Evaluation of Common Use Brominated Flame Retardant (BFR) Toxicity Using a Zebrafish Embryo Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Care and Maintenance
2.2. Chemicals and Materials Used
2.3. BFR Exposure Method
2.4. Spontaneous Movement Evaluation
2.5. Acetylcholinesterase Activity
2.6. GST Activity
2.7. Statistical Analysis
3. Results
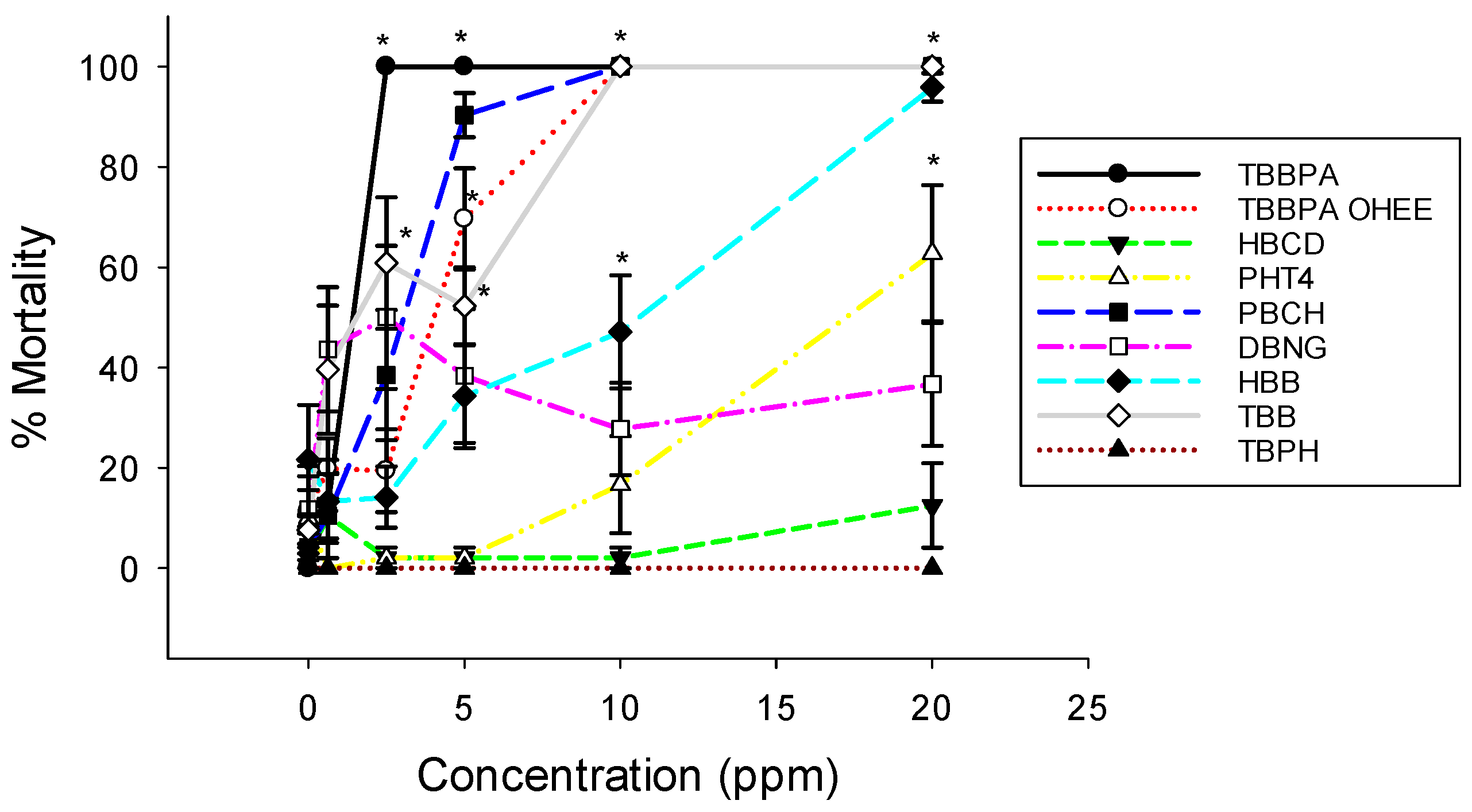
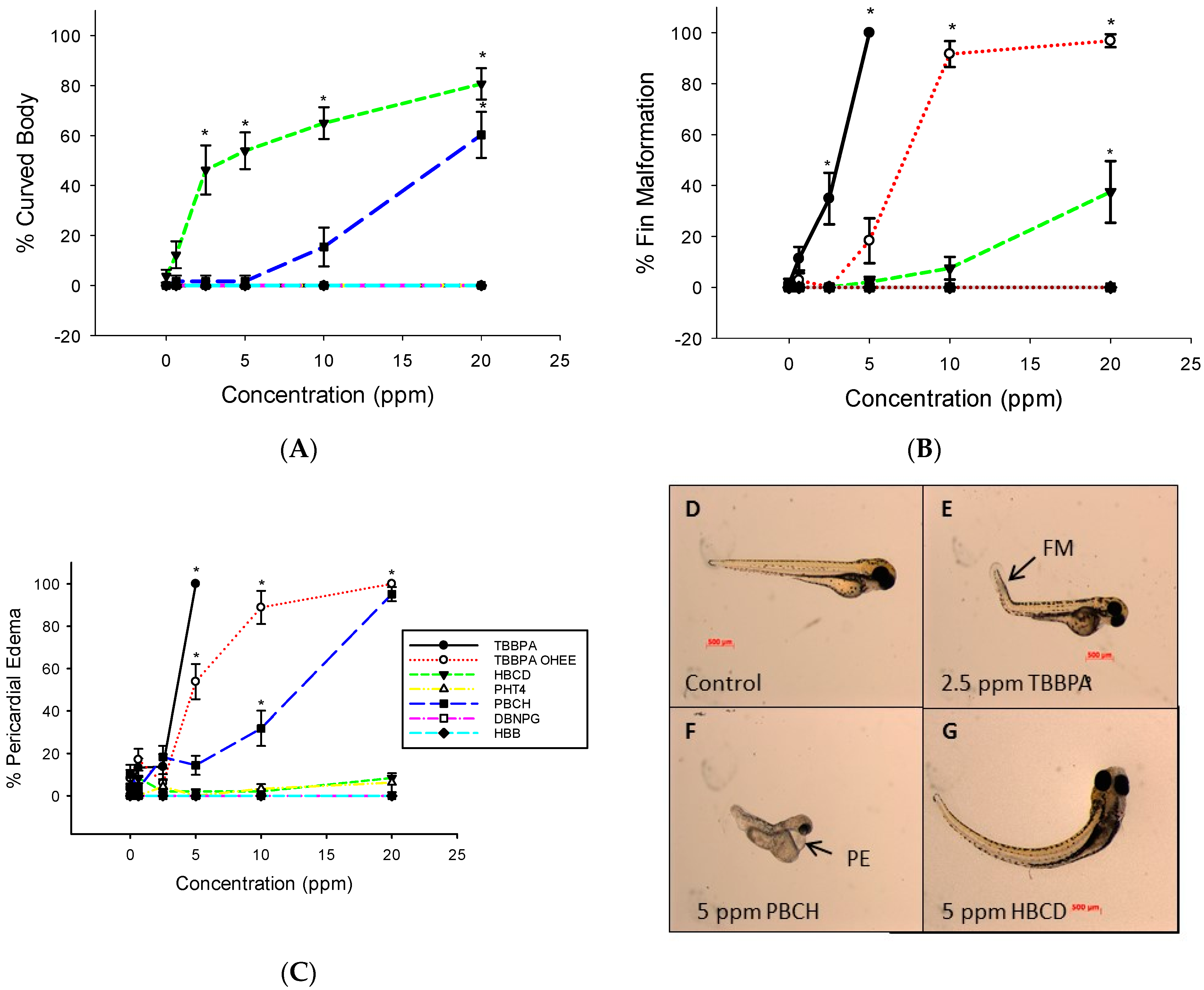
3.1. Embryo Mortality and Malformations
3.2. Spontaneous Movement and Acetylcholinesterase Evaluation
3.3. GST Activity Evaluation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Covaci, A.; Harrad, S.; Herzke, D.; Abdallah, M.A.; Fernie, K.; Toms, L.M.; Takigami, H. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environ. Int. 2014, 65, 147–158. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP). Listing of Pops in the Stockholm Convention; United Nations Environment Programme, Ed.; Stockholm Convention: Châtelaine, Switzerland, 2009. [Google Scholar]
- Liu, L.Y.; Salamova, A.; Venier, M.; Hites, R.A. Trends in the levels of halogenated flame retardants in the great lakes atmosphere over the period 2005–2013. Environ. Int. 2016, 92–93, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.Y.; Bi, X.H.; Sheng, G.Y.; Lu, S.Y.; Fu, H.; Yuan, J.; Li, L.P. Exposure to polybrominated diphenyl ethers among workers at an electronic waste dismantling region in Guangdong, China. Environ. Int. 2007, 33, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Law, K.; Halldorson, T.; Danell, R.; Stern, G.; Gewurtz, S.; Alaee, M.; Marvin, C.; Whittle, M.; Tomy, G. Bioaccumulation and trophic transfer of some brominated flame retardants in a lake winnipeg (Canada) food web. Environ. Toxicol. Chem. 2006, 25, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.X.; Jiao, Y.; Hu, Y.; Sun, Z.W.; Zhou, X.Q.; Feng, J.F.; Li, J.G.; Wu, Y.N. Levels of tetrabromobisphenol a, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Sci. Total Environ. 2013, 452, 10–18. [Google Scholar] [CrossRef] [PubMed]
- De Wit, C.A.; Herzke, D.; Vorkamp, K. Brominated flame retardants in the arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885–2918. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Allen, J.G.; Kelly, S.M.; Konstantinov, A.; Klosterhaus, S.; Watkins, D.; McClean, M.D.; Webster, T.F. Alternate and new brominated flame retardants detected in us house dust. Environ. Sci. Technol. 