Comparison of Neurological Function in Males and Females from Two Substrains of C57BL/6 Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Handling
2.2. Open Field Locomotor Activity
2.3. Rotarod
2.4. Novel Object Recognition
2.5. Morris Water Maze
| Schedule | Platform Size | Platform Location | Cues |
|---|---|---|---|
| Week 1-Cued | 10 cm | Varies each day | Proximal cue on platform |
| Week 2-Acquistion | 10 cm | Southwest | Distal cues on walls |
| Week 3-Reversal | 7 cm | Northeast | Distal cues on walls |
| Week 4-Shift-reduced | 5 cm | Northwest | Distal cues on walls |
2.6. Fear Conditioning
2.7. Statistical Analysis
3. Results
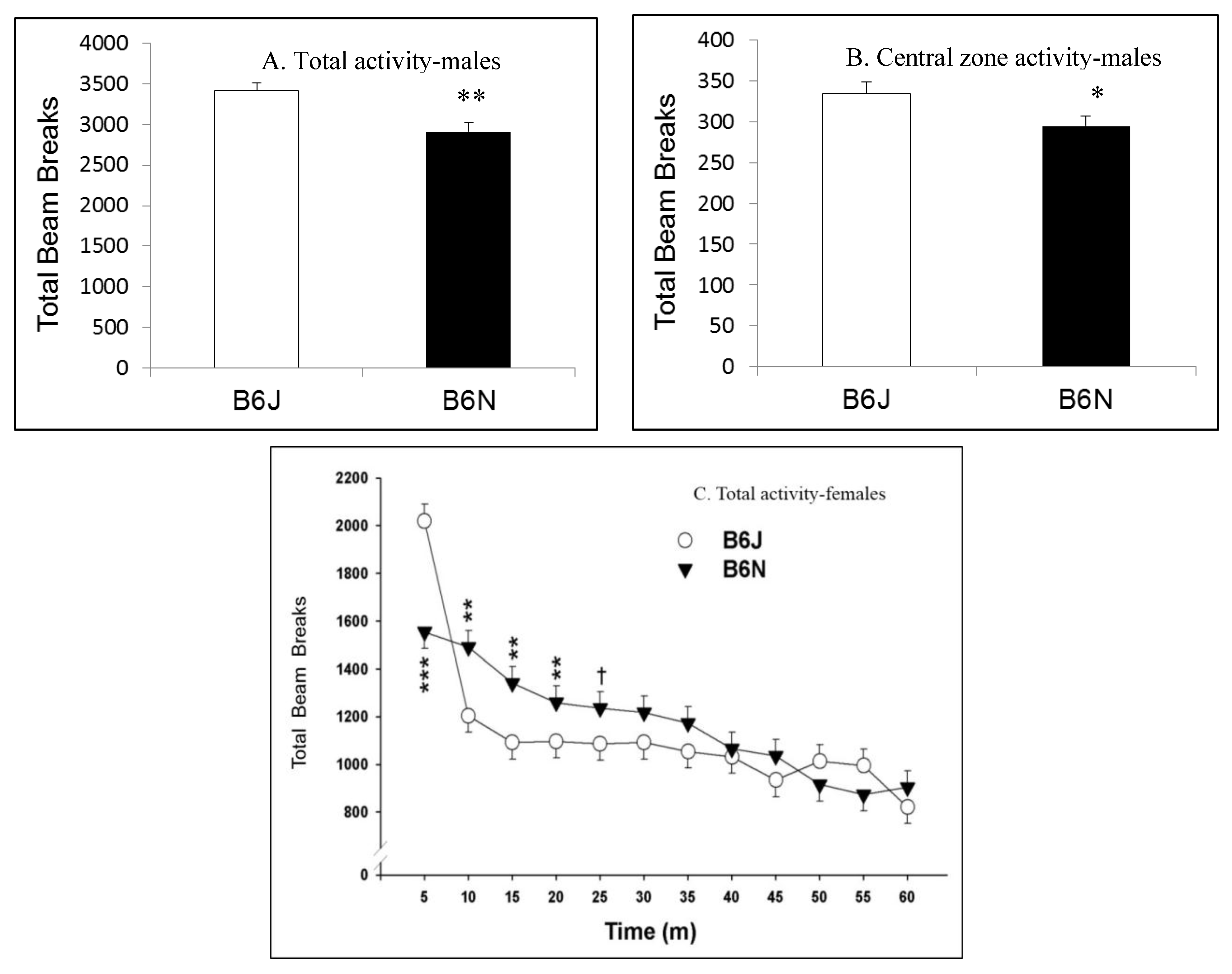
