Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage
Abstract
:1. Introduction
2. Material and Methods
2.1. Fresh Meat Samples
2.2. Protein Content and pH Determination
2.3. Microbiological Analysis
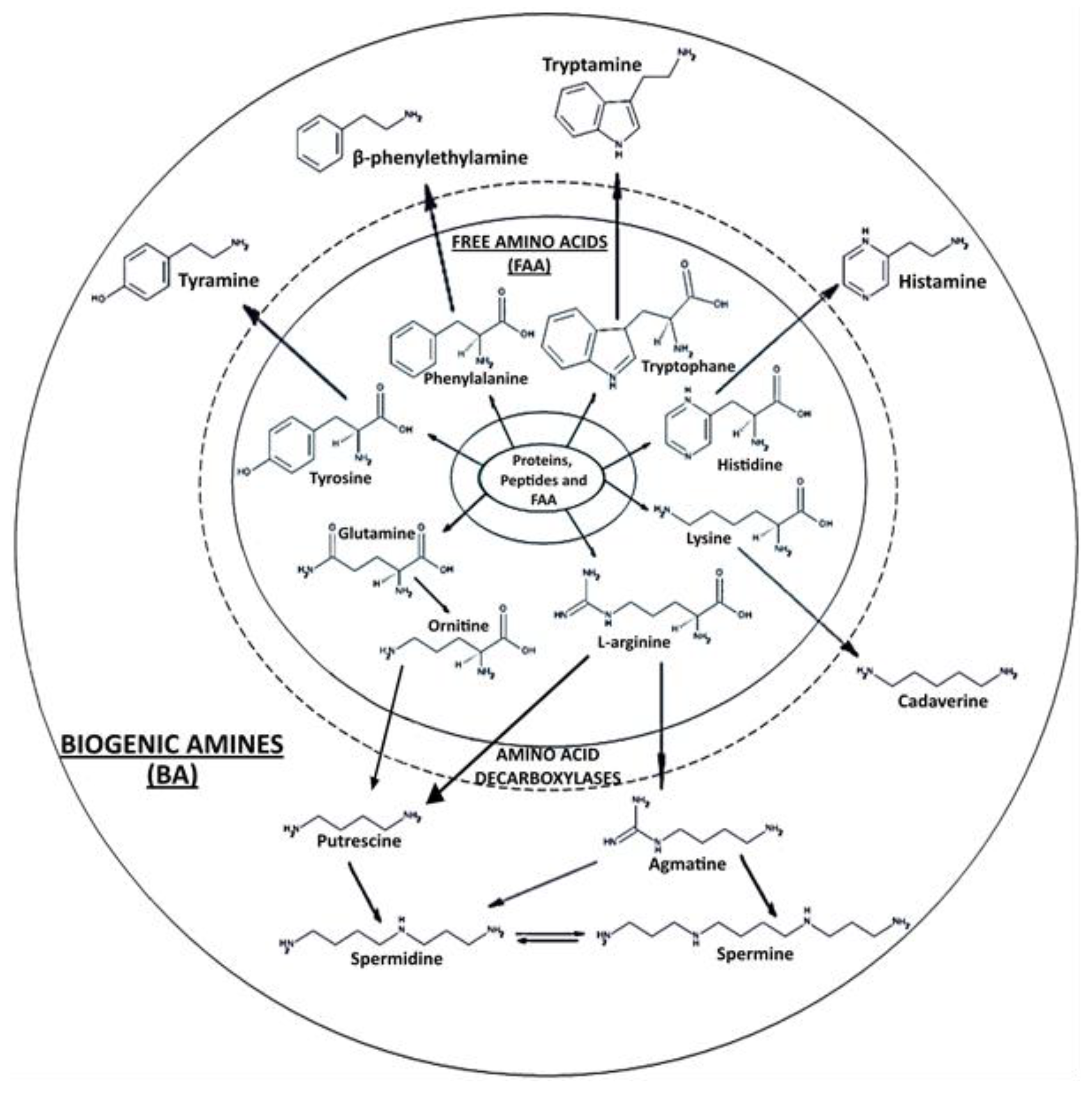
2.4. Determination of Free Amino Acids (FAA)
2.5. Determination of Biogenic Amines (BA)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Protein Content and pH
3.2. Microbiology
3.3. Free Amino Acids (FAA)
3.4. Biogenic Amines
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez-Colmenero, F.; Herrero, A.; Cofrades, S.; Ruiz-Capillas, C. Meat: Eating Quality and Preservation. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Oxford Academic Press: Kidlington, UK, 2016; Volume 3, pp. 685–692. [Google Scholar]
- Shabbir, M.A.; Raza, A.; Anjum, F.M.; Khan, M.R.; Suleria, H.A.R. Effect of Thermal Treatment on Meat Proteins with Special Reference to Heterocyclic Aromatic Amines (HAAs). Crit. Rev. Food Sci. Nutr. 2015, 55, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Colmenero, F.; Herrero, A.M.; Ruiz-Capillas, C.; Cofrades, S. Meat and functional foods. In Handbook of Meat and Meat Processing; Hui, Y.H., Ed.; CRC Press. Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 225–248. [Google Scholar]
- Mietz, J.L.; Karmas, E. Polyamine and histamine content of rockfish, salmon, lobster, and shrimp as an indicator of decomposition. J. Assoc. Off. Anal. Chem. 1978, 61, 139–145. [Google Scholar]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Moral, A. Changes in free amino acids during chilled storage of hake (Merluccius merluccius L.) in controlled atmospheres and their use as a quality control index. Eur. Food Res. Technol. 2001, 212, 302–307. [Google Scholar] [CrossRef]
- Rabie, M.A.; Peres, C.; Malcata, F.X. Evolution of amino acids and biogenic amines throughout storage in sausages made of horse, beef and turkey meats. Meat Sci. 2014, 96, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Amino acid availability as an influential factor on the biogenic amine formation in dry fermented sausages. Food Control 2014, 36, 76–81. [Google Scholar] [CrossRef]
- Jairath, G.; Singh, P.K.; Dabur, R.S.; Rani, M.; Chaudhari, M. Biogenic amines in meat and meat products and its public health significance: A review. J. Food Sci. Technol. 2015, 52, 6835–6846. [Google Scholar] [CrossRef]
- Hernández-Jover, T.; Izquierdo-Pulido, M.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biogenic Amine Sources in Cooked Cured Shoulder Pork. J. Agric. Food Chem. 1996, 44, 3097–3101. [Google Scholar] [CrossRef]
- Silva, C.M.G.; Glória, M.B.A. Bioactive amines in chicken breast and thigh after slaughter and during storage at 4 ± 1 °C and in chicken-based meat products. Food Chem. 2002, 78, 241–248. [Google Scholar] [CrossRef]
- Vinci, G.; Antonelli, M.L. Biogenic amines: Quality index of freshness in red and white meat. Food Control 2002, 13, 519–524. [Google Scholar] [CrossRef]
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Joint FAO/WHO Expert Meeting on the Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products: Meeting Report. Available online: http://www.fao.org/fileadmin/user_upload/agns/news_events/Histamine_Final_Report.pdf (accessed on 10 July 2018).
