ayotte, P.; Dewailly, E.; Jacobson, S.W.; Jacobson, J.L. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ. Health Perspect. 2001, 109, 1291–1299. [Google Scholar] [PubMed]
- Marques, R.C.; Bernardi, J.V.; Dórea, J.G.; Brandão, K.G.; Bueno, L.; Leão, R.S.; Malm, O. Fish consumption during pregnancy, mercury transfer, and birth weight along the Madeira River Basin in Amazonia. Int. J. Environ. Res. Public Health 2013, 10, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Cordier, S.; Garel, M.; Mandereau, L.; Morcel, H.; Doineau, P.; Gosme-Seguret, S.; Josse, D.; White, R.; Amiel-Tison, C. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ. Res. 2002, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Deroma, L.; Parpinel, M.; Tognin, V.; Channoufi, L.; Tratnik, J.; Horvat, M.; Valent, F.; Barbone, F. Neuropsychological assessment at school-age and prenatal low-level exposure to mercury through fish consumption in an Italian birth cohort living near a contaminated site. Int. J. Hyg. Environ. Health 2013, 216, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.B.; Pagulayan, O.; Akagi, H.; Francisco Rivera, A.; Lee, L.V.; Berroya, A.; Vince Cruz, M.C.; Casintahan, D. Tagum study II: Follow-up study at two years of age after prenatal exposure to mercury. Pediatrics 2003, 111, e289-95. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, C.H.; Tian, Y.; Wang, Y.; Xie, H.F.; Zhou, X.; Yu, X.D.; Yu, X.G.; Tong, S.; Zhou, Q.X.; et al. Prenatal exposure to mercury and neurobehavioral development of neonates in Zhoushan City, China. Environ. Res. 2007, 105, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Fröhlich, S.; Graf-Rohrmeister, K.; Eibenberger, B.; Jessenig, V.; Gicic, D.; Prinz, S.; Wittmann, K.J.; Zeisler, H.; Vallant, B.; et al. Perinatal lead and mercury exposure in Austria. Sci. Total Environ. 2010, 408, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Holzman, C.; Rahbar, M.H.; Trosko, K.; Fischer, L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ. Health Perspect. 2007, 115, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Kawabata, T.; Tatsuta, N.; Kimura, F.; Miyazawa, T.; Mizuno, S.; Nishigori, H.; Arima, T.; Kagawa, Y.; Yoshimasu, K.; et al. Determinants of polyunsaturated fatty acid concentrations in erythrocytes of pregnant Japanese women from a birth cohort study: Study protocol and baseline findings of an adjunct study of the Japan Environment & Children’s Study. Environ. Health Prev. Med. 2017, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J.; Davidson, P.W.; Shamlaye, C.F. A review of methylmercury and child development. Neurotoxicology 1998, 19, 313–328. [Google Scholar] [PubMed]
- McKeown-Eyssen, G.E.; Ruedy, J.; Neims, A. Methylmercury exposure in northern Quebec. II. Neurologic findings in children. Am. J. Epidemiol. 1983, 118, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.E.; Lynch, M.; Myers, G.J.; Shamlaye, C.F.; Thurston, S.W.; Zareba, G.; Clarkson, T.W.; Davidson, P.W. Prenatal exposure to dental amalgam: Evidence from the Seychelles Child Development Study main cohort. J. Am. Dent. Assoc. 2011, 142, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, N.; Murata, K.; Budtz-Jørgensen, E.; Weihe, P.; Grandjean, P. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 1999, 10, 370–375. [Google Scholar] [CrossRef] [PubMed]
