Increasing the Yield of Irish Brown Crab (Cancer pagurus) during Processing without Adversely Affecting Shelf-Life
Abstract
:1. Introduction
2. Materials and Method
2.1. Sample Preparation
2.2. Microbiological Analysis
2.3. Measuring pH, aw and WHC
2.4. Statistical Analysis
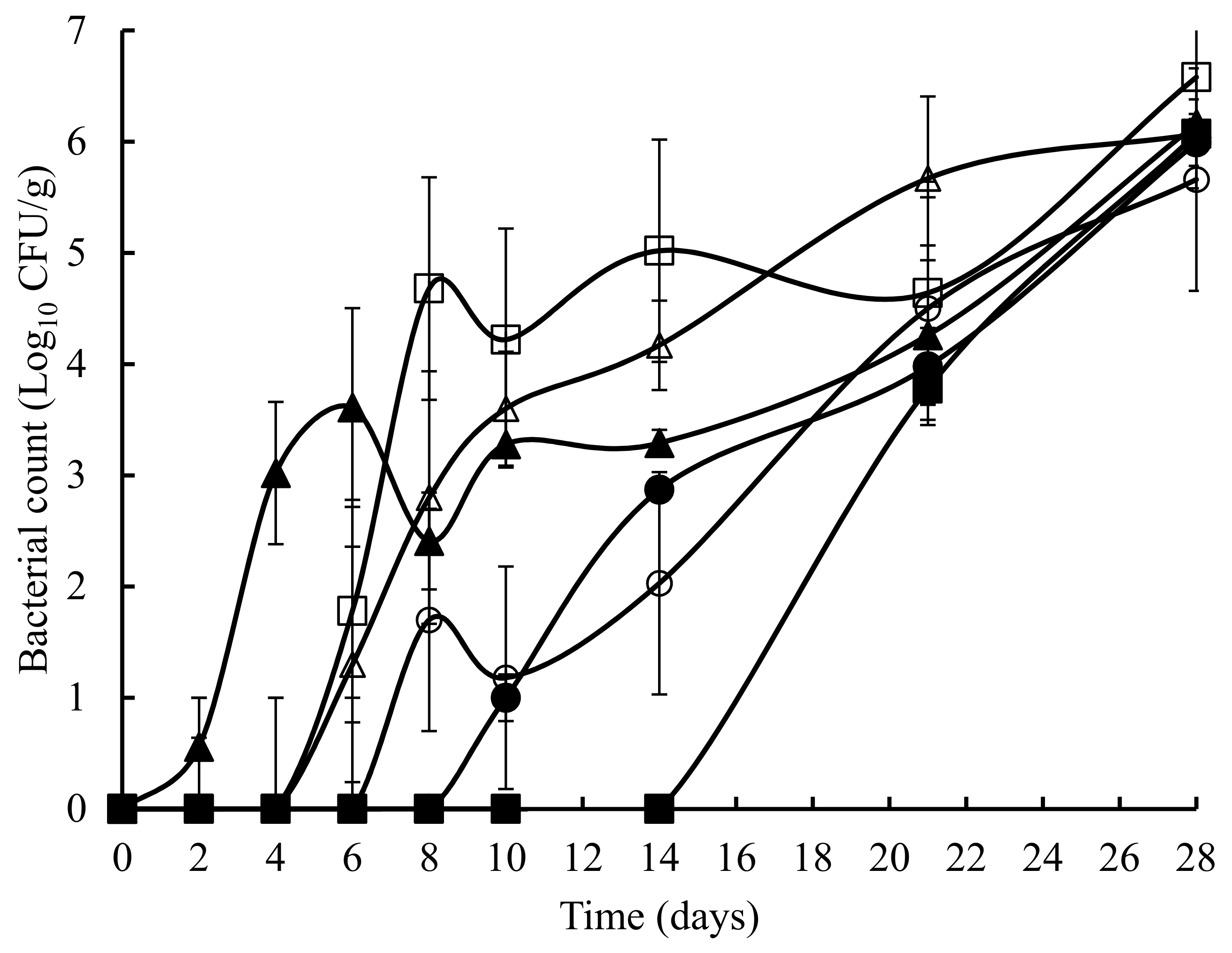
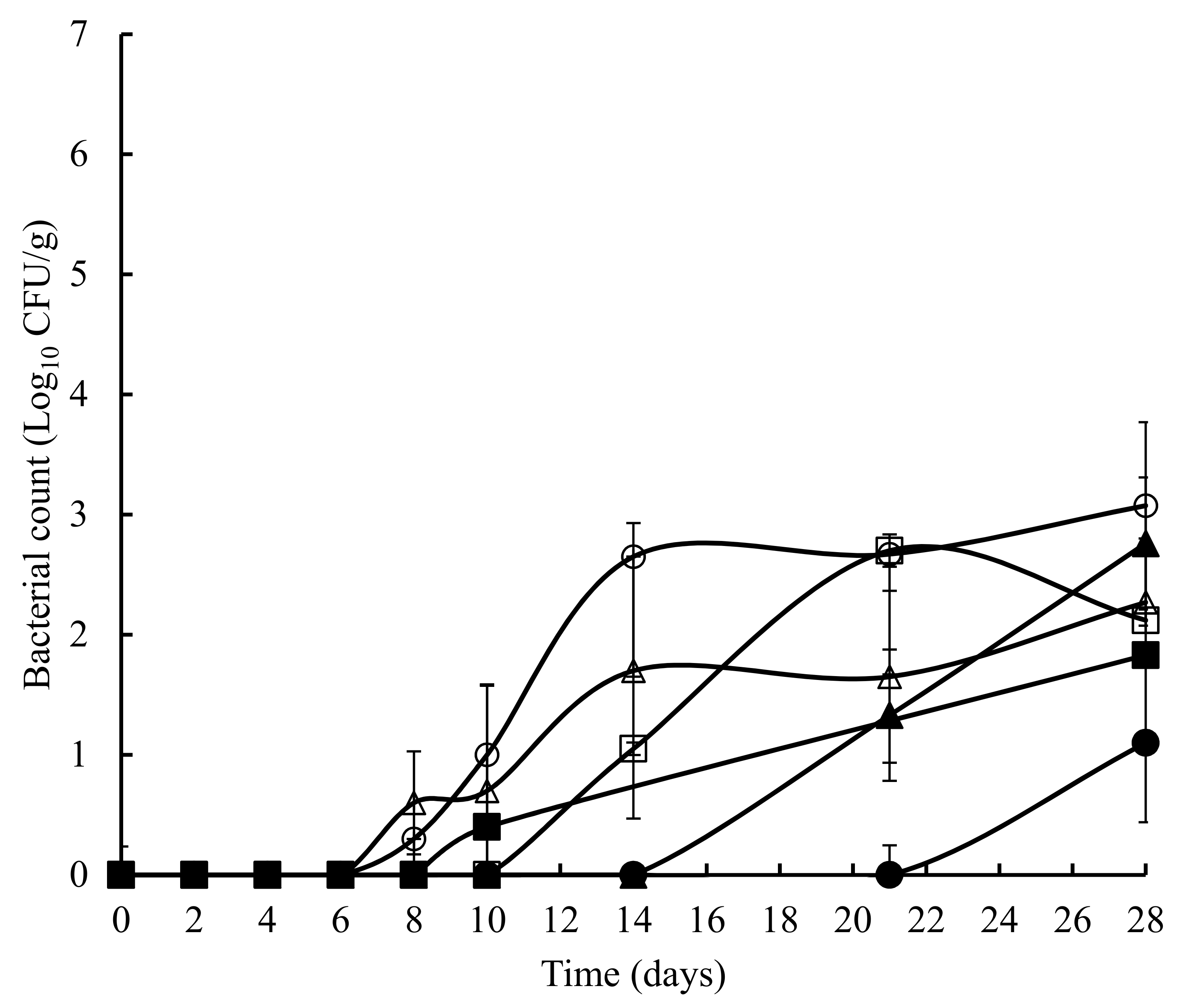
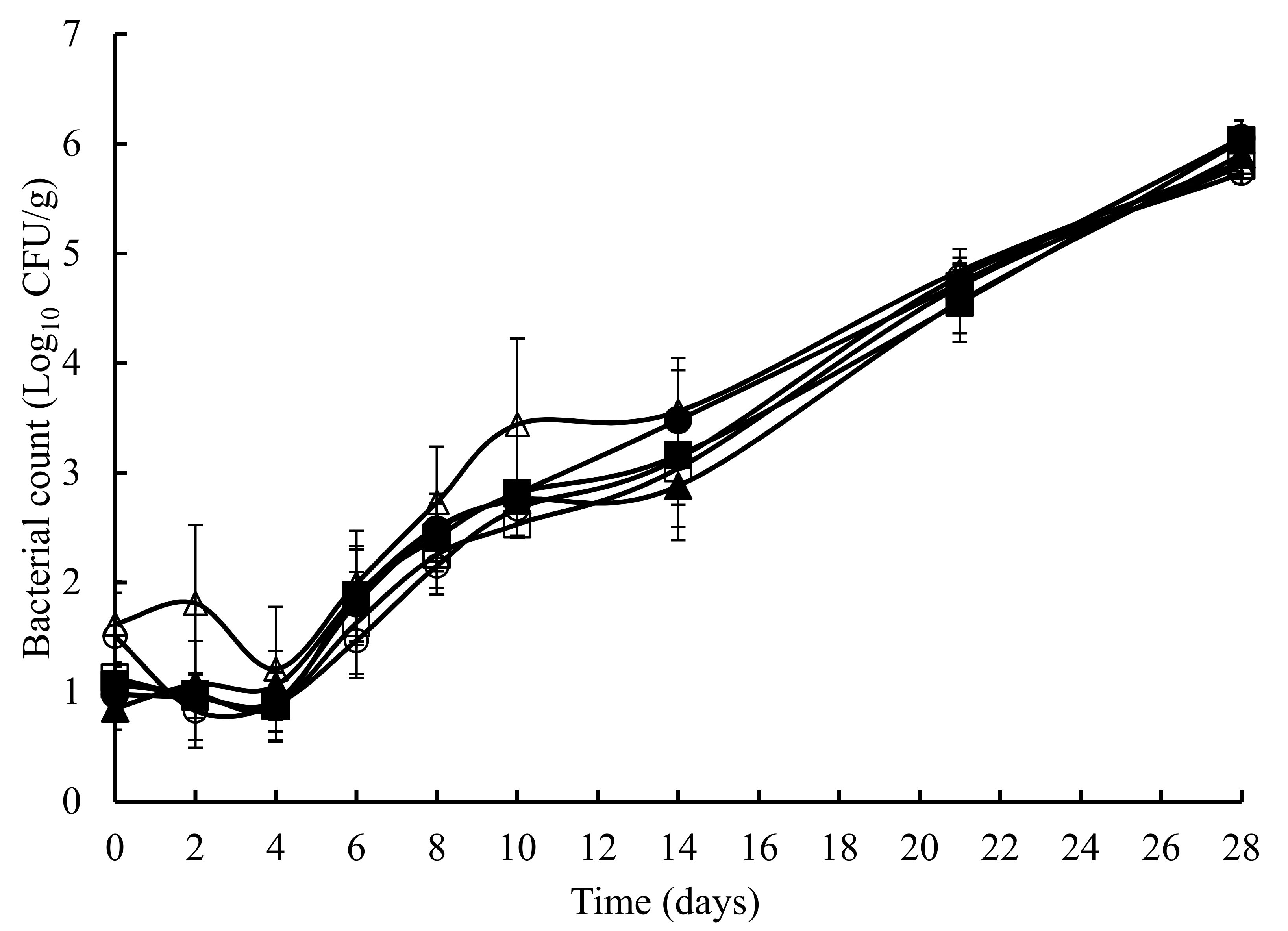
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BIM. Business of Seafood; Marine Institute and Bord Iascaigh Mhara: Dublin, Ireland, 2017. [Google Scholar]
- EUROSTAT. 2009. Available online: http://epp.eurostat.ec.europa.eu/portal/page/fisheries/data/database (accessed on 22 June 2018).