- Cofrades, S.; López-López, I.; Ruiz-Capillas, C.; Triki, M.; Jiménez-Colmenero, F. Quality characteristics of low-salt restructured poultry with microbial transglutaminase and seaweed. Meat Sci. 2011, 87, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leggio, A.; Belsito, E.L.; De Marco, R.; Di Gioia, M.L.; Liguori, A.; Siciliano, C.; Spinella, M. Dry fermented sausages of Southern Italy: A comparison of free amino acids and biogenic amines between industrial and homemade products. J. Food Sci. 2012, 77, S170–S175. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pando, G.; Cofrades, S.; Ruiz-Capillas, C.; Solas, M.T.; Triki, M.; Jiménez-Colmenero, F. Low-fat frankfurters formulated with a healthier lipid combination as functional ingredient: Microstructure, lipid oxidation, nitrite content, microbiological changes and biogenic amine formation. Meat Sci. 2011, 89, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Storage stability of low-fat sodium reduced fresh merguez sausage prepared with olive oil in konjac gel matrix. Meat Sci. 2013, 94, 438–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Effect of preformed konjac gels, with and without olive oil, on the technological attributes and storage stability of merguez sausage. Meat Sci. 2013, 93, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triki, M.; Jiménez-Colmenero, F.; Herrero, A.M.; Ruiz-Capillas, C. Optimisation of a chromatographic procedure for determining biogenic amine concentrations in meat and meat products employing a cation-exchange column with a post-column system. Food Chem. 2012, 130, 1066–1073. [Google Scholar] [CrossRef] [Green Version]
- ANSES Ciqual Table de Composition Nutritionnelle Des Aliments. Available online: https://ciqual.anses.fr/ (accessed on 10 July 2018).
- Obanor, F.O. Biochemical Basis of the Effect of Pre-Slaughter Stress and Post-Slaughter Processing Conditions on Meat Tenderness. Master’s Thesis, Lincoln University, Christchurch, New Zealand, 2002. [Google Scholar]
- Page, J.K.; Wulf, D.M.; Schwotzer, T.R. A survey of beef muscle color and pH. J. Anim. Sci. 2001, 79, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.; Kozačinski, Ž.L.; Njari, B.; Marenčić, D.; Mršić, G.; Špiranec, K.; Špoljarić, D.; Čop, M.J.; Živković, M.; Popović, M. Technological properties and chemical composition of the meat of sheep fed with Agaricus bisporus supplement. Vet. Arh. 2015, 85, 591–600. [Google Scholar]
- Van Laack, R.L.J.M.; Kauffman, R.G.; Sybesma, W.; Smulders, F.J.M.; Eikelenboom, G.; Pinheiro, J.C. Is color brightness (L-value) a reliable indicator of water holding capacity in porcine muscle? Meat Sci. 1994, 38, 193–201. [Google Scholar] [CrossRef]
- Patterson, B.A.; Matarneh, S.K.; Stufft, K.M.; England, E.M.; Scheffler, T.L.; Preisser, R.H.; Shi, H.; Stewart, E.C.; Eilert, S.; Gerrard, D.E. Pectoralis major muscle of turkey displays divergent function as correlated with meat quality. Poult. Sci. 2017, 96, 1492–1503. [Google Scholar] [PubMed]
- Cortez-Vega, W.R.; Pizato, S.; Prentice, C. Quality of raw chicken breast stores at 5 °C and packaged under different modified atmospheres. J. Food Saf. 2012, 32, 360–368. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, T.C. Effects of Refrigerated Storage, pH Adjustment, and Marinade on Color of Raw and Microwave Cooked Chicken Meat. Poult. Sci. 1993, 72, 355–362. [Google Scholar] [CrossRef]
- Debut, M.; Berri, C.; Baeza, E.; Sellier, N.; Arnould, C.; Guemene, D.; Jehl, N.; Boutten, B.; Jego, Y.; Beaumont, C. Variation of chicken technological meat quality in relation to genotype and preslaughter stress conditions. Poult. Sci. 2003, 82, 1829–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lázaro, C.A.; Conte-Júnior, C.A.; Canto, A.C.; Monteiro, M.L.G.; Costa-Lima, B.; da Cruz, A.G.; Mársico, E.T.; Franco, R.M. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT Food Sci. Technol. 2015, 60, 15–21. [Google Scholar] [CrossRef]
- Laranjo, M.; Gomes, A.; Agulheiro-Santos, A.C.; Potes, M.E.; Cabrita, M.J.; Garcia, R.; Rocha, J.M.; Roseiro, L.C.; Fernandes, M.J.; Fraqueza, M.J.; et al. Impact of salt reduction on biogenic amines, fatty acids, microbiota, texture and sensory profile in traditional blood dry-cured sausages. Food Chem. 2017, 218, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2005R2073:20060101:EN:PDF (accessed on 14 August 2018).
- USDA. USDA National Nutrient Database for Standard Reference. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 14 August 2018).