- White, R.F.; Palumbo, C.L.; Yurgelun-Todd, D.A.; Heaton, K.J.; Weihe, P.; Debes, F.; Grandjean, P. Functional MRI approach to developmental methylmercury and polychlorinated biphenyl neurotoxicity. Neurotoxicology 2011, 32, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Amarasiriwardena, C.; Korrick, S.A. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch. Pediatr. Adolesc. Med. 2012, 166, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.E.; Evans, K.; Thurston, S.W.; van Wijngaarden, E.; Wallace, J.M.; McSorley, E.M.; Bonham, M.P.; Mulhern, M.S.; McAfee, A.J.; Davidson, P.W.; et al. Prenatal exposure to dental amalgam in the Seychelles Child Development Nutrition Study: Associations with neurodevelopmental outcomes at 9 and 30 months. Neurotoxicology 2012, 33, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Guxens, M.; Murcia, M.; Lertxundi, A.; Ramon, R.; Riaño, I.; Rebagliato, M.; Ibarluzea, J.; Tardon, A.; Sunyer, J.; et al. Prenatal exposure to mercury and infant neurodevelopment in a multicenter cohort in Spain: Study of potential modifiers. Am. J. Epidemiol. 2012, 175, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Sørensen, H.T. The Danish national birth cohort—A valuable tool for pharmacoepidemiology in pregnancy. Int. J. Risk Saf. Med. 1997, 10, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Magnus, P.; Irgens, L.M.; Haug, K.; Nystad, W.; Skjaerven, R.; Stoltenberg, C.; MoBa Study Group. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol. 2006, 35, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Salles, T.; Mendez, M.A.; Morales, E.; Bustamante, M.; Rodríguez-Vicente, A.; Kogevinas, M.; Sunyer, J. Dietary benzo(a)pyrene and fetal growth: Effect modification by vitamin C intake and glutathione S-transferase P1 polymorphism. Environ. Int. 2012, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Hardy, R.; Kuh, D.; Wadsworth, M.E. Birth weight and cognitive function in the British 1946 birth cohort: Longitudinal population based study. BMJ 2001, 322, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Baghurst, P.; Vimpani, G.; McMichael, A. Socioeconomic position, maternal IQ, home environment, and cognitive development. J. Pediatr. 2007, 151, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Debes, F.; Budtz-Jørgensen, E.; Weihe, P.; White, R.F.; Grandjean, P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006, 28, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Debes, F.; Weihe, P.; Grandjean, P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex 2016, 74, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Interpreting epidemiologic studies of developmental neurotoxicity: Conceptual and analytic issues. Neurotoxicol. Teratol. 2009, 31, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; Burse, V.W.; Needham, L.L.; Storr-Hansen, E.; Heinzow, B.; Debes, F.; Murata, K.; Simonsen, H.; Ellefsen, P.; et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol. Teratol. 2001, 23, 305–317. [Google Scholar] [CrossRef]
