Tannery Effluent Treatment by Yeast Species Isolates from Watermelon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of the Samples
2.2. Isolation of Yeast
2.3. Characterization of the Yeast Isolates
2.4. Experimental Procedure for Effluent Treatment
2.5. Analysis of Physicochemical Properties
2.5.1. Nitrate
2.5.2. Phosphate
2.5.3. Sulphate
2.5.4. Chemical Oxygen Demand (COD)
2.5.5. Turbidity
2.5.6. Biochemical Oxygen Demand (BOD)
- D1 = DO of diluted sample immediately after preparation (mg·LG−1)
- D2 = DO of diluted sample after 5 days incubation at 20 °C (mg·LG−1)
- P = Decimal volumetric fraction of sample used (mL of the sample taken (90 mL) divided by total volume of the BOD bottle (250 mL).
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- EPA (Environmental Protection Agency). Quality Criteria for Water; Environmental Protection Agency United State of American: Washington, DC, USA, 2013; Volume 140.
- Bound, J.P.; Voulvoulis, N. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environ. Health Perspect. 2005, 113, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.I.; Musa, A.E.; Ibrahim, E.H.; Salma, A.S.; Babiker, M.E. Evaluation and Characterization of Tannery Wastewater. J. For. Prod. Ind. 2014, 3, 141–150. [Google Scholar]
- Chowdhury, M.; Mostafa, M.G.; Biswas, T.K.; Saha, A.K. Treatment of leather industrial effluents and coagulation process. Water Resour. Ind. 2013, 3, 11–22. [Google Scholar] [CrossRef]
- Vijayanand, S.; Hemapriya, J. Biosorption and Detoxification of Cr (VI) BY Tannery Effluent Acclimatized Halotolerant Bacterial Strain. Int. J. Curr. Microbiol. Appl. Sci. 2004, 3, 971–982. [Google Scholar]
- Goyal, N.; Jain, S.C.; Banerjee, U.C. Comparative studies on the microbial adsorption of heavy metals. Adv. Environ. Resour. 2003, 7, 311–319. [Google Scholar] [CrossRef]