2008, 42, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Liu, A.; Wang, T.; Zhang, C.; Fu, J.; Yu, M.; Sun, J.; Zhu, N.; Li, Z.; Wei, G.; et al. Identification of tetrabromobisphenol a allyl ether and tetrabromobisphenol a 2,3-dibromopropyl ether in the ambient environment near a manufacturing site and in mollusks at a coastal region. Environ. Sci. Technol. 2013, 47, 4760–4767. [Google Scholar] [CrossRef] [PubMed]
- Vorkamp, K.; Riget, F.F. A review of new and current-use contaminants in the arctic environment: Evidence of long-range transport and indications of bioaccumulation. Chemosphere 2014, 111, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; Venier, M.; Hites, R.A. 2-ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate flame retardants in the great lakes atmosphere. Environ. Sci. Technol. 2012, 46, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Branchi, G.; Capone, F.; Vitalone, A.; Madia, F.; Santucci, D.; Alleva, E.; Costa, L.G. Early developmental exposure to BDE 99 or Aroclor 1254 affects neurobehavioural profile: Interference from the administration route. Neurotoxicology 2005, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Fernie, K.J.; Shutt, J.L.; Mayne, G.; Hoffman, D.; Letcher, R.J.; Drouillard, K.G.; Ritchie, I.J. Exposure to polybrominated diphenyl ethers (PBDEs): Changes in thyroid, vitamin a, glutathione homeostasis, and oxidative stress in American Kestrels (falco sparverius). Toxicol. Sci. 2005, 88, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book; University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.D.; Andres, V.; Featherstone, R.M. Citation classics—New and rapid colorimetric determination of acetylcholinesterase activity. Curr. Contents 1977, 7. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases—First enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Usenko, C.Y.; Robinson, E.M.; Usenko, S.; Brooks, B.W.; Bruce, E.D. PBDE developmental effects on embryonic zebrafish. Environ. Toxicol. Chem. 2011, 30, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, F.; Vicini, S.; Schuetze, S.M. Embryonic acetylcholine-receptors guarantee spontaneous contractions in rat developing muscle. Nature 1988, 335, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Abel, E.L.; Kudela, M.; Janise, A.; Bruce, E.D. Comparison of PBDE congeners as inducers of oxidative stress in Zebrafish. Environ. Toxicol. Chem. 2015, 34, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Hopkins, D.C.; Trumble, S.J.; Bruce, E.D. Hydroxylated PBDEs induce developmental arrest in zebrafish. Toxicol. Appl. Pharmacol. 