- Stewart, P.; Sargent, D.; Reihman, J.; Gump, B.; Lonky, E.; Darvill, T.; Hicks, H.; Pagano, J. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ. Health Perspect. 2006, 114, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Debes, F.; Weihe, P.; Grandjean, P. Prenatal exposure to lead and cognitive deficit in 7- and 14-year-old children in the presence of concomitant exposure to similar molar concentration of methylmercury. Neurotoxicol. Teratol. 2011, 33, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Nakai, K.; Murata, K.; Suzuki, K.; Iwai-Shimada, M.; Yaginuma-Sakurai, K.; Kurokawa, N.; Nakamura, T.; Hosokawa, T.; Satoh, H. Prenatal exposures to environmental chemicals and birth order as risk factors for child behavior problems. Environ. Res. 2012, 114, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Nakai, K.; Murata, K.; Suzuki, K.; Iwai-Shimada, M.; Kurokawa, N.; Hosokawa, T.; Satoh, H. Impacts of prenatal exposures to polychlorinated biphenyls, methylmercury and lead on intellectual ability of 42-month-old children in Japan. Environ. Res. 2014, 133, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.L.; Mogensen, U.B.; Bjerve, K.S.; Debes, F.; Weihe, P.; Grandjean, P.; Budtz-Jørgensen, E. Negative confounding by essential fatty acids in methylmercury neurotoxicity associations. Neurotoxicol. Teratol. 2014, 42, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.J.; Davidson, P.W.; Bonham, M.P.; Duffy, E.M.; Stokes-Riner, A.; Thurston, S.W.; Wallace, J.M.; Robson, P.J.; Shamlaye, C.F.; Georger, L.A.; et al. Associations of maternal long-chain polyunsaturated fatty acids, methylmercury and infant development in the Seychelles child development nutrition study. Neurotoxicology 2008, 29, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ha, E.H.; Park, H.; Ha, M.; Kim, Y.; Hong, Y.C.; Lee, E.J.; Kim, H.; Chang, N.; Kim, B.N. Prenatal mercury exposure, fish intake and neurocognitive development during first three years of life: Prospective cohort mothers and Children’s environmental health (MOCEH) study. Sci. Total Environ. 2018, 615, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
| Candidate Area | n | Geometric Mean | Min | Max |
|---|---|---|---|---|
| Candidate area A | 100 | 3.27 | 1.00 | 13.3 |
| Candidate area B | 94 | 1.99 | 0.66 | 10.3 |
| Candidate area C | 99 | 1.80 | 0.55 | 5.35 |
| Candidate area D | 100 | 2.01 | 0.67 | 8.15 |
| Yasutake et al. Tohoku J. Exp. Med. 2003, 199(3), 161–169 | ||||
| Minamata | 594 | 1.23 | 0.09 | 7.33 |
| Kumamoto | 327 | 1.33 | 0.14 | 6.20 |
| Tottori | 209 | 1.40 | 0.26 | 12.5 |
| Wakayama | 303 | 1.40 | 0.00 | 8.09 |
| Chiba | 233 | 2.30 | 0.14 | 25.8 |
| Child Age | Neurobehavioral Development Assessment |
|---|---|
| 3 days | Neonatal Behavioral Assessment Scale |
| 7 months | Kyoto Scale of Psychological Development (KSPD) |
| Bayley Scales of Infant Development second edition (BSID-II) | |
| Fagan Test of Infant Intelligence | |
| 18 months (1.5 years) | KSPD, BSID-II, Evaluation of Environmental Stimulation (EES) |
| Raven standard progressive matrices | |
| 30 months (2.5 years) | Child Behavior Checklist age for 2–3, EES |
| 42 months (3.5 years) | Kaufman Assessment Battery for Children |
| 66 months (5.5 years) | Social-Maturity Skill Scale (S-M scale) |
| 84 months (7 years) | Wechsler Intelligence Scale for Children Third edition |
| 120 months (10 years) | S-M scale |
| 144 months (12 years) | Wechsler Intelligence Scale for Children Forth edition |
| Basal Characterisctics | Urban Area | Coastal Area | p-Value ** |
|---|---|---|---|
| Mean ± SD * | Mean ± SD * | ||
| (or %) | (or %) | ||
| Maternal characteristics | |||
