Improvements on the Stability and Vitamin Content of Acerola Juice Obtained by Ultrasonic Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Ultrasonic Processing and Experimental Design
2.3. Determination of Vitamins
2.4. Cloud Stability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Vitamin Content
3.2. Cloud Stability
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Montilla, A.; Soria, A.C.; Villamiel, M. Effects of conventional and ultrasound blanching on enzyme inactivation and carbohydrate content of carrots. Eur. Food. Res. Technol. 2012, 234, 1071–1079. [Google Scholar] [CrossRef]
- Jang, J.-H.; Moon, K.-D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
- Lee, H.; Zhou, B.; Feng, H.; Martin, S.E. Effect of pH on inactivation of escherichia coli K12 by sonication, manosonication, thermosonication, and manothermosonication. J. Food Sci. 2009, 74, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.G.M.; Fonteles, T.V.; Jesus, A.L.T.; Almeida, F.D.L.; Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. High-Intensity Ultrasound Processing of Pineapple Juice. Food Bioprocess Technol. 2011, 6, 997–1006. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. Power ultrasound processing of cantaloupe melon juice: Effects on quality parameters. Food Res. Int. 2012, 48, 41–48. [Google Scholar] [CrossRef]
- Anese, M.; Mirolo, G.; Beraldo, P.; Lippe, G. Effect of ultrasound treatments of tomato in vitro bioaccessibility. Food Chem. 2013, 136, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Ball, G.F.M. Vitamins in Foods: Analysis, Bioavailability, and Stability; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Fernandes, F.A.N.; Rodrigues, S.; Cárcel, J.A.; García-Pérez, J.V. Ultrasound-Assisted Air-Drying of Apple (Malus domestica L.) and Its Effects on the Vitamin of the Dried Product. Food Bioprocess Technol. 