3.1. Open Field Locomotor Activity
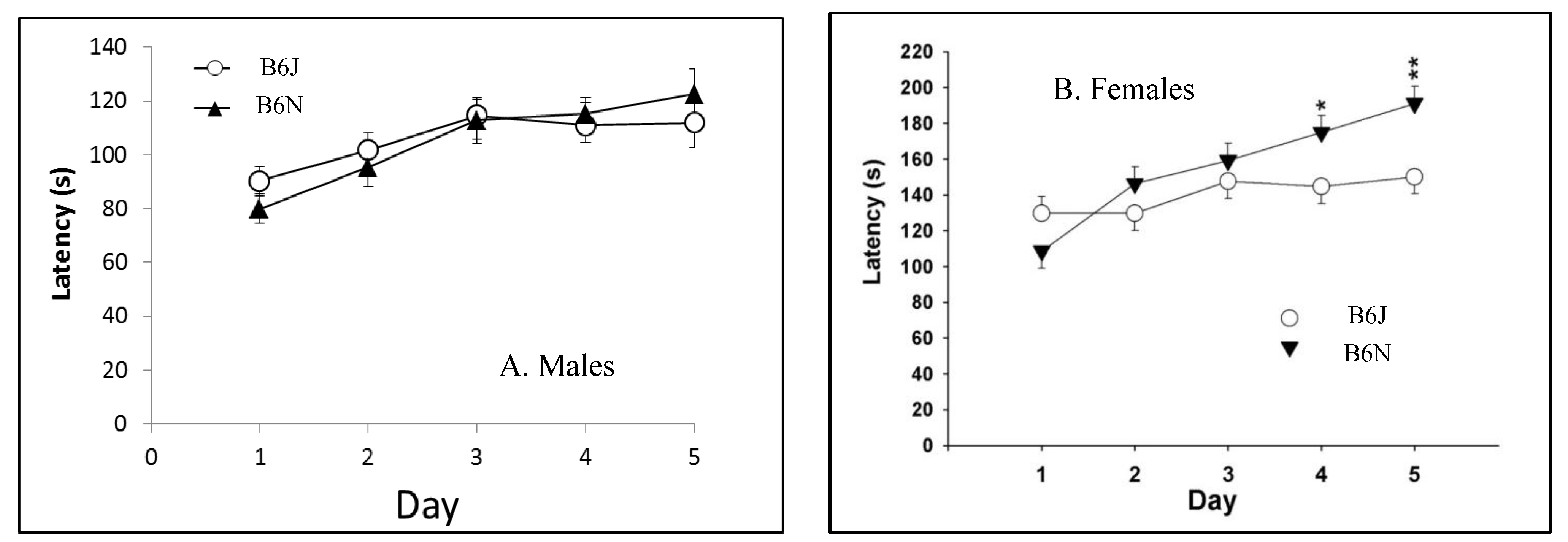
3.2. Rotarod

3.3. Novel Object Recognition
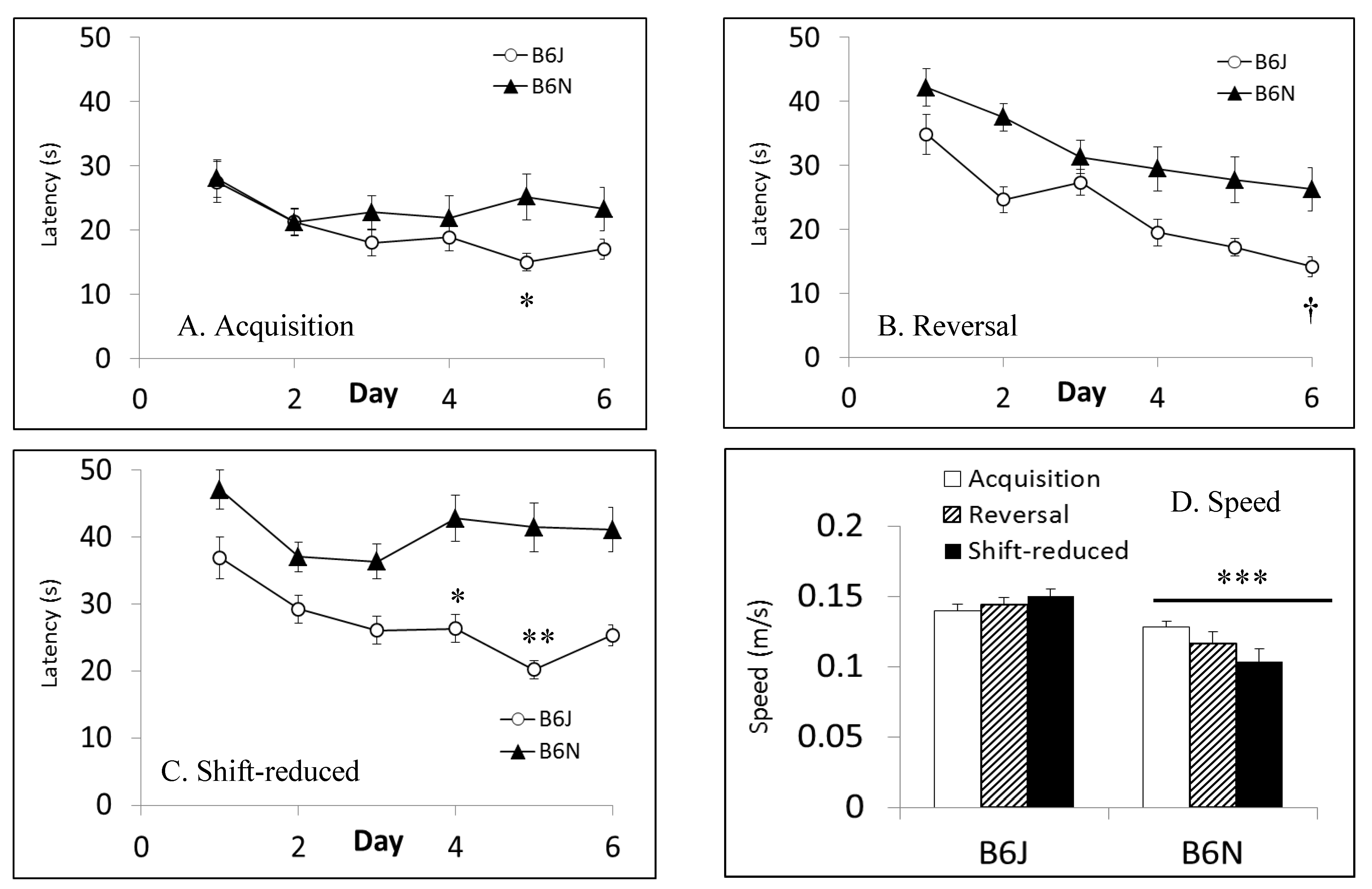
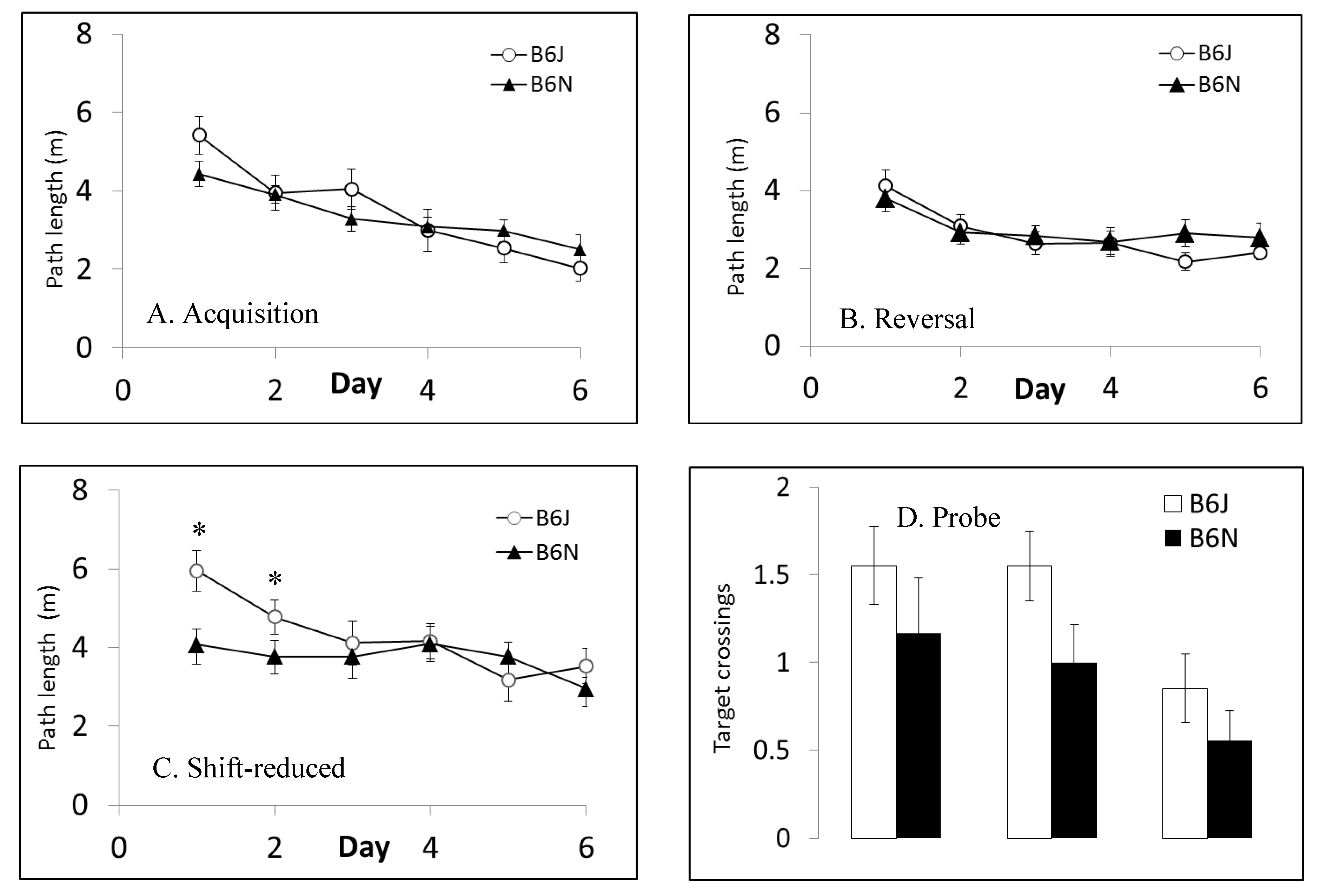
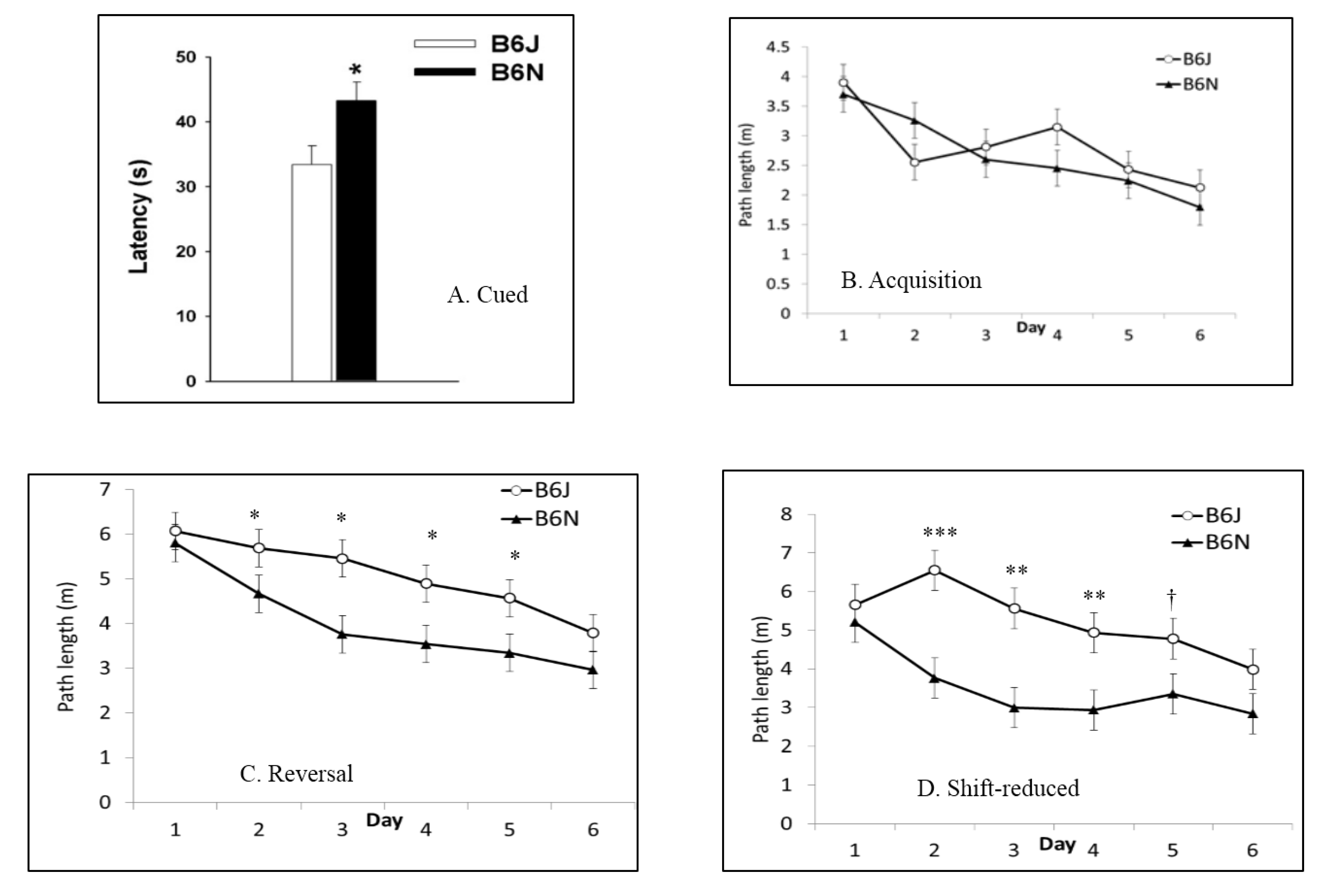
3.4. Morris Water Maze

3.5. Fear Conditioning

| Behavior Test | Function | Males | Females |
|---|---|---|---|
| Open field locomotor | Baseline activity | Higher activity in B6J | Slower habituation in B6N |
| Rotarod | Balance and coordination | Greater improvement in B6N over five test days | Significantly longer latencies in B6N; greater improvement in B6N over five tests days |