- Liu, X.F.; Supek, F.; Nelson, N.; Culotta, V.C. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 Gene. J. Biol. Chem. 1997, 272, 11763–11769. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Janssens, S.; Soares, H.M.; Soares, E.V. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae, Advantage of using dead biomas. J. Appl. Microbiol. 2009, 106, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Zang, K.; Li, F. Isolation and Characterization of a chromium-resistant bacterium serratia sp. Cr-10 from a chromate contaminated site. Appl. Microbiol. Biotechnol. 2011, 90, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Palamthodi, S.; Patil, D.; Patil, Y. Microbial degradation of textile industrial effluents. Afr. J. Biotechnol. 2011, 10, 12687–12691. [Google Scholar]
- Abioye, O.P.; Mustapha, O.T.; Aransiola, S.A. Biological Treatment of Textile Effluent Using Candida zeylanoides and Saccharomyces cerevisiae Isolated from Soil. Adv. Biol. 2014, 2014, 670394. [Google Scholar] [CrossRef]
- Pang, C.; Liu, Y.; Cao, X.; Li, M.; Huang, G.; Hua, R.; Wang, C. Biosorption of uranium (VI) from aqueous solution by dead fungal biomass of Penicilliumcitrinum. Chem. Eng. J. 2010, 7437, 1–30. [Google Scholar]
- Min, K.S.; Yu, J.J.; Kim, Y.J.; Yun, Z. Removal of ammonium from tannery wastewater by electrochemical treatment. J. Environ. Sci. Health 2004, 39, 1867–1879. [Google Scholar] [CrossRef]
- Sundarapandiyan, S.; Chandrasekar, R.; Ramanaiah, B.; Krishnan, S.; Saravanan, P. Electrochemical oxidation and reuse of tannery saline wastewater. J. Hazard. Mater. 2010, 180, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, G.M.; Hamzeh, A.; Semerjian, L. Post treatment of tannery wastewater using lime/bittern coagulation and activated carbon adsorption. Desalination 2011, 273, 359–365. [Google Scholar] [CrossRef]