| Maternal age at parturition (years) | 31.3 ± 4.4 | 29.5 ± 4.9 | p < 0.001 |
| Body mass index before pregnancy (kg/m2) | 21.0 ± 2.8 | 21.5 ± 3.3 | 0.002 |
| Drinkers during pregnancy (%, yes) | 31.7 | 16.6 | p < 0.001 |
| Smokers during pregnancy (%, yes) | 7.8 | 12.5 | p < 0.001 |
| Maternal education level (%, >12 y) | 74.9 | 41.5 | p < 0.001 |
| Raven score *** | 51.5 ± 6.3 | 49.9 ± 6.0 | p < 0.001 |
| EES score at 18 months *** | 28.2 ± 3.4 | 26.6 ± 3.8 | p < 0.001 |
| Child characteristics | |||
| Child gender (%, boys) | 52.6 | 50.9 | 0.547 |
| Birth order (%, first child) | 51.4 | 42.1 | 0.001 |
| Gestational duration (weeks) | 39.5 ± 1.3 | 39.7 ± 1.2 | 0.038 |
| Birth weight (g) | 3073 ± 338 | 3141 ± 365 | p < 0.001 |
| Delivery type (%, vaginal delivery) | 83.6 | 84.4 | 0.765 |
| Apgar score (1 min) | 8.2 ± 0.8 | 8.4 ± 0.8 | p < 0.001 |
| Exposures | Urban Area | Coastal Area | p-Value * |
|---|---|---|---|
| n, Median, 5–95 Percentiles | n, Median, 5–95 Percentiles | ||
| Exposure biomarkers: | |||
| Cord-blood THg (ng/g) ** | 562, 10.0, 4.2–22.4 | 731, 16.0, 5.6–39.3 | p < 0.001 |
| Maternal hair THg (μg/g) ** | 595, 2.0, 0.9–4.4 | 748, 2.6, 0.9–6.0 | p < 0.001 |
| Breast milk THg (ng/g) ** | - | 27, 0.8, 0.1–1.8 | - |
| Cord-blood PCB (ng/g-lipid) ** | 518, 45.8, 18.4–112.2 | - | - |
| Breast milk PCB (ng/g-lipid) ** | 544, 93.1, 42.4–185.9 | - | - |
| Cord-blood lead (ng/dL) | 555, 1.0, 0.6–1.8 | 664, 0.7, 0.4–1.4 | p < 0.001 |
| Cord-blood selenium (ng/mL) | 555, 192.7 (130.3–271.9) | - | - |
| Cord-plasma selenium (ng/g) | - | 709, 66.3, 51.0–271.9 | - |
| Maternal-plasma DHA ** | - | 742, 169.7, 101.1–256.9 | - |
| Seafood intake during pregnancy (kg/y) | 598, 44.4, 12.6–110.8 | 749, 47.7, 10.5–140.6 | 0.089 |
| Fish Species | Urban Area (n = 598) | Coastal Area (n = 749) | p-Value ** |
|---|---|---|---|
| Median (Min-Max) | Median (Min-Max) | ||
| Tuna | 4.1 (0.0–123.7) | 4.4 (0.0–105.0) | 0.161 |
| Bonito | 2.1 (0.0–41.8) | 2.7 (0.0–108.3) | p < 0.001 |
| Whale | 0.0 (0.0–17.5) | 0.0 (0.0–2.3) | 0.004 |
| Salmon | 3.1 (0.0–34.7) | 3.1 (0.0–92.5) | 0.037 |
| Eel | 0.3 (0.0–32.1) | 0.0 (0.0–12.5) | p < 0.001 |
| Yellowtail | 0.6 (0.0–70.0) | 0.0) (0.0–70.0) | p < 0.001 |
| Silvery blue fish | 5.8 (0.0–55.0) | 5.8 (0.0–70.0) | 0.546 |
| White-meat fish | 7.2 (0.0–87.0) | 7.2 (0.0–87.0) | 0.918 |
| Other fish | 0.0 (0.0–57.9) | 3.0 (0.0–90.0) | p < 0.001 |
| Squid/Octopus | 2.0 (0.0–30.0) | 2.0 (0.0–60.0) | 0.264 |
| Shellfish | 1.7 (0.0–39.3) | 2.3 (0.0–50.0) | p < 0.001 |
| Salmon roe | 0.0 (0.0–37.5) | 0.0 (0.0–37.5) | 0.162 |
| Canned tuna | 1.7 (0.0–60.0) | 2.0 (0.0–60.0) | 0.524 |
| Time of Each Examination | Urban Area | Coastal Area | ||||
|---|---|---|---|---|---|---|
| Registrants | Participants | % | Registrants | Participants | % | |
| 3 days | 599 | 587 | 98.0 | 749 | 709 | 94.7 |
| 7 months | 594 | 516 | 86.9 | 749 | 653 | 87.2 |
| 18 months (1.5 years) | 589 | 477 | 81.0 | 747 | 617 | 82.6 |
| 30 months (2.5 years) | 595 | 499 | 83.9 | 739 | 649 | 87.8 |
| 42 months (3.5 years) | 566 | 472 | 83.4 | 733 | 597 | 81.3 |
| 66 months (5.5 years) | 580 | 456 | 78.6 | 727 | 614 | 84.5 |
| 84 months (7 years) | 546 | 457 | 83.7 | 720 | 498 | 69.2 |
| 120 months (10 years) | 711 | 569 | 80.0 | |||
| 144 months (12 years) | 699 | 385 | 55.0 | |||
| BSID-II Scores | Urban Area (n = 416) | Coastal Area (n = 600) | p-Value ** |