- Niewiarowicz, A. Meat Anomalies in Broilers. Poult. Int. 1978, 17, 50–51. [Google Scholar]
- Cowieson, A.J.; Acamovic, T.; Bedford, M.R. The effects of phytase and phytic acid on the loss of endogenous amino acids and minerals from broiler chickens. Br. Poult. Sci. 2004, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, K.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese black cattle. Meat Sci. 2016, 112, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F. Biogenic amines in low- and reduced-fat dry fermented sausages formulated with konjac gel. J. Agric. Food Chem. 2012, 60, 9242–9248. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Moral, A. Free amino acids and biogenic amines in red and white muscle of tuna stored in controlled atmospheres. Amino Acids 2004, 26, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freiding, S.; Gutsche, K.A.; Ehrmann, M.A.; Vogel, R.F. Genetic screening of Lactobacillus sakei and Lactobacillus curvatus strains for their peptidolytic system and amino acid metabolism, and comparison of their volatilomes in a model system. Syst. Appl. Microbiol. 2011, 34, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.A.; Kang, D.R.; Belal, S.A.; Cho, E.-S.-R.; Jung, J.-H.; Jung, Y.-C.; Choi, Y.-I.; Shim, K.-S. Meat Quality and Physicochemical Trait Assessments of Berkshire and Commercial 3-way Crossbred Pigs. Korean J. Food Sci. Anim. Resour. 2016, 36, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dermiki, M.; Phanphensophon, N.; Mottram, D.S.; Methven, L. Contributions of non-volatile and volatile compounds to the umami taste and overall flavour of shiitake mushroom extracts and their application as flavour enhancers in cooked minced meat. Food Chem. 2013, 141, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Nollet, L.M.L. Flow Injection Analysis of Food Additives; CRC Press. Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Fraqueza, M.J.; Alfaia, C.M.; Barreto, A.S. Biogenic amine formation in turkey meat under modified atmosphere packaging with extended shelf life: Index of freshness. Poult. Sci. 2012, 91, 1465–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadáková, E.; Pelikánová, T.; Kalač, P. Concentration of biologically active polyamines in meat and liver of sheep and lambs after slaughter and their changes in mutton during storage and cooking. Meat Sci. 2011, 87, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Rokka, M.; Eerola, S.; Smolander, M.; Alakomi, H.-L.; Ahvenainen, R. Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions: B. Biogenic amines as quality-indicating metabolites. Food Control 2004, 15, 601–607. [Google Scholar] [CrossRef]
- Roig-Roig-Sagués, A.X.; Ruiz-Capillas, C.; Espinosa, D.; Hernández, M. The decarboxylating bacteria present in foodstuffs and the effect of emerging technologies on their formation. In Biological Aspects of Biogenic Amines, Polyamines and Conjugates; Dandrifosse, G., Ed.; Transworld Research Network: Kerala, India, 2009. [Google Scholar]
- Ten Brink, B.; Damink, C.; Joosten, H.M.; Huis in’t Veld, J.H. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Gardini, F.; Martuscelli, M.; Caruso, M.C.; Galgano, F.; Crudele, M.A.; Favati, F.; Guerzoni, M.E.; Suzzi, G. Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. Int. J. Food Microbiol. 2001, 64, 105–117. [Google Scholar] [CrossRef]