- Ganesh, R.; Balaji, G.; Ramanujam, R.A. Biodegradation of tannery wastewater using sequencing batch reactor—Respirometric assessment. Bioresour. Technol. 2006, 97, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Assefa, F.; Gumaelius, L.; Dalhammar, G. Biological nitrogen and organic matter removal from tannery wastewater in pilot plant operations in Ethiopia. Appl. Microbiol. Biotechnol. 2004, 66, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Kandelbauer, A.; Erlacher, A.; Cavaco, P.; Gubitz, G.M. A new alkali-thermostable azoreductase from Bacillus spstarin SF. Appl. Environ. Microbial. 2004, 70, 837–844. [Google Scholar] [CrossRef]
- Preetha, B.; Viruthagiri, T. Biosorption of Zinc (ii) by Rhizopusarrhizus; equilibrium and Kinetic modelling. Afr. J. Biotechnol. 2005, 4, 506–508. [Google Scholar]
- Rahim, M.K.; Mostafa, C.; Hossein, M.M.; Yuse, K.K.; Shahin, O. Biosorption of Cd and Ni by Inactivated Bacteria Isolation from Agricultural soil treated with sewage sludge. Ecohydrol. Hydrobiol. 2012, 12, 191–198. [Google Scholar]
- Magyarosy, A.; Laidlaw, R.D.; Kilaas, R.; Echer, C.; Clark, D.S.; Keasling, J.D. Nickel accumulation and Nickel oxalate precipitation by Aspergillus niger. Appl. Microbiol. Biotechnol. 2002, 59, 382–388. [Google Scholar] [PubMed]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2006. [Google Scholar]
- Saratale, R.G.; Saratale, G.D.; Kalyani, D.C.; Chang, J.S.; Govindwar, S.P. Enhanced decolorization and biodegradation of textile azo dye scarlet R by using developed microbial Syndicate-GR. Bioresour. Technol. 2009, 100, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association-American Water Works Association; Water Environment Federation: Washington, DC, USA, 1998; ISBN-13: 9780875532356. [Google Scholar]