| Novel object recognition | Non-spatial learning and memory | No significant differences | No significant differences |
| Morris water maze | Spatial learning and memory | Shorter latencies to escape in B6J; faster swim speeds in B6J; shorter path lengths in shift-reduced in B6N | Shorter path lengths in reversal and shift-reduced in B6N; faster swim speeds in B6J |
| Fear conditioning | Fear learning | More freezing in cued phase before tone by B6N | Not tested |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chadman, K.K.; Yang, M.; Crawley, J.N. Criteria for validating mouse models of psychiatric diseases. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 1–11. [Google Scholar] [CrossRef]
- Crawley, J.N.; Belknap, J.K.; Collins, A.; Crabbe, J.C.; Frankel, W.; Henderson, N.; Hitzemann, R.J.; Maxson, S.C.; Miner, L.L.; Silva, A.J.; et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology 1997, 132, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Behavioral phenotyping of rodents. Comp. Med. 2003, 53, 140–146. [Google Scholar] [PubMed]
- Bolivar, V.J.; Walters, S.R.; Phoenix, J.L. Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behav. Brain Res. 2007, 176, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Molenhuis, R.T.; de, V.L.; Bruining, H.; Kas, M.J. Enhancing the value of psychiatric mouse models; differential expression of developmental behavioral and cognitive profiles in four inbred strains of mice. Eur. Neuropsychopharmacol. 2014, 24, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Waterston, R.H.; Lindblad-Toh, K.; Birney, E.; Rogers, J.; Abril, J.F.; Agarwal, P.; Agarwala, R.; Ainscough, R.; Alexandersson, M.; An, P.; et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Takao, K.; Nakanishi, K.; Yamasaki, N.; Tanda, K.; Miyakawa, T. Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 2010, 4, 29. [Google Scholar] [PubMed]
- The Jackson Laboratory New Resource Illustrates Divergence of C57BL/6 Laboratory Mouse Substrains; The Jackson Laboratory: Bar Harbor, ME, USA, 2008.