- Curiel, J.A.; Ruiz-Capillas, C.; de Las Rivas, B.; Carrascosa, A.V.; Jiménez-Colmenero, F.; Muñoz, R. Production of biogenic amines by lactic acid bacteria and enterobacteria isolated from fresh pork sausages packaged in different atmospheres and kept under refrigeration. Meat Sci. 2011, 88, 368–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Capillas, C.; Herrero, A.; Triki, M.; Jiménez-Colmenero, F. Biogenic Amine Formation in Reformulated Cooked Sausage Without Added Nitrite. J. Nutr. Med. Diet Care 2017, 3, 2–6. [Google Scholar] [CrossRef]
- James, S.; James, C. Raw material selection: meat and poultry. In Chilled Foods: A Comprehensive Guide, 3rd ed.; Brown, M., Ed.; Woodhead Publishing: Cambridge, England, 2008; pp. 61–82. [Google Scholar]
| Meat Type | Chilling Storage at 2 °C | |||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 10 | |
| Pork leg (5 muscles) | 6.70 ± 0.02 c5 | 5.89 ± 0.02 a2 | 6.26 ± 0.03 b3 | 6.32 ± 0.01 b3 |
| Lamb leg (7 muscles) | 5.90 ± 0.01 b2 | 6.05 ± 0.01 c3 | 5.77 ± 0.01 a2 | 6.01 ± 0.03 c1 |
| Turkey leg (4 muscles) | 6.54 ± 0.01 a4 | 6.89 ± 0.01 b5 | 6.63 ± 0.02 a4 | 7.34 ± 0.01 c5 |
| Chicken breast (1 muscle) | 6.39 ± 0.26 b3 | 6.30 ± 0.02 a, b4 | 6.26 ± 0.01 a3 | 6.67 ± 0.00 c4 |
| Beef leg (4 muscles) | 5.71 ± 0.01 a1 | 5.71 ± 0.10 a1 | 5.52 ± 0.02 a1 | 6.20 ± 0.01 b2 |
| Microorganisms | Meat Type | Chilling Storage at 2 °C | |||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 10 | ||
| Total Viable Count (TVC) | Pork leg (5 muscles) | 5.53 ± 0.12 a4 | 7.79 ± 0.05 b3 | 9.74 ± 0.01 c4 | 10.04 ± 0.01 d1, 2 |
| Lamb leg (7 muscles) | 5.64 ± 0.25 a4 | 7.54 ± 0.40 b2 | 9.99 ± 0.17 c5 | 10.04 ± 0.02 c2 | |
| Turkey leg (4 muscles) | 4.98 ± 0.01 a3 | 6.45 ± 0.13 b1 | 8.96 ± 0.05 c1 | 9.91 ± 0.09 d1 | |
| Chicken breast (1 muscle) | 4.13 ± 0.17 a1 | 7.79 ± 0.03 b3 | 9.55 ± 0.02 c3 | 10.04 ± 0.05 d1, 2 | |
| Beef leg (4 muscles) | 4.74 ± 0.10 a2 | 6.55 ± 0.09 b1 | 9.25 ± 0.04 c2 | 10.22 ± 0.03 d2 | |
| Lactic acid bacteria (LAB) | Pork leg (5 muscles) | 3.76 ± 0.07 a3 | 5.64 ± 0.01 b3 | 7.57 ± 0.03 c5 | 8.34 ± 0.08 d2 |
| Lamb leg (7 muscles) | 4.24 ± 0.01 a4 | 5.70 ± 0.05 b3 | 6.05 ± 0.11 c3 | 8.26 ± 0.09 d2 | |
| Turkey leg (4 muscles) | 4.65 ± 0.03 a5 | 5.61 ± 0.10 b3 | 7.40 ± 0.02 c4 | 8.08 ± 0.13 d1 | |
| Chicken breast (1 muscle) | 2.96 ± 0.02 a1 | 5.38 ± 0.06 b2 | 6.26 ± 0.02 b1 | 8.99 ± 0.09 c1 | |
| Beef leg (4 muscles) | 3.20 ± 0.14 a2 | 4.34 ± 0.06 b1 | 5.89 ± 0.01 c2 | 8.04 ± 0.06 d1 | |
| Enterobacteriaceae | Pork leg (5 muscles) | 2.95 ± 0.00 a1 | 4.95 ± 0.04 b1 | 6.11 ± 0.05 c1 | 7.53 ± 0.10 d1 |
| Lamb leg (7 muscles) | 4.71 ± 0.00 a4 | 6.55 ± 0.05 b3, 4 | 6.37 ± 0.02 b3 | 7.99 ± 0.04 c2 | |
| Turkey leg (4 muscles) | 4.24 ± 0.02 a3 | 5.88 ± 0.06 b2 | 6.79 ± 0.02 c4 | 7.68 ± 0.03 d4 | |
| Chicken breast (1 muscle) | 4.07 ± 0.76 a2, 3 | 6.73 ± 0.03 b4 | 6.72 ± 0.04 b2 | 7.27 ± 0.09 c1 | |
| Beef leg (4 muscles) | 3.92 ± 0.03 a2 | 6.42 ± 0.12 b3 | 6.62 ± 0.01 c2 | 7.66 ± 0.05 d3 | |
| FAA | Meat Type | Chilling Storage at 2 °C | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 10 | |||
| FAA no precursors of BA | Aspartic acid | Pork leg (5 muscles) | 2.96 ± 0.04 a2 | 6.82 ± 0.86 c3 | 9.46 ± 2.59 d2 | 4.87 ± 0.02 b1 |
| Lamb leg (7 muscles) | 1.72 ± 0.00 a1 | 1.80 ± 0.31 a1 | 4.00 ± 0.12 b1 | 5.98 ± 0.04 c2 | ||