- Ong, S.A.; Toorisaka, E.; Harata, M.; Hano, T. Decolorization of orange II using an anaerobic sequencing batch reactor with and without co-substrates. J. Environ. Sci. 2012, 24, 291–296. [Google Scholar] [CrossRef]
- Abioye, O.P.; Afolayan, E.O.; Aransi, S.A. Treatment of Pharmaceutical Effluent by Saccharomyces cerevisiae and Torulaspora delbrueckii Isolated from Spoilt Watermelon. Res. J. Environ. Toxicol. 2015, 9, 188–195. [Google Scholar]
- CPCB (Central Pollution Control Board). Pollution Control: Acts, Rules and Modifications; Central Pollution Control Board: New Delhi, India, 1995. [Google Scholar]
- Noorjaham, C.M. Physicochemical Characteristics, Identification of Fungi and Biodegradation of Industrial Effluent. J. Environ. Earth Sci. 2014, 4, 4. [Google Scholar]

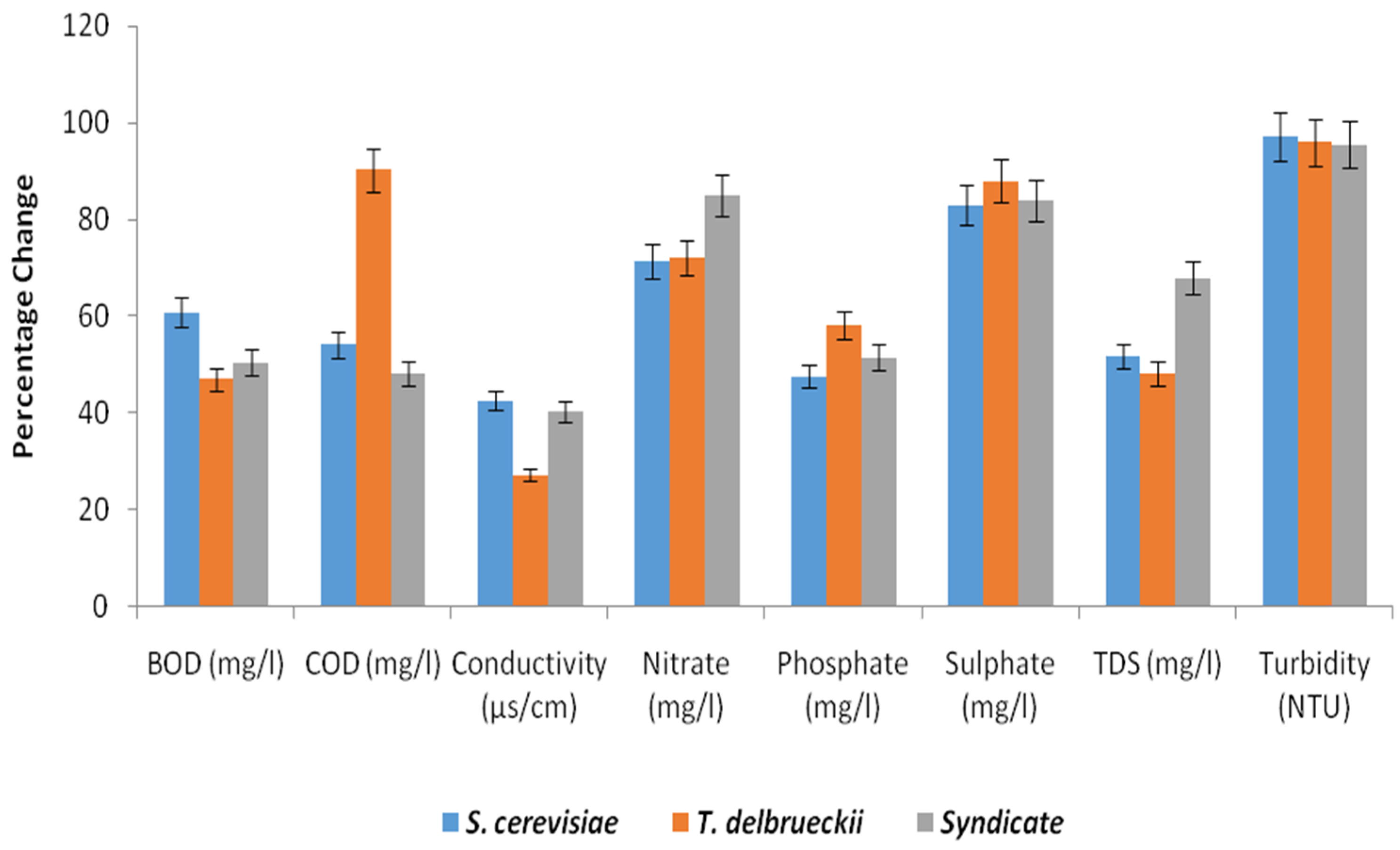
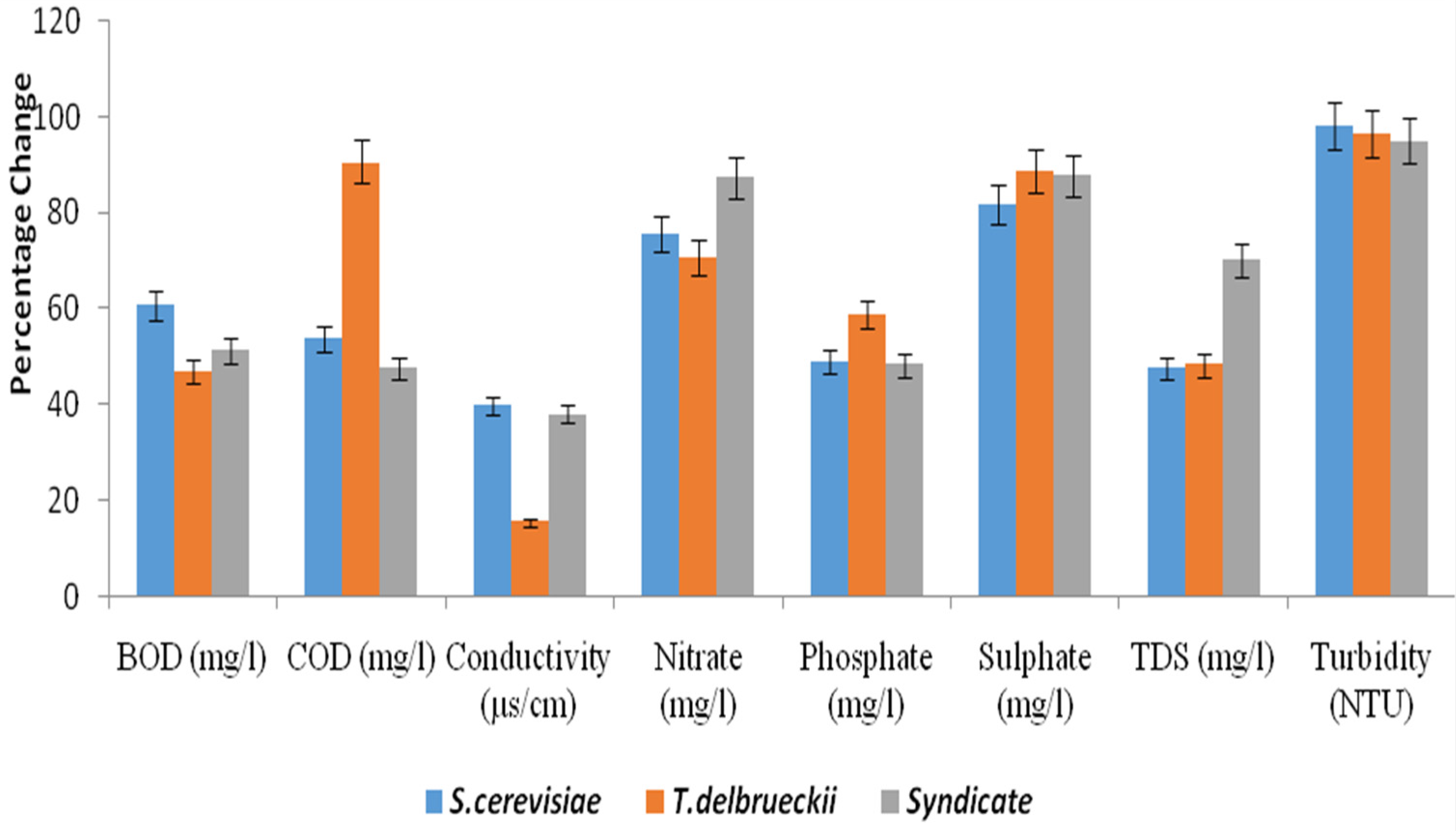
| Parameters | Conc. of Effluents | Post Treatment Days | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| BOD (mg/L) | 100% | 4249 ± 6.52 | 2990 ± 7.24 | 3142 ± 11.10 | 1671 ± 3.65 |
| COD (mg/L) | 10,607 ± 11.02 | 7475 ± 9.25 | 7863 ± 7.06 | 4910 ± 7.15 | |
| Conductivity (µs/cm) | 412 ± 5.25 | 515 ± 11.24 | 527 ± 4.51 | 248 ± 12.71 | |
| Nitrates (mg/L) | 262 ± 4.53 | 105 ± 9.72 | 60 ± 4.64 | 64 ± 9.27 | |
| Phosphates (mg/L) | 3.56 ± 7.12 | 3.48 ± 9.34 | 2.74 ± 5.24 | 1.82 ± 6.52 | |
| Sulphates(mg/L) | 115 ± 6.42 | 35 ± 2.78 | 27 ± 6.83 | 21 ± 9.11 | |
| TDS (mg/L) | 248 ± 11.23 | 173 ± 4.67 | 130 ± 5.45 | 130 ± 6.15 | |
| Turbidity (NTU) | 474 ± 4.67 | 27 ± 7.86 | 20 ± 9.04 | 9 ± 5.81 | |
| BOD (mg/L) | 75% | 4237 ± 5.20 | 2969 ± 6.03 | 3176 ± 5.50 | 1664 ± 2.75 |
| COD (mg/L) | 10,656 ± 9.42 | 7485 ± 7.06 | 7940 ± 6.05 | 4879 ± 9.14 | |
| Conductivity (µs/cm) | 378 ± 7.05 | 521 ± 9.64 | 521 ± 6.47 | 217 ± 11.42 | |