- Condon-Abanto, S.; Arroyo, C.; Alvarez, I.; Brunton, N.; Whyte, P.; Lyng, J.G. An assessment of the application of ultrasound in the processing of ready-to-eat whole brown crab (Cancer pagurus). Ultrason. Sonochem. 2018, 40, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Hanover, L.M.; Webb, N.B.; Howell, A.J.; Thomas, F.B. Effects of Cooking and Rinsing on the Protein Losses from Blue Crabs. J. Milk Food Technol. 1973, 36, 409–413. [Google Scholar] [CrossRef]
- Day, L.; Seymour, R.B.; Pitts, K.F.; Konczak, I.; Lundin, L. Incorporation of functional ingredients into foods. Trends Food Sci. Technol. 2009, 20, 388–395. [Google Scholar] [CrossRef]
- O’Grady, M.N.; Kerry, J.P. The effect of non-meat ingredients on quality parameters in meat and poultry. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Sawston, UK, 2010; Volume 23, pp. 701–725. [Google Scholar]
- Hart, M.R.; Quin, B.F.; Nguyen, M.L. Phosphorus runoff from agricultural land and direct fertilizer effects: A review. J. Environ. Qual. 2004, 33, 1954–1972. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Vieira, H.; Lourenço, H.; Gonçalves, S.; Martins, M.F.; Mendes, R. Control of phosphate levels in seafood products from the Portuguese market: Is there a need for concern? J. Food Compos. Anal. 2017, 62, 94–102. [Google Scholar] [CrossRef]
- Petracci, M.; Bianchi, M.; Mudalal, S.; Cavani, C. Functional ingredients for poultry meat products. Trends Food Sci. Technol. 2013, 33, 27–39. [Google Scholar] [CrossRef]
- GGoncalves, A.A.; Ribeiro, J.L.D. Do phosphates improve the seafood quality? Reality and legislation. Pan-Am. J. Aquat. Sci. 2008, 3, 237–247. [Google Scholar]
- Lampila, L.E. Functions and Uses of Phosphates in the Seafood Industry. J. Aquat. Prod. Technol. 1993, 1, 29–41. [Google Scholar] [CrossRef]
- Joly, G. Starches. In Ingredients in Meat Products: Properties, Functionality and Applications; Tarté, R., Ed.; Springer Science & Business Media: Berlin, Germany, 2009; pp. 41–55. [Google Scholar]
- Mills, E.W. Nonmeat binders for use in cook-in-bag and smoked ham. J. Muscle Foods 1995, 6, 23–35. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Elmore, A.R. Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics. Int. J. Toxicol. 2005, 24 (Suppl. S2), 51–111. [Google Scholar] [PubMed]
- Coglianese, M.; Neff, J.M. Evaluation of the ascorbic acid status of two estuarine crustaceans. The blue crab, Callinectes sapidus and the grass shrimp, Palaemonetes pugio. Comp. Biochem. Physiol. Part A Physiol. 1981, 68, 451–455. [Google Scholar] [CrossRef]
- Julio, C.; Karina, P.; Giselda, B.; Leda, G. The Use of Chloride, Citric and Ascorbic Acid Dip and Packaged Film to Extend the Shelf Life of Pejerrey Odonthested bonaerensis during Storage at Different Temperatures. Food Nutr. Sci. 2014, 5, 15. [Google Scholar] [CrossRef]
- Kilic, A.; Oztan, A. Effect of Ascorbic Acid Utilization on Cold Smoked Fish Quality (Oncorhynchus mykiss) during Process and Storage. Food Sci. Technol. Res. 2013, 19, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Nordic Committee on Food Analysis. Aerobic Count and Specific Spoilage Organisms in Fish and Fish Products. NKML Newsl. 2006, 184, 1–6. [Google Scholar]
- Gilbert, R.J.; Donovan, T.; Little, C.; Nye, K.; Ribeiro, C.D.; Richards, J.; Roberts, D.; Bolton, F.J. Guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of sale. Commun. Dis. Public Health 2000, 3, 163–167. [Google Scholar] [PubMed]
- Zapata, J.F.; Price, J.F. Evaluation of texture and water holding capacity in cooked minced fish. J. Food Process. Preserv. 1982, 6, 189–195. [Google Scholar] [CrossRef]
- Stevenson, J.R. Dynamics of the integument. In Integument, Pigments, and Hormonal Processes. The Biology of Crustacea; Academic Press: Cambridge, MA, USA, 1985; pp. 1–42. [Google Scholar]
- Martínez, M.A.; Velazquez, G.; Cando, D.; Núñez-Flores, R.; Borderías, A.J.; Moreno, H.M. Effects of high pressure processing on protein fractions of blue crab (Callinectes sapidus) meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 323–329. [Google Scholar] [CrossRef]