| Turkey leg (4 muscles) | 7.95 ± 0.41 a3 | 13.61 ± 0.32 b4 | 20.68 ± 0.02 d3 | 15.92 ± 0.39 c3 | ||
| Chicken breast (1 muscle) | 15.38 ± 0.83 b3 | 23.87 ± 0.16 c5 | 29.18 ± 0.29 d4 | 4.57 ± 0.11 a1 | ||
| Beef leg (4 muscles) | 2.37 ± 1.00 a1, 2 | 5.15 ± 0.18 b2 | 3.99 ± 0.00 b1 | 4.96 ± 0.11 b1 | ||
| Threonine | Pork leg (5 muscles) | 2.21 ± 0.04 a2 | 4.23 ± 0.23 b3 | 11.23 ± 2.71 c3 | 12.30 ± 0.04 c2 | |
| Lamb leg (7 muscles) | 2.67 ± 0.04 a b2 | 2.10 ± 0.02 a1 | 4.08 ± 0.05 b c2 | 4.97 ± 0.19 c1 | ||
| Turkey leg (4 muscles) | 8.30 ± 0.80 a3 | 11.06 ± 0.48 b4 | 12.40 ± 0.18 b3 | 24.41 ± 0.14 c3 | ||
| Chicken breast (1 muscle) | 13.71 ± 0.59 a4 | 21.17 ± 0.34 b5 | 30.82 ± 0.23 c4 | 12.51 ± 0.26 a2 | ||
| Beef leg (4 muscles) | 1.40 ± 0.04 a1 | 2.58 ± 0.07 b2 | 2.75 ± 0.07 b1 | 5.10 ± 0.10 c1 | ||
| Serine | Pork leg (5 muscles) | 5.18 ± 0.45 a1 | 9.12 ± 0.74 b1 | 10.51 ± 2.41 c2 | 8.45 ± 0.06 b3 | |
| Lamb leg (7 muscles) | ND | ND | 5.84 ± 0.12 b1 | 4.36 ± 0.00 a1 | ||
| Turkey leg (4 muscles) | 37.63 ± 1.04 c3 | 41.59 ± 0.32 d2 | 31.69 ± 0.17 b3 | 15.26 ± 0.19 a4 | ||
| Chicken breast (1 muscle) | 27.67 ± 1.42 b2 | 41.41 ± 0.52 d2 | 36.99 ± 0.73 c4 | 8.85 ± 0.28 a3 | ||
| Beef leg (4 muscles) | ND | ND | ND | 7.40 ± 0.04 a2 | ||
| Glutamic acid | Pork leg (5 muscles) | 8.09 ± 0.96 a2 | 16.78 ± 0.94 b3 | 48.67 ± 1.93 d3 | 28.97 ± 0.19 c1 | |
| Lamb leg (7 muscles) | 5.94 ± 0.14 a1 | 11.05 ± 0.26 b2 | 19.09 ± 0.39 c2 | 27.66 ± 1.73 d1 | ||
| Turkey leg (4 muscles) | 28.95 ± 1.66 a4 | 50.35 ± 0.09 b5 | 55.64 ± 0.04 b4 | 87.58 ± 0.19 c3 | ||
| Chicken breast (1 muscle) | 21.07 ± 0.88 a3 | 36.66 ± 0.54 c4 | 51.58 ± 0.73 d3 | 30.52 ± 0.66 b1 | ||
| Beef leg (4 muscles) | 4.42 ± 0.38 a1 | 8.81 ± 0.08 b1 | 15.06 ± 0.30 c1 | 65.41 ± 0.14 d2 | ||
| Glycine | Pork leg (5 muscles) | 6.17 ± 0.37 a2 | 12.65 ± 0.77 b c2 | 13.71 ± 3.14 c2 | 11.23 ± 0.06 b2 | |
| Lamb leg (7 muscles) | 18.10 ± 0.02 a4 | 19.89 ± 0.06 b3 | 17.88 ± 0.33 a3 | 17.69 ± 0.42 a3 | ||
| Turkey leg (4 muscles) | 18.24 ± 0.99 a4 | 20.88 ± 0.22 b3,4 | 18.62 ± 0.18 a3 | 28.28 ± 0.01 c4 | ||
| Chicken breast (1 muscle) | 16.76 ± 0.75 b3 | 21.93 ± 0.44 c4 | 27.41 ± 0.45 d4 | 11.74 ± 0.21 a2 | ||
| Beef leg (4 muscles) | 3.14 ± 0.07 a1 | 10.31 ± 0.08 c1 | 6.73 ± 0.18 b1 | 7.04 ± 0.03 b1 | ||
| β-alanine | Pork leg (5 muscles) | 18.70 ± 1.06 a1 | 29.68 ± 1.51 b1 | 38.51 ± 8.70 c2 | 27.69 ± 0.03 b1 | |
| Lamb leg (7 muscles) | 30.83 ± 0.00 a3 | 36.65 ± 0.09 c2 | 32.65 ± 0.68 b1 | 41.50 ± 0.87 d2 | ||
| Turkey leg (4 muscles) | 37.64 ± 2.14 a4 | 42.34 ± 0.10 b3 | 41.90 ± 0.45 b2 | 48.33 ± 0.11 c3 | ||
| Chicken breast (1 muscle) | 22.68 ± 0.86 a2 | 38.59 ± 0.67 c2 | 52.54 ± 0.87 d3 | 28.75 ± 0.72 b1 | ||
| Beef leg (4 muscles) | 29.09 ± 0.20 a3 | 47.69 ± 0.34 c4 | 41.59 ± 0.77 b2 | 42.99 ± 0.30 b2 | ||
| Cysteine | Pork leg (5 muscles) | 0.53 ± 0.12 a2 | 0.92 ± 0.04 b2 | 1.61 ± 0.27 c3 | 1.71 ± 0.03 c3 | |
| Lamb leg (7 muscles) | 0.20 ± 0.04 a1 | 1.05 ± 0.09 b2 | 1.17 ± 0.00 b2 | 1.20 ± 0.04 b1 | ||
| Turkey leg (4 muscles) | 0.33 ± 0.00 a1 | 0.39 ± 0.00 a1 | 0.42 ± 0.04 a1 | 2.01 ± 0.09 b4 | ||
| Chicken breast (1 muscle) | 0.22 ± 0.00a1 | 0.33 ± 0.00 a1 | 3.95 ± 0.04 c4 | 1.74 ± 0.09 b3 | ||
| Beef leg (4 muscles) | 0.98 ± 0.04 a3 | 0.98 ± 0.12 a2 | 1.06 ± 0.00 a2 | 1.42 ± 0.03 b2 | ||