|---|---|---|---|
| Mean ± SD * | Mean ± SD * | ||
| MDI *** | 89.8 ± 11.9 | 86.9 ± 10.6 | <0.001 |
| PDI *** | 84.6 ± 10.6 | 84.4 ± 10.6 | 0.793 |
| Major Independent Variables | MDI ** | PDI ** | ||
|---|---|---|---|---|
| β | p-Value | β | p-Value | |
| Cord-blood THg * | −0.028 | 0.380 | −0.053 | 0.104 |
| Child gender | −0.230 | <0.001 | −0.111 | <0.001 |
| Birth weight | 0.034 | 0.261 | −0.012 | 0.696 |
| Birth order | −0.031 | 0.312 | 0.049 | 0.113 |
| Drinking habit during pregnancy | 0.035 | 0.253 | −0.044 | 0.164 |
| Smoking habit during pregnancy | 0.000 | 0.997 | 0.040 | 0.197 |
| Raven score *** | 0.036 | 0.238 | 0.063 | 0.044 |
| EES score at 18 months *** | 0.134 | <0.001 | 0.071 | 0.025 |
| Contribution rate, R2 | 0.101 | <0.001 | 0.080 | <0.001 |
| Major Independent Variables | Boys (n = 523) | Girls (n = 493) | ||||||
|---|---|---|---|---|---|---|---|---|
| MDI * | PDI * | MDI * | PDI * | |||||
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Cord-blood THg ** | −0.036 | 0.437 | −0.122 | 0.008 | −0.017 | 0.729 | 0.024 | 0.616 |
| Birth weight | 0.093 | 0.036 | 0.045 | 0.307 | −0.051 | 0.262 | −0.085 | 0.057 |
| Birth order | 0.007 | 0.873 | 0.026 | 0.554 | −0.085 | 0.061 | 0.066 | 0.142 |
| Drinking habit during pregnancy | −0.021 | 0.639 | −0.039 | 0.379 | 0.085 | 0.062 | −0.053 | 0.236 |
| Smoking habit during pregnancy | 0.000 | 0.999 | 0.033 | 0.440 | 0.012 | 0.782 | 0.057 | 0.203 |
| Raven score *** | 0.052 | 0.239 | 0.036 | 0.407 | 0.006 | 0.888 | 0.090 | 0.045 |
| EES score at 18 months *** | 0.092 | 0.038 | 0.086 | 0.052 | 0.204 | <0.001 | 0.068 | 0.137 |
| Contribution rate, R2 | 0.058 | <0.001 | 0.078 | <0.001 | 0.061 | <0.001 | 0.082 | <0.001 |
| Major Independent Variables | Urban Area (n = 220) | Coastal Area (n = 303) | ||
|---|---|---|---|---|
| β | p-Value | β | p-Value | |
| Cord-blood THg * | −0.033 | 0.606 | −0.18 | 0.002 |
| Birth weight | −0.045 | 0.477 | 0.091 | 0.128 |
| Birth order | −0.081 | 0.202 | 0.091 | 0.124 |
| Drinking habit during pregnancy | −0.058 | 0.362 | −0.044 | 0.450 |
| Smoking habit during pregnancy | −0.016 | 0.806 | 0.062 | 0.282 |
| Raven score *** | −0.098 | 0.126 | 0.124 | 0.033 |
| EES score at 18 months *** | 0.039 | 0.543 | 0.116 | 0.048 |
| Contribution rate, R2 | 0.148 | <0.001 | 0.052 | 0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsuta, N.; Nakai, K.; Sakamoto, M.; Murata, K.; Satoh, H. Methylmercury Exposure and Developmental Outcomes in Tohoku Study of Child Development at 18 Months of Age. Toxics 2018, 6, 49. https://doi.org/10.3390/toxics6030049
Tatsuta N, Nakai K, Sakamoto M, Murata K, Satoh H. Methylmercury Exposure and Developmental Outcomes in Tohoku Study of Child Development at 18 Months of Age. Toxics. 2018; 6(3):49. https://doi.org/10.3390/toxics6030049
Chicago/Turabian StyleTatsuta, Nozomi, Kunihiko Nakai, Mineshi Sakamoto, Katsuyuki Murata, and Hiroshi Satoh. 2018. "Methylmercury Exposure and Developmental Outcomes in Tohoku Study of Child Development at 18 Months of Age" Toxics 6, no. 3: 49. https://doi.org/10.3390/toxics6030049
APA StyleTatsuta, N., Nakai, K., Sakamoto, M., Murata, K., & Satoh, H. (2018). Methylmercury Exposure and Developmental Outcomes in Tohoku Study of Child Development at 18 Months of Age. Toxics, 6(3), 49. https://doi.org/10.3390/toxics6030049