| Nitrates (mg/L) | 249 ± 6.72 | 97 ± 6.72 | 65 ± 7.50 | 71 ± 5.67 | |
| Phosphates (mg/L) | 3.74 ± 9.67 | 3.62 ± 4.23 | 3.23 ± 6.81 | 1.96 ± 4.78 | |
| Sulphates(mg/L) | 112 ± 6.81 | 32 ± 4.93 | 26 ± 5.92 | 19 ± 9.56 | |
| TDS (mg/L) | 265 ± 3.56 | 169 ± 6.86 | 136 ± 7.85 | 128 ± 2.98 | |
| Turbidity (NTU) | 463 ± 8.23 | 24 ± 6.97 | 17 ± 4.98 | 13 ± 7.34 | |
| BOD (mg/L) | 50% | 4267 ± 11.03 | 2964 ± 9.05 | 3137 ± 7.06 | 1693 ± 5.07 |
| COD (mg/L) | 10,593 ± 7.04 | 7439 ± 6.05 | 7926 ± 7.15 | 4863 ± 11.03 | |
| Conductivity (μs/cm) | 399 ± 9.24 | 503 ± 7.71 | 519 ± 7.60 | 206 ± 9.53 | |
| Nitrates (mg/L) | 256 ± 7.62 | 111 ± 7.54 | 72 ± 8.04 | 77 ± 6.65 | |
| Phosphates (mg/L) | 4.25 ± 7.98 | 3.17 ± 6.87 | 2.68 ± 2.98 | 1.68 ± 6.12 | |
| Sulphates(mg/L) | 111 ± 4.67 | 34 ± 4.98 | 24 ± 5.70 | 23 ± 7.09 | |
| TDS (mg/L) | 253 ± 4.90 | 181 ± 8.09 | 142 ± 11.15 | 123 ± 6.87 | |
| Turbidity (NTU) | 447 ± 6.98 | 21 ± 5.87 | 19 ± 11.03 | 12 ± 5.89 | |
| Parameters | Conc. of Effluents | Post Treatment Days | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| BOD (mg/L) | 100% | 4249 ± 6.52 | 2950 ± 7.89 | 2836 ± 4.78 | 2250 ± 9.50 |
| COD (mg/L) | 10,607 ± 11.02 | 7395 ± 6.98 | 7083 ± 4.98 | 997 ± 6.98 | |
| Conductivity (µs/cm) | 412 ± 7.90 | 541 ± 5.98 | 585 ± 7.12 | 476 ± 4.56 | |
| Nitrates (mg/L) | 262 ± 5.87 | 196 ± 7.98 | 143 ± 5.87 | 76.8 ± 9.23 | |
| Phosphates (mg/L) | 3.56 ± 6.34 | 3.16 ± 7.98 | 2.17 ± 6.78 | 1.47 ± 9.67 | |
| Sulphates (mg/L) | 115 ± 11.12 | 9.6 ± 11.45 | 18.9 ± 8.13 | 12.9 ± 6.89 | |
| TDS (mg/L) | 248 ± 11.23 | 211.8 ± 6.89 | 169.4 ± 4.87 | 128.3 ± 7.90 | |
| Turbidity (NTU) | 474 ± 6.45 | 88.3 ± 7.11 | 27.2 ± 5.98 | 15.9 ± 6.09 | |
| BOD (mg/L) | 75% | 4237 ± 5.20 | 2958 ± 5.78 | 2828 ± 7.90 | 2244 ± 9.89 |
| COD (mg/L) | 10,656 ± 9.42 | 7363 ± 8.90 | 7049 ± 5.89 | 1032 ± 9.80 | |