- Morse, H.C. Origins of Inbred Mice; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Freeman, H.C.; Hugill, A.; Dear, N.T.; Ashcroft, F.M.; Cox, R.D. Deletion of nicotinamide nucleotide transhydrogenase: A new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 2006, 55, 2153–2156. [Google Scholar] [CrossRef] [PubMed]
- Specht, C.G.; Schoepfer, R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC. Neurosci. 2001, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kim, K.; Joseph, C.; Kourrich, S.; Yoo, S.H.; Huang, H.C.; Vitaterna, M.H.; de Villena, F.P.; Churchill, G.; Bonci, A.; et al. C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 2013, 342, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Mekada, K.; Abe, K.; Murakami, A.; Nakamura, S.; Nakata, H.; Moriwaki, K.; Obata, Y.; Yoshiki, A. Genetic differences among C57BL/6 substrains. Exp. Anim. 2009, 58, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zurita, E.; Chagoyen, M.; Cantero, M.; Alonso, R.; Gonzalez-Neira, A.; Lopez-Jimenez, A.; Lopez-Moreno, J.A.; Landel, C.P.; Benitez, J.; Pazos, F.; et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011, 20, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.D.; Zhang, N.N.; Sokoloff, G.; Fanselow, M.S.; Ennes, H.S.; Palmer, A.A.; McRoberts, J.A. Behavioral differences among C57BL/6 substrains: Implications for transgenic and knockout studies. J. Neurogenet. 2008, 22, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.D. The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann. N.Y. Acad. Sci. 2011, 1245, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Bourdi, M.; Davies, J.S.; Pohl, L.R. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem. Res. Toxicol. 2011, 24, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Zelikowsky, M.; Hersman, S.; Chawla, M.K.; Bartnes, C.A.; Fanselow, M.S. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J. Neurosci. 2014, 34, 8462–8466. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Miller, M.M.; Filipski, S.B.; Tolwani, R.J. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 479–483. [Google Scholar] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Sorge, R.E.; Martin, L.J.; Isbester, K.A.; Sotocinal, S.G.; Rosen, S.; Tuttle, A.H.; Wieskopf, J.S.; Acland, E.L.; Dokova, A.; Kadoura, B.; et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 2014, 11, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.P.; Altenhofen, E.; Ashworth, A.; Brown, A.; Kamau-Cheggeh, C.; Curran, M.; Evans, A.; Floyd, R.; Fowler, J.; Garber, H.; et al. Ahrd Cyp1a2(-/-) mice show increased susceptibility to PCB-induced developmental neurotoxicity. Neurotoxicology 2012, 33, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.P.; Nebert, D.W.; Genter, M.B.; Patel, K.V.; Schaefer, T.L.; Skelton, M.R.; Williams, M.T.; Vorhees, C.V. In utero and lactational exposure to PCBs in mice: Adult offspring show altered learning and memory depending on Cyp1a2 and Ahr genotypes. Environ. Health Perspect. 2011, 119, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, M.E.; Boeckman, R.; Krochmal, D.; Fernando, H.; Ahrens, R.; Csernansky, J.G. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Res. Bull. 2003, 60, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Bardgett, M.E.; Csernansky, J.G. Kainic acid lesions disrupt fear-mediated memory processing. Neurobiol. Learn. Mem. 2002, 77, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Bothe, G.W.; Bolivar, V.J.; Vedder, M.J.; Geistfeld, J.G. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004, 3, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Clapcote, S.J.; Roder, J.C. Survey of embryonic stem cell line source strains in the water maze reveals superior reversal learning of 129S6/SvEvTac mice. Behav. Brain Res. 2004, 152, 35–48. [Google Scholar] [PubMed]