| Valine | Pork leg (5 muscles) | 5.77 ± 0.04 a2 | 8.17 ± 0.45 b3 | 13.16 ± 2.74 c3 | 16.25 ± 0.06 d3 | |
| Lamb leg (7 muscles) | 2.59 ± 0.08 a1 | 4.64 ± 0.08 b1 | 7.58 ± 0.14 c1 | 7.09 ± 0.11 c1 | ||
| Turkey leg (4 muscles) | 8.29 ± 0.37 a3 | 11.28 ± 0.43 b4 | 11.49 ± 0.18 b2 | 23.19 ± 0.14 c4 | ||
| Chicken breast (1 muscle) | 12.97 ± 0.47 a4 | 20.28 ± 0.34 c5 | 23.45 ± 0.18 d4 | 16.67 ± 0.61 b3 | ||
| Beef leg (4 muscles) | 5.30 ± 0.08 a2 | 6.23 ± 0.56 b2 | 8.26 ± 0.16 c1 | 9.37 ± 0.04 d2 | ||
| Methionine | Pork leg (5 muscles) | 2.67 ± 0.29 a2 | 3.17 ± 0.10 a2 | 9.31 ± 1.94 b3 | 12.30 ± 0.04 c3 | |
| Lamb leg (7 muscles) | 1.10 ± 0.00 a1 | 1.44 ± 0.30 a1 | 3.97 ± 0.01 b1 | 4.12 ± 0.10 b1 | ||
| Turkey leg (4 muscles) | 3.19 ± 0.07 a2 | 4.84 ± 0.34 b3 | 5.00 ± 0.04 b2 | 16.79 ± 0.01 c4 | ||
| Chicken breast (1 muscle) | 7.10 ± 0.30 a3 | 11.58 ± 0.27 b4 | 14.63 ± 0.21 c4 | 12.34 ± 0.29 b3 | ||
| Beef leg (4 muscles) | 1.31 ± 0.00 a1 | 2.20 ± 0.68 a1, 2 | 4.09 ± 0.05 b1 | 6.62 ± 0.08 c2 | ||
| Isoleucine | Pork leg (5 muscles) | 2.98 ± 0.12 a3 | 4.36 ± 0.25 b2 | 8.28 ± 1.74 c2 | 9.88 ± 0.08 d3 | |
| Lamb leg (7 muscles) | 1.73 ± 0.13 a1 | 2.40 ± 0.10 b1 | 3.92 ± 0.08 c1 | 4.05 ± 0.00 c1 | ||
| Turkey leg (4 muscles) | 5.25 ± 0.21 a4 | 7.24 ± 0.18 b3 | 6.97 ± 0.08 b2 | 16.33 ± 0.00 c4 | ||
| Chicken breast (1 muscle) | 8.16 ± 0.35a5 | 13.98 ± 0.23 c4 | 15.75 ± 0.40 d3 | 10.08 ± 0.20 b3 | ||
| Beef leg (4 muscles) | 2.21 ± 0.04 a2 | 3.02 ± 0.51 a b1 | 3.90 ± 0.05 b1 | 5.88 ± 0.07 c2 | ||
| Leucine | Pork leg (5 muscles) | 5.35 ± 0.08 a2 | 6.88 ± 0.38 b3 | 14.18 ± 2.92 c3 | 16.65 ± 0.06 d3 | |
| Lamb leg (7 muscles) | 3.09 ± 0.34 a1 | 4.20 ± 0.14 b1 | 7.05 ± 0.19 c1 | 7.22 ± 0.04 c1 | ||
| Turkey leg (4 muscles) | 7.66 ± 0.40 a3 | 11.28 ± 0.10 b4 | 10.84 ± 0.07 b2 | 26.19 ± 0.06 c4 | ||
| Chicken breast (1 muscle) | 14.29 ± 0.44 a4 | 24.10 ± 0.46 c5 | 26.76 ± 0.46 d4 | 16.86 ± 0.60 b3 | ||
| Beef leg (4 muscles) | 3.79 ± 0.04 a1 | 5.65 ± 0.54 b2 | 6.99 ± 0.05 c1 | 9.99 ± 0.09 d2 | ||
| FAA precursors of BA | Tyrosine | Pork leg (5 muscles) | 3.82 ± 0.24 a3 | 5.02 ± 0.35 b3 | 7.90 ± 1.79 c3 | 8.94 ± 0.27 c3 |
| Lamb leg (7 muscles) | 2.13 ± 0.06 a2 | 2.36 ± 0.04 b1 | 2.62 ± 0.17 c1 | 3.09 ± 0.47 c1 | ||
| Turkey leg (4 muscles) | 6.21 ± 0.34 a4 | 8.75 ± 0.13 b4 | 8.20 ± 0.41 b3 | 9.24 ± 0.07 a3 | ||
| Chicken breast (1 muscle) | 11.23 ± 0.54 b5 | 18.14 ± 0.43 d5 | 15.80 ± 0.20 c4 | 17.17 ± 0.27 c4 | ||
| Beef leg (4 muscles) | 1.63 ± 0.18 a1 | 2.96 ± 0.17 b2 | 5.22 ± 0.18 c2 | 6.04 ± 0.56 d2 | ||
| Phenylalanine | Pork leg (5 muscles) | 3.94 ± 0.33 a3 | 4.39 ± 0.37 a3 | 14.82 ± 3.31 b3 | 17.41 ± 0.22 b3 | |
| Lamb leg (7 muscles) | 2.36 ± 0.22 a2 | 2.50 ± 0.12 a1 | 4.86 ± 0.63 b1 | 5.82 ± 0.59 b1 | ||
| Turkey leg (4 muscles) | 4.10 ± 0.46 a3 | 6.87 ± 0.28 b4 | 6.45 ± 0.15 b2 | 19.92 ± 0.05 c4 | ||
| Chicken breast (1 muscle) | 8.24 ± 0.12 a4 | 13.70 ± 0.35 b5 | 15.74 ± 0.34 c3 | 18.41 ± 0.78 d3 | ||
| Beef leg (4 muscles) | 1.72 ± 0.27 a1 | 3.54 ± 0.06 b2 | 4.72 ± 0.22 c1 | 13.18 ± 0.00 d2 | ||
| Histidine | Pork leg (5 muscles) | ND | 3.26 ± 0.16 a1 | 3.85 ± 0.91 a1 | 5.00 ± 0.03 b1 | |
| Lamb leg (7 muscles) | ND | ND | ND | ND | ||
| Turkey leg (4 muscles) | ND | ND | ND | ND | ||
| Chicken breast (1 muscle) | ND | ND | ND | 5.62 ± 0.78 a1 | ||
| Beef leg (4 muscles) | ND | ND | ND | ND | ||