| Conductivity (µs/cm) | 378 ± 6.89 | 535 ± 4.78 | 582 ± 7.23 | 481 ± 8.90 | |
| Nitrates (mg/L) | 249 ± 4.89 | 187 ± 9.23 | 138 ± 5.23 | 69.8 ± 9.04 | |
| Phosphates (mg/L) | 3.74 ± 2.98 | 3.13 ± 6.75 | 2.11 ± 3.98 | 1.56 ± 7.09 | |
| Sulphates (mg/L) | 112 ± 5.56 | 7.9 ± 4.97 | 21.7 ± 7.45 | 13.4 ± 8.34 | |
| TDS (mg/L) | 265 ± 3.56 | 203.5 ± 7.34 | 178.3 ± 4.89 | 137.2 ± 7.39 | |
| Turbidity (NTU) | 463 ± 3.89 | 98.5 ± 5.67 | 23.8 ± 6.87 | 18.3 ± 5.89 | |
| BOD (mg/L) | 50% | 4267 ± 11.03 | 2981 ± 6.89 | 2815 ± 7.45 | 2217 ± 11.90 |
| COD (mg/L) | 10,593 ± 7.04 | 7406 ± 2.34 | 7070 ± 4.87 | 1045 ± 5.79 | |
| Conductivity (µs/cm) | 399 ± 9.86 | 524 ± 8.56 | 572 ± 4.89 | 489 ± 9.87 | |
| Nitrates (mg/L) | 256 ± 4.97 | 176 ± 6.98 | 127 ± 4.23 | 65.3 ± 2.12 | |
| Phosphates (mg/L) | 4.25 ± 5.66 | 3.24 ± 5.78 | 2.09 ± 6.77 | 1.62 ± 11.02 | |
| Sulphates (mg/L) | 111 ± 2.45 | 8.4 ± 7.32 | 14.9 ± 8.10 | 11.6 ± 7.34 | |
| TDS (mg/L) | 253 ± 4.90 | 205.7 ± 4.85 | 183.7 ± 5.85 | 131.6 ± 5.90 | |
| Turbidity (NTU) | 447 ± 4.21 | 76.4 ± 6.89 | 20.3 ± 6.45 | 16.8 ± 7.34 | |
| Parameters | Conc. of Effluents | Post Treatment Days | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| BOD (mg/L) | 100% | 4249 ± 6.52 | 3229 ± 5.87 | 2635 ± 6.98 | 2075 ± 7.89 |
| COD (mg/L) | 10,607 ± 11.02 | 7805 ± 5.56 | 6315 ± 3.56 | 5560 ± 4.78 | |
| Conductivity (µs/cm) | 665 ± 5.78 | 579 ± 11.45 | 479 ± 6.89 | 412 ± 3.78 | |
| Nitrates (mg/L) | 262 ± 6.89 | 115 ± 4.67 | 78 ± 4.67 | 33 ± 5.34 | |
| Phosphates (mg/L) | 3.56 ± 4.98 | 2.88 ± 9.22 | 2.06 ± 5.98 | 1.84 ± 6.87 | |
| Sulphates (mg/L) | 115 ± 2.67 | 40 ± 6.87 | 16 ± 2.45 | 14 ± 8.13 | |
| TDS (mg/L) | 248 ± 11.23 | 176 ± 5.87 | 101 ± 5.11 | 74 ± 4.78 | |
| Turbidity (NTU) | 474 ± 2.11 | 67 ± 5.89 | 37 ± 3.67 | 23 ± 5.87 | |
| BOD (mg/L) | 75% | 4237 ± 5.20 | 3243 ± 1.34 | 2614 ± 5.89 | 2097 ± 4.78 |