2012, 262, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.X.; Pan, L.Q.; Xiu, M.; Jin, Q.; Wang, G.H.; Wang, C. Bioaccumulation and detoxification responses in the scallop chlamys farreri exposed to tetrabromobisphenol A (TBBPA). Environ. Toxicol. Pharmacol. 2015, 39, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Gorga, M.; Martinez, E.; Ginebreda, A.; Eljarrat, E.; Barcelo, D. Determination of PBDES, HBB, PBEB, DBDPE, HBCD, TBBPA and related compounds in sewage sludge from Catalonia (Spain). Sci. Total Environ. 2013, 444, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.G.; Zeng, H. HBCD and TBBPA in particulate phase of indoor air in Shenzhen, China. Sci. Total Environ. 2013, 458, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Labadie, P.; Tlili, K.; Alliot, F.; Bourges, C.; Desportes, A.; Chevreuil, M. Development of analytical procedures for trace-level determination of polybrominated diphenyl ethers and tetrabromobisphenol a in river water and sediment. Anal. Bioanal. Chem. 2010, 396, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Wang, S.R.; Wu, F.C.; Yan, Z.G.; Liu, H.L. Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ. Sci. Pollut. Res. Int. 2012, 19, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Wang, S.R.; Sun, F.C.; Zhang, M.M.; Wu, F.C.; Xu, F.F.; Ding, Z.S. Protective effects of puerarin against tetrabromobisphenol a-induced apoptosis and cardiac developmental toxicity in zebrafish embryo-larvae. Environ. Toxicol. 2015, 30, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ji, G.; Liu, J.; Zhang, S.; Gong, Y.; Shi, L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; Haggard, D.E.; Gonnerman, G.D.; Tanguay, R.L. Advanced morphological—Behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 2015, 145, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Du, M.M.; Zhang, D.D.; Yan, C.Z.; Zhang, X. Developmental toxicity evaluation of three hexabromocyclododecane diastereoisomers on zebrafish embryos. Aquat. Toxicol. 2012, 112–113, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, F.; Xu, C.; Liu, W.; Wen, S.; Xu, Y. Cytotoxicity evaluation of three pairs of hexabromocyclododecane (HBCD) enantiomers on Hep G2 cell. Toxicol. in Vitro 2008, 22, 1520–1527. [Google Scholar] [CrossRef]