| Lysine | Pork leg (5 muscles) | 4.96 ± 0.29 a1 | 9.92 ± 056 b1 | 24.46 ± 5.63 c3 | 25.84 ± 0.04 c3 | |
| Lamb leg (7 muscles) | 7.69 ± 0.00 a3 | 9.64 ± 0.06 b1 | 12.69 ± 0.16 c1 | 12.76 ± 0.24 c1 | ||
| Turkey leg (4 muscles) | 15.09 ± 0.92 a4 | 20.66 ± 0.17 b3 | 26.19 ± 0.26 c3 | 60.33 ± 0.04 d5 | ||
| Chicken breast (1 muscle) | 32.38 ± 1.74 b5 | 37.50 ± 0.73 c4 | 69.68 ± 0.89 d4 | 27.13 ± 0.52 a4 | ||
| Beef leg (4 muscles) | 5.91 ± 0.05 a2 | 12.09 ± 0.17 b2 | 13.58 ± 0.16 c2 | 18.56 ± 1.63 d2 | ||
| Arginine | Pork leg (5 muscles) | 4.19 ± 0.40 b1 | 7.56 ± 0.58 c1 | 0.12 ± 0.01 a2 | ND | |
| Lamb leg (7 muscles) | 6.39 ± 0.28 b2 | 7.06 ± 0.12 c1 | 0.08 ± 0.01 a1 | ND | ||
| Turkey leg (4 muscles) | 12.49 ± 0.89 b3 | 16.17 ± 0.13 c2 | 1.45 ± 0.23 a3, 4 | 0.72 ± 0.04 d5 | ||
| Chicken breast (1 muscle) | 22.44 ± 1.21 b4 | 26.32 ± 0.62 c3 | 1.41 ± 0.06 a3 | ND | ||
| Beef leg (4 muscles) | 4.27 ± 0.11 b1 | 6.74 ± 0.33 c1 | 1.57±0.06 a4 | ND | ||
| TFAAP of BA | Pork leg (5 muscles) | 16.91 ± 1.25 a2 | 30.15 ± 0.55 b3 | 51.15 ± 11.76 c3 | 57.18 ± 0.04 c3 | |
| Lamb leg (7 muscles) | 18.58 ± 0.56 a2 | 21.56 ± 0.02 c1 | 20.25 ± 0.85 b1 | 20.68 ± 0.12 b1 | ||
| Turkey leg (4 muscles) | 37.89 ± 2.61 a3 | 52.46 ± 0.70 c4 | 42.29 ± 0.52 b3 | 90.21 ± 0.27 d5 | ||
| Chicken breast (1 muscle) | 74.29 ± 3.60 b4 | 95.66 ± 2.12 c5 | 102.63 ± 1.48 d4 | 62.71 ± 1.37 a4 | ||
| Beef leg (4 muscles) | 13.53 ± 0.61 a1 | 25.33 ± 0.27 b2 | 25.09 ± 0.62 b2 | 37.79 ± 1.07 c2 | ||
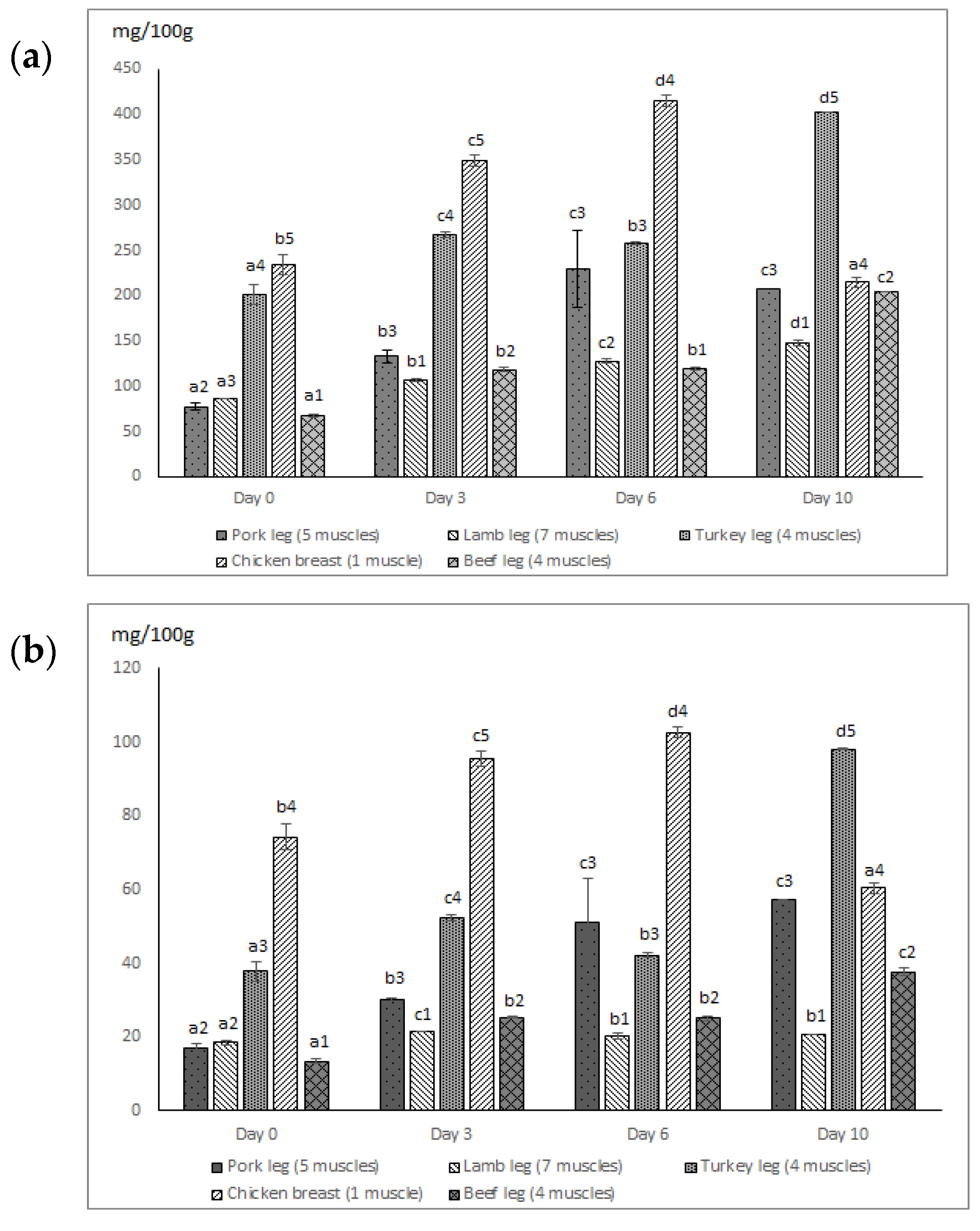
| TFAA | Pork leg (5 muscles) | 77.50 ± 4.35 a2 | 132.94 ± 6.63 b3 | 229.78 ± 42.86 c3 | 207.48 ± 0.23 c3 | |
| Lamb leg (7 muscles) | 86.56 ± 0.10 a3 | 106.78 ± 0.98 b1 | 127.48 ± 2.46 c2 | 147.51 ± 3.50 d1 | ||
| Turkey leg (4 muscles) | 201.33 ± 10.70 a4 | 267.32 ± 3.10 c4 | 257.94 ± 1.62 b3 | 394.50 ± 0.36 d5 | ||
| Chicken breast (1 muscle) | 234.30 ± 10.49 b5 | 349.56 ± 6.08 c5 | 415.68 ± 6.08 d4 | 217.34 ± 5.40 a4 | ||
| Beef leg (4 muscles) | 67.54 ± 0.94 a1 | 117.96 ± 2.37 b2 | 119.51 ± 2.12 b1 | 203.98 ± 0.26 c2 | ||
| BA | Meat Type | Chilling Storage at 2 °C | |||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 10 | ||
| Tyramine | Pork leg (5 muscles) | 0.67 ± 0.03 a3 | 1.10 ± 0.02 b3 | 11.20 ± 0.04 c4 | 16.58 ± 0.04 d4 |
| Lamb leg (7 muscles) | 0.10 ± 0.00 a1 | 0.19 ± 0.01 a1 | 7.05 ± 0.25 b3 | 10.71 ± 0.01 c3 | |
| Turkey leg (4 muscles) | ND | 0.40 ± 0.04 a2 | 1.72 ± 0.02 b2 | 6.88 ± 0.14 c2 | |
| Chicken breast (1 muscle) | ND | 0.47 ± 0.07 a2 | 27.54 ± 0.86 b5 | 35.16 ± 0.36 c5 | |
| Beef leg (4 muscles) | 0.34 ± 0.04 a2 | 0.42 ± 0.02 a2 | 0.53 ± 0.03 b1 | 1.57 ± 0.07 c1 | |
| Histamine | Pork leg (5 muscles) | ND | ND | ND | ND |
| Lamb leg (7 muscles) | ND | ND | ND | ND | |
| Turkey leg (4 muscles) | ND | ND | ND | ND | |
| Chicken breast (1 muscle) | 0.53 ± 0.03 a1 | 1.23 ± 0.03 b2 | 1.73 ± 0.03 c2 | 2.11 ± 0.01 d2 | |
| Beef leg (4 muscles) | ND | 0.10 ± 0.00 a1 | 0.21 ± 0.01 b1 | 0.50 ± 0.02 c1 | |
| Phenylethylamine | Pork leg (5 muscles) | ND | 0.77 ± 0.03 a1 | 1.28 ± 0.02 b1 | 1.66 ± 0.06 c1 |
| Lamb leg (7 muscles) | 0.76 ± 0.00 a3 | 4.80 ± 0.08 b3 | 7.57 ± 0.27 c3 | 9.05 ± 0.15 d3 | |
| Turkey leg (4 muscles) | 0.21 ± 0.05 a1 | 15.07 ± 0.01 d4 | 12.85 ± 0.47 c4 | 11.33 ± 0.29 b4 | |
| Chicken breast (1 muscle) | ND | 16.87 ± 0.13 c5 | 17.99 ± 0.15 b5 | 12.81 ± 0.05 a5 | |