- Fridgeirsdottir, G.A.; Hillered, L.; Clausen, F. Escalated handling of young C57BL/6 mice results in altered Morris water maze performance. Ups. J. Med. Sci. 2014, 119, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Menendez, L.; Karamanlidis, G.; Kolwicz, S.; Tian, R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. Am. J. Physiol Heart Circ. Physiol. 2013, 305, H397–H402. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, F.; Lauzier, B.; Poirier, I.; Gelinas, R.; Rivard, M.E.; Robillard, F.I.; Thorin, E.; Des, R.C. Mouse strain differences in metabolic fluxes and function of ex vivo working hearts. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H78–H87. [Google Scholar] [CrossRef] [PubMed]
- Mattapallil, M.J.; Wawrousek, E.F.; Chan, C.C.; Zhao, H.; Roychoudhury, J.; Ferguson, T.A.; Caspi, R.R. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2921–2927. [Google Scholar] [CrossRef]
- Pritchett-Corning, K.R.; Clifford, C.B.; Elder, B.J.; Vezina, M. Retinal lesions and other potential confounders of ocular research in inbred mice. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3764–3765. [Google Scholar] [CrossRef]
- Sundberg, J.P.; Taylor, D.; Lorch, G.; Miller, J.; Silva, K.A.; Sundberg, B.A.; Roopenian, D.; Sperling, L.; Ong, D.; King, L.E.; et al. Primary follicular dystrophy with scarring dermatitis in c57bl/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet. Pathol. 2011, 48, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Mitzner, W.; Watson, J. Variation in airway responsiveness of male C57BL/6 mice from 5 vendors. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 401–406. [Google Scholar] [PubMed]
- Balogh, S.A.; McDowell, C.S.; Stavnezer, A.J.; Denenberg, V.H. A behavioral and neuroanatomical assessment of an inbred substrain of 129 mice with behavioral comparisons to C57BL/6J mice. Brain Res. 1999, 836, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Montkowski, A.; Poettig, M.; Mederer, A.; Holsboer, F. Behavioural performance in three substrains of mouse strain 129. Brain Res. 1997, 762, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Eisener-Dorman, A.F.; Lawrence, D.A.; Bolivar, V.J. Behavioral and genetic investigations of low exploratory behavior in Il18rl−/−mice: We can’t always blame it on the targeted gene. Brain Behav. Immun. 2010, 24, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Arguello, P.A.; Kvajo, M.; Karayiorgou, M.; Gogos, J.A. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3693–3697. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sintes, R.; Kvajo, M.; Gogos, J.A.; Lucas, J.J. Mice with a naturally occurring DISC1 mutation display a broad spectrum of behaviors associated to psychiatric disorders. Front. Behav. Neurosci. 2014, 8, 1–12. [Google Scholar] [PubMed]
- Kiselycznyk, C.; Holmes, A. All (C57BL/6) mice are not created equal. Front. Neurosci. 2011, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Soliman, F.; Glatt, C.E.; Bath, K.G.; Levita, L.; Jones, R.M.; Pattwell, S.S.; Jing, D.; Tottenham, N.; Amso, D.; Somerville, L.H.; et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 2010, 327, 863–866. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashworth, A.; Bardgett, M.E.; Fowler, J.; Garber, H.; Griffith, M.; Curran, C.P. Comparison of Neurological Function in Males and Females from Two Substrains of C57BL/6 Mice. Toxics 2015, 3, 1-17. https://doi.org/10.3390/toxics3010001
Ashworth A, Bardgett ME, Fowler J, Garber H, Griffith M, Curran CP. Comparison of Neurological Function in Males and Females from Two Substrains of C57BL/6 Mice. Toxics. 2015; 3(1):1-17. https://doi.org/10.3390/toxics3010001
Chicago/Turabian StyleAshworth, Amy, Mark E. Bardgett, Jocelyn Fowler, Helen Garber, Molly Griffith, and Christine Perdan Curran. 2015. "Comparison of Neurological Function in Males and Females from Two Substrains of C57BL/6 Mice" Toxics 3, no. 1: 1-17. https://doi.org/10.3390/toxics3010001