- Baron, E.; Manez, M.; Andreu, A.C.; Sergio, F.; Hiraldo, F.; Eljarrat, E.; Barcelo, D. Bioaccumulation and biomagnification of emerging and classical flame retardants in bird eggs of 14 species from donana natural space and surrounding areas (South-Western Spain). Environ. Int. 2014, 68, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, X.J.; Wu, J.P.; Liu, J.; Wang, J.; Chen, S.J.; Mai, B.X. Contaminant pattern and bioaccumulation of legacy and emerging organhalogen pollutants in the aquatic biota from an e-waste recycling region in south China. Environ. Toxicol. Chem. 2010, 29, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.; Hauler, C.; Gallistl, C.; Gimenez, J.; Gauffier, P.; Castillo, J.J.; Fernandez-Maldonado, C.; de Stephanis, R.; Vetter, W.; Eljarrat, E.; et al. Halogenated natural products in dolphins: Brain-blubber distribution and comparison with halogenated flame retardants. Environ. Sci. Technol. 2015, 49, 9073–9083. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.B.; Qu, R.J.; Wang, C.; Wang, L.S.; Wang, Z.Y. Comparative antioxidant status in freshwater fish carassius auratus exposed to six current-use brominated flame retardants: A combined experimental and theoretical study. Aquat. Toxicol. 2013, 140, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Pape, P.G.; Sanger, J.E.; Nametz, R.C. Tetrabromophthalic anhydride in flame-retardant urethane foams. J. Cell. Plast. 1968, 4, 438–442. [Google Scholar] [CrossRef]
- Segev, O.; Meusel, W.; Friedenberger, M.; Brenner, A.; Kushmaro, A. Aerobic biodegradation of the brominated flame retardants, dibromoneopentyl glycol and tribromoneopentyl alcohol. Biodegradation 2009, 20, 621–627. [Google Scholar] [CrossRef] [PubMed]
- La Guardia, M.J.; Hale, R.C.; Harvey, E.; Mainor, T.M.; Ciparis, S. In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima). Environ. Sci. Technol. 2012, 46, 5798–5805. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; He, K.; Hites, R.A.; Salamova, A. Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ. Sci. Technol. 2016, 50, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.M.; Levin, E.D. Neurotoxicity of fire master 550 (r) in zebrafish (Danio rerio): Chronic developmental and acute adolescent exposures. Neurotoxicol. Teratol. 