2015, 8, 1503–1511. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S.; García-Pérez, J.V.; Cárcel, J.A. Effects of Ultrasound-Assisted Air Drying on Vitamins and Carotenoids of Cherry Tomatoes. Dry Technol. 2016, 34, 986–996. [Google Scholar] [CrossRef]
- USDA. USDA National Nutrient Database for Standard Reference. Release 28; US Department of Agriculture: Washington, DC, USA, 2015.
- Jedlicka, A.; Klimes, J. Determination of Water- and Fat-Soluble Vitamins in Different Matrices Using High-Performance Liquid Chromatography. Chem. Pap. 2005, 59, 202–222. [Google Scholar] [CrossRef]
- Rizzolo, A.; Polesello, S. Chromatographic determination of vitamins in foods. J. Chromatogr. 1992, 624, 103–152. [Google Scholar] [CrossRef]
- Selimović, A.; Salkić, M.; Selimović, A. Direct Spectrophotometric Determination of l—Ascorbic acid in Pharmaceutical Preparations using Sodium Oxalate as a Stabilizer. Int. J. Basic Appl. Sci. 2011, 11, 106–109. [Google Scholar]
- Versteeg, C.; Rombouts, F.M.; Spaansen, C.H.; Pilnik, W. Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. J. Food Sci. 1980, 45, 969–971. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications, 2nd ed.; Ellis Horwood Publishers: New York, NY, USA, 2002; p. 166. [Google Scholar]
- Wall, J.S.; Carpenter, K.J. Variation in availability of niacin in grain products. Food Technol. 1988, 42, 198–204. [Google Scholar]