- Resconi, V.C.; Keenan, D.F.; Garcia, E.; Allen, P.; Kerry, J.P.; Hamill, R.M. The effects of potato and rice starch as substitutes for phosphate in and degree of comminution on the technological, instrumental and sensory characteristics of restructured ham. Meat Sci. 2016, 121, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atughonu, A.G.; Zayas, J.F.; Herald, T.J.; Harbers, L.H. Thermo-rheology, quality characteristics, and microstructure of frankfurters prepared with selected plant and milk additives1. J. Food Qual. 1998, 21, 223–238. [Google Scholar] [CrossRef]
- Tsai, S.; Unklesbay, N.; Unklesbay, K.; Clarke, A. Water and absorptive properties of restructured beef with five binders of four isothermal temperatures. LWT Food Sci. Technol. 1998, 31, 78–83. [Google Scholar] [CrossRef]
- Su, Y.K.; Bowers, J.A.; Zayas, J.F. Physical characteristics and microstructure of reduced-fat frankfurters as affected by salt and emulsified fats stabilized with non meat. proteins. J. Food Sci. 2000, 65, 128–132. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Jarmoluk, A. Effect of sodium caseinate and κ-carrageenan on binding and textural properties of pork muscle gels enhanced by microbial transglutaminase addition. Food Res. Int. 2003, 36, 285–294. [Google Scholar] [CrossRef]
- Petridis, D.; Ritzoulis, C.; Tzivanos, I.; Vlazakis, E.; Derlikis, E.; Patroklos, V. Effect of fat volume fraction, sodium caseinate, and starch on the optimization of the sensory properties of frankfurter sausages. Food Sci. Nutr. 2013, 1, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Quiroga Ledezma, C.C. Chapter 20—Starch Interactions With Native and Added Food Components A2—Sjöö, Malin, in Starch in Food, 2nd ed.; Nilsson, L., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 769–801. [Google Scholar]
- Maulvault, A.L.; Anacleto, P.; Lourenço, H.M.; Carvalho, M.L.; Nunes, M.L.; Marques, A. Nutritional quality and safety of cooked edible crab (Cancer pagurus). Food Chem. 2012, 133, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V.; Maulvault, A.L.; Alves, R.N.; Kwadijk, C.; Kotterman, M.; Tediosi, A.; Fernandez-Tejedor, M.; Sloth, J.J.; Granby, K.; Rasmussen, R.R.; et al. Effects of steaming on contaminants of emerging concern levels in seafood. Food Chem. Toxicol. 2018, 118, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Wiech, M.; Vik, E.; Duinker, A.; Frantzen, S.; Bakke, S.; Maage, A. Effects of cooking and freezing practices on the distribution of cadmium in different tissues of the brown crab (Cancer pagurus). Food Control 2017, 75, 14–20. [Google Scholar] [CrossRef]
- Zargar, M.; Yeganeh, S.; Razavi, S.H.; Ojagh, S.M. The Effect of Sodium Caseinate Coating Incorporated with Zataria moltiflora Essential Oil on the Quality and Shelf Life of Rainbow Trout During Refrigerated Storage. J. Aquat. Food Prod. Technol. 2016, 25, 1311–1322. [Google Scholar] [CrossRef]



| Mean | S.E.M 1 (n = 5) | Significance | |
|---|---|---|---|
| Water (control 1) | 11.1 | 2.21 | |
| AA 2 (control 2) | 8.0 | 4.86 | NS5 |
| SC 3 | 3.5 | 3.71 | p < 0.025 |
| PS 4 | 4.7 | 4.55 | p < 0.01 |
| SC + AA | 5.8 | 3.26 | p < 0.05 |
| PS + AA | 2.3 | 3.52 | p < 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDermott, A.; Whyte, P.; Brunton, N.; Lyng, J.; Bolton, D.J. Increasing the Yield of Irish Brown Crab (Cancer pagurus) during Processing without Adversely Affecting Shelf-Life. Foods 2018, 7, 99. https://doi.org/10.3390/foods7070099
McDermott A, Whyte P, Brunton N, Lyng J, Bolton DJ. Increasing the Yield of Irish Brown Crab (Cancer pagurus) during Processing without Adversely Affecting Shelf-Life. Foods. 2018; 7(7):99. https://doi.org/10.3390/foods7070099
Chicago/Turabian StyleMcDermott, Aoife, Paul Whyte, Nigel Brunton, James Lyng, and Declan J. Bolton. 2018. "Increasing the Yield of Irish Brown Crab (Cancer pagurus) during Processing without Adversely Affecting Shelf-Life" Foods 7, no. 7: 99. https://doi.org/10.3390/foods7070099