2015, 52, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ren, X.; Long, Y.; Hu, L.; Qu, G.; Zhou, Q.; Jiang, G. The potential neurotoxicity of emerging tetrabromobisphenol A derivatives based on rat pheochromocytoma cells. Chemosphere 2016, 154, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.H.; Wang, X.R.; Luo, Y.; Su, Y. Electron paramagnetic resonance evidence of hydroxyl radical generation and oxidative damage induced by tetrabromobisphenol a in carassius auratus. Aquat. Toxicol. 2005, 74, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Reistad, T.; Mariussen, E.; Fonnum, F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: The involvement of the map kinase pathway and protein kinase C. Toxicol. Sci. 2005, 83, 89–100. [Google Scholar] [CrossRef] [PubMed]



| BFR 1 | CAS | Structure |
|---|---|---|
| Tetrabromobisphenol A (TBBPA) | 79-94-7 |  |
| Hexabromocyclododecane (HBCD) | 3194-55-6 |  |
| Tetrabromophthalic anhydride (PHT4) | 632-79-1 |  |
| Di(2-ethylhexyl) tetrabromophthalate (TBPH) | 26040-51-7 |  |
| 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB) | 183658-27-7 |  |
| Dibromoneopentyl glycol (DBNPG) | 3296-90-0 |  |
| Hexabromobenzene (HBB) | 87-82-1 |  |
| Pentabromochlorocyclohexane (PBCH) | 87-84-3 |  |
| 4,4′-isopropylidenebis[2-(2,6-dibromophenoxyl)ethanol] (TBBPA-OHEE) | 4162-45-2 |  |
| BFR | Log P | LC50 (ppm) | EC50 (ppm) |
|---|---|---|---|
| TBBPA | 7.46 | 1.45 | 0.99 (PE) |
| HBCD | 7.47 | 7.7 | 3.35 (CB) |
| PHT4 | 4.75 | 18 | 18 (M) |
| TBPH | 10.77 | >20 | >20 |
| TBB | 8.06 | 7.0 | 7.0 (M) |
| DBNPG | 2.14 | >20 | >20 |
| HBB | 7.01 | 10.7 | 10.7 (M) |
| PBCH | 4.86 | 2.6 | 2.6 (M) |
| TBBPA-OHEE | 6.96 | 2.2 | 1.85 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usenko, C.Y.; Abel, E.L.; Hopkins, A.; Martinez, G.; Tijerina, J.; Kudela, M.; Norris, N.; Joudeh, L.; Bruce, E.D. Evaluation of Common Use Brominated Flame Retardant (BFR) Toxicity Using a Zebrafish Embryo Model. Toxics 2016, 4, 21. https://doi.org/10.3390/toxics4030021
Usenko CY, Abel EL, Hopkins A, Martinez G, Tijerina J, Kudela M, Norris N, Joudeh L, Bruce ED. Evaluation of Common Use Brominated Flame Retardant (BFR) Toxicity Using a Zebrafish Embryo Model. Toxics. 2016; 4(3):21. https://doi.org/10.3390/toxics4030021
Chicago/Turabian StyleUsenko, Crystal Y., Erika L. Abel, Aaron Hopkins, Gerardo Martinez, Jonathan Tijerina, Molly Kudela, Nick Norris, Lana Joudeh, and Erica D. Bruce. 2016. "Evaluation of Common Use Brominated Flame Retardant (BFR) Toxicity Using a Zebrafish Embryo Model" Toxics 4, no. 3: 21. https://doi.org/10.3390/toxics4030021