| COD (mg/L) | 10,656 ± 9.42 | 7791 ± 5.87 | 6309 ± 6.88 | 5533 ± 2.65 | |
| Conductivity (µs/cm) | 633 ± 7.89 | 568 ± 5.98 | 493 ± 3.33 | 378 ± 4.90 | |
| Nitrates (mg/L) | 249 ± 3.78 | 107 ± 3.78 | 96 ± 2.54 | 37 ± 11.90 | |
| Phosphates (mg/L) | 3.74 ± 4.89 | 2.81 ± 2.11 | 2.15 ± 7.90 | 1.81 ± 9.09 | |
| Sulphates (mg/L) | 112 ± 3.09 | 26 ± 5.98 | 22 ± 5.98 | 18 ± 5.23 | |
| TDS (mg/L) | 265 ± 3.56 | 169 ± 5.98 | 110 ± 4.87 | 85 ± 6.89 | |
| Turbidity (NTU) | 463 ± 2.56 | 55 ± 3.76 | 43 ± 3.23 | 21 ± 4.78 | |
| BOD (mg/L) | 50% | 4267 ± 11.03 | 3243 ± 4.90 | 2625 ± 9.89 | 2188 ± 7.98 |
| COD (mg/L) | 10,593 ± 7.04 | 7780 ± 4.89 | 6322 ± 2.78 | 5547 ± 6.87 | |
| Conductivity (µs/cm) | 643 ± 7.34 | 582 ± 3.89 | 476 ± 5.87 | 399 ± 2.23 | |
| Nitrates (mg/L) | 256 ± 4.98 | 117 ± 4.98 | 81 ± 4.78 | 39 ± 3.98 | |
| Phosphates (mg/L) | 4.25 ± 9.45 | 2.72 ± 6.34 | 2.17 ± 2.56 | 1.74 ± 4.78 | |
| Sulphates (mg/L) | 111 ± 7.23 | 35 ± 2.11 | 21 ± 5.89 | 12 ± 4.45 | |
| TDS (mg/L) | 253 ± 4.90 | 181 ± 4.34 | 98 ± 7.67 | 77 ± 1.45 | |
| Turbidity (NTU) | 447 ± 6.78 | 48 ± 2.76 | 34 ± 4.44 | 17 ± 2.78 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoduwa, S.I.R.; Igiri, B.; Udeh, C.B.; Edenta, C.; Gauje, B. Tannery Effluent Treatment by Yeast Species Isolates from Watermelon. Toxics 2017, 5, 6. https://doi.org/10.3390/toxics5010006
Okoduwa SIR, Igiri B, Udeh CB, Edenta C, Gauje B. Tannery Effluent Treatment by Yeast Species Isolates from Watermelon. Toxics. 2017; 5(1):6. https://doi.org/10.3390/toxics5010006
Chicago/Turabian StyleOkoduwa, Stanley Irobekhian Reuben, Bernard Igiri, Chinyere Blessing Udeh, Chidi Edenta, and Balli Gauje. 2017. "Tannery Effluent Treatment by Yeast Species Isolates from Watermelon" Toxics 5, no. 1: 6. https://doi.org/10.3390/toxics5010006