| Beef leg (4 muscles) | 0.47 ± 0.01 a2 | 2.33 ± 0.05 b2 | 2.47 ± 0.05 b2 | 2.62 ± 0.02 c2 | |
| Putrescine | Pork leg (5 muscles) | 0.57 ± 0.09 a1 | 0.72 ± 0.02 a1 | 5.10 ± 0.48 b2 | 14.55 ± 0.09 c3 |
| Lamb leg (7 muscles) | 1.19 ± 0.07 a2 | 3.22 ± 0.00 b4 | 6.40 ± 0.24 c3 | 10.11 ± 0.01 d2 | |
| Turkey leg (4 muscles) | 1.23 ± 0.03 a2 | 4.70 ± 0.08 b5 | 8.44 ± 0.44 c5 | 68.72 ± 0.02 d5 | |
| Chicken breast (1 muscle) | 1.23 ± 0.03 a2 | 1.83 ± 0.01 b2 | 7.47 ± 0.07 c4 | 51.99 ± 0.29 d4 | |
| Beef leg (4 muscles) | 1.34 ± 0.02 a3 | 2.07 ± 0.03 b3 | 3.99 ± 0.03 c1 | 7.40 ± 0.04 d1 | |
| Cadaverine | Pork leg (5 muscles) | ND | ND | 1.11 ± 0.07 a1 | 16.16 ± 0.28 b4 |
| Lamb leg (7 muscles) | ND | ND | 3.42 ± 0.02 a2 | 5.08 ± 0.12 b1 | |
| Turkey leg (4 muscles) | ND | ND | 1.27 ± 0.03 a1 | 13.25 ± 0.27 b2 | |
| Chicken breast (1 muscle) | ND | ND | 3.98 ± 0.10 a3 | 14.31 ± 0.11 b3 | |
| Beef leg (4 muscles) | ND | ND | ND | ND | |
| Tryptamine | Pork leg (5 muscles) | ND | ND | 6.37 ± 0.07 a1 | 6.56 ± 0.10 a2 |
| Lamb leg (7 muscles) | ND | ND | ND | ND | |
| Turkey leg (4 muscles) | ND | ND | ND | ND | |
| Chicken breast (1 muscle) | 3.82 ± 0.12 b1 | 15.78 ± 0.16 d1 | 7.47 ± 0.07 c2 | 0.37 ± 0.01 a1 | |
| Beef leg (4 muscles) | ND | ND | ND | ND | |
| Agmatine | Pork leg (5 muscles) | ND | ND | ND | ND |
| Lamb leg (7 muscles) | ND | 0.15 ± 0.01 a1 | 1.14 ± 0.02 b1 | 2.30 ± 0.08 c1 | |
| Turkey leg (4 muscles) | ND | ND | ND | ND | |
| Chicken breast (1 muscle) | ND | ND | ND | ND | |
| Beef leg (4 muscles) | ND | ND | ND | ND | |
| Spermidine | Pork leg (5 muscles) | 2.63 ± 0.13 a2 | 2.70 ± 0.00 a1 | 3.18 ± 0.14 b1 | 3.88 ± 0.18 c1 |
| Lamb leg (7 muscles) | 8.09 ± 0.13 a4 | 11.99 ± 0.13 c4 | 8.69 ± 0.21 a4 | 10.16 ± 0.12 b5 | |
| Turkey leg (4 muscles) | 7.33 ± 0.05 a3 | 18.27 ± 0.07 d5 | 12.14 ± 0.10 c5 | 9.67 ± 0.09 b4 | |
| Chicken breast (1 muscle) | 9.78 ± 0.06 d5 | 6.69 ± 0.13 b3 | 6.24 ± 0.20 a3 | 7.02 ± 1.10 c3 | |
| Beef leg (4 muscles) | 2.29 ± 0.05 a1 | 3.39 ± 0.05 b2 | 4.35 ± 0.01 c2 | 5.39 ± 0.05 d2 | |
| Spermine | Pork leg (5 muscles) | 27.60 ± 0.96 b1 | 27.10 ± 0.12 b1 | 25.23 ± 1.17 a1 | 26.87 ± 0.31 b2 |
| Lamb leg (7 muscles) | 31.36 ± 0.58 a2 | 40.85 ± 0.37 c3 | 31.60 ± 0.98 a3 | 36.42 ± 0.00 b4 | |
| Turkey leg (4 muscles) | 35.44 ± 1.28 b3 | 49.20 ± 0.40 d4 | 36.95 ± 0.53 c4 | 32.55 ± 0.09 a3 | |
| Chicken breast (1 muscle) | 45.03 ± 0.81 b4 | 53.60 ± 0.24 d5 | 47.26 ± 0.54 c5 | 41.92 ± 0.16a5 | |
| Beef leg (4 muscles) | 30.86 ± 0.36 b2 | 33.02 ± 0.24 c2 | 29.54 ± 0.40 b2 | 25.08 ± 0.04 a1 | |
| BAI | Pork leg (5 muscles) | 1.24 | 1.82 | 17.41 | 47.29 |
| Lamb leg (7 muscles) | 1.29 | 3.41 | 16.87 | 25.90 | |
| Turkey leg (4 muscles) | 1.23 | 5.10 | 11.43 | 88.85 | |
| Chicken breast (1 muscle) | 1.76 | 3.53 | 40.72 | 103.57 | |
| Beef leg (4 muscles) | 1.68 | 2.59 | 4.73 | 9.47 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage. Foods 2018, 7, 132. https://doi.org/10.3390/foods7090132
Triki M, Herrero AM, Jiménez-Colmenero F, Ruiz-Capillas C. Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage. Foods. 2018; 7(9):132. https://doi.org/10.3390/foods7090132
Chicago/Turabian StyleTriki, Mehdi, Ana M. Herrero, Francisco Jiménez-Colmenero, and Claudia Ruiz-Capillas. 2018. "Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage" Foods 7, no. 9: 132. https://doi.org/10.3390/foods7090132