- Ghosh, H.P.; Sarkar, P.K.; Guha, B.C. Distribution of the bound form of nicotinic acid in natural materials. J. Nutr. 1963, 79, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Plesovsky-Vig, N. Pantothenic acid. In Modern Nutrition in Health and Disease, 9th ed.; Shils, M.E., Olson, J.A., Shike, M., Ross, A.C., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1999; p. 423. [Google Scholar]
- Fernandes, F.A.N.; Oliveira, V.S.; Gomes, W.F.; Rodrigues, S. Degradation kinetics of vitamin E during ultrasound application and the adjustment in avocado puree by tocopherol acetate addition. LWT Food Sci. Technol. 2016, 69, 342–347. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Inactivation kinetics of pectin methylesterase and cloud retention in sonicated orange juice. Innov. Food Sci. Emerg. Technol. 2009, 10, 166–171. [Google Scholar] [CrossRef]


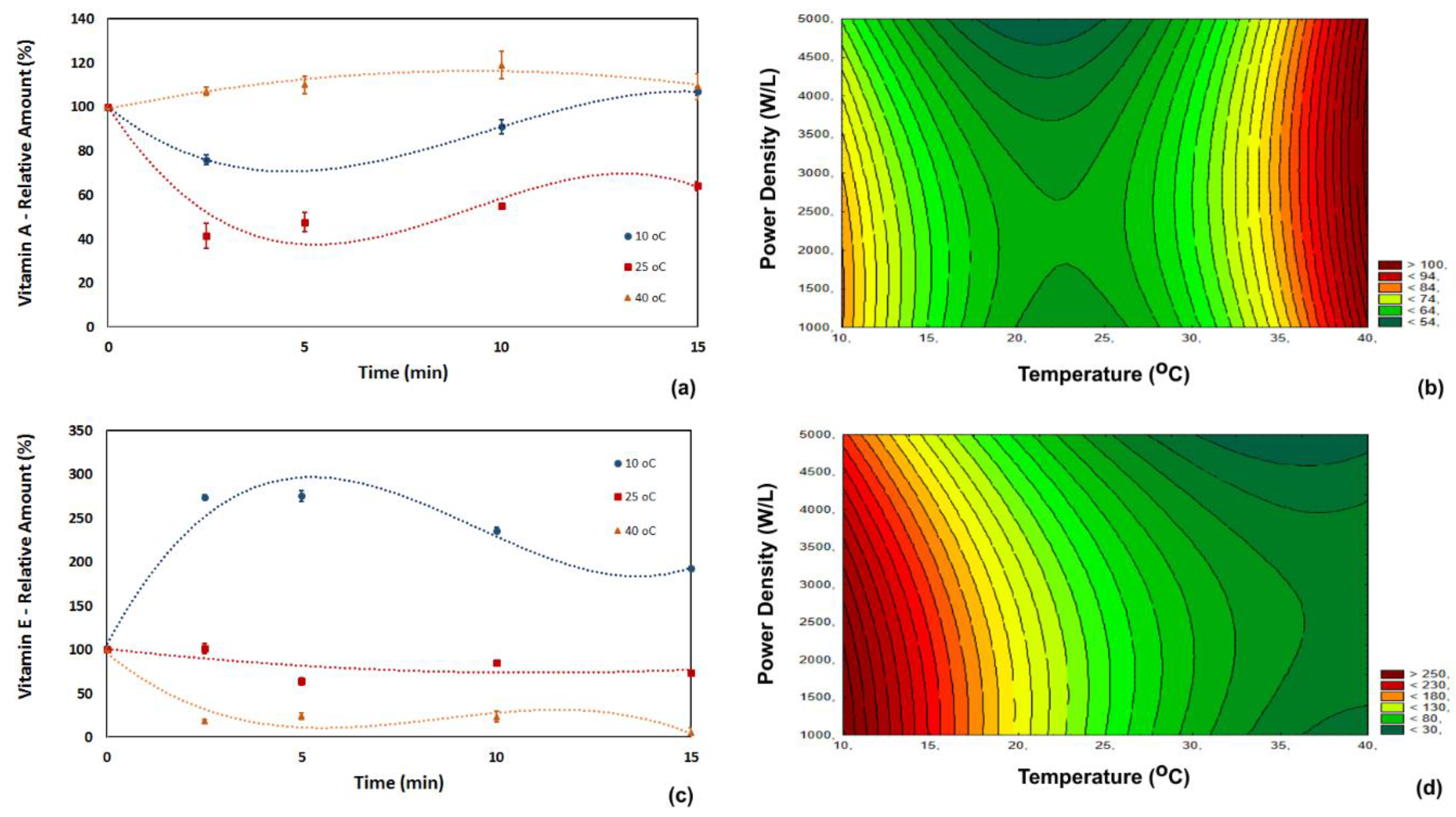
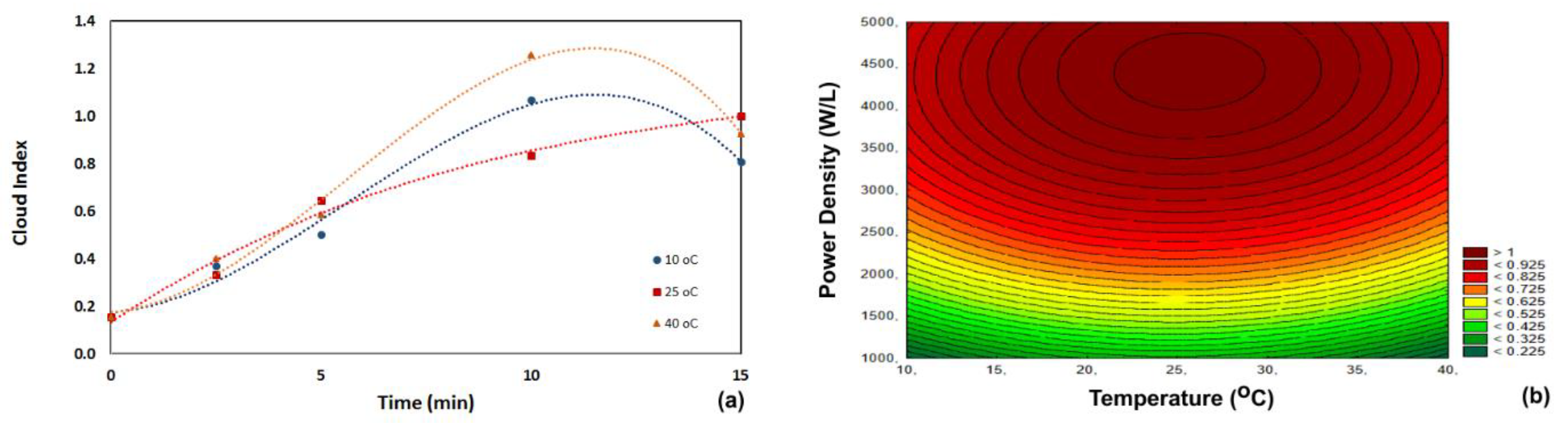
| Run | Power Density (W/L) | Temperature (°C) | Time (min) |
|---|---|---|---|
| 1 | 1000 | 10 | 2.5, 5, 10, 15 |
| 2 | 1000 | 25 | 2.5, 5, 10, 15 |
| 3 | 1000 | 40 | 2.5, 5, 10, 15 |
| 4 | 3000 | 10 | 2.5, 5, 10, 15 |
| 5 (C) | 3000 | 25 | 2.5, 5, 10, 15 |
| 6 | 3000 | 40 | 2.5, 5, 10, 15 |
| 7 | 5000 | 10 | 2.5, 5, 10, 15 |
| 8 | 5000 | 25 | 2.5, 5, 10, 15 |
| 9 | 5000 | 40 | 2.5, 5, 10, 15 |
| 10 (C) | 3000 | 25 | 2.5, 5, 10, 15 |
| Factor | Effect | Standard Error | p |
|---|---|---|---|
| Vitamin B2 | |||
| Mean | 77.91 | 1.26 | 0 |
| Temperature | 19.62 | 2 | 0.0002 |
| Temperature 2 | 27.77 | 3.09 | 0.0003 |
| Power Density | 6.45 | 2.01 | 0.0237 |
| Power Density 2 | −1.84 | 3.09 | 0.578 |
| Temp × Power Density | −1.2 | 2.46 | 0.6455 |
| Vitamin B3 | |||
| Mean | 74.04 | 2.22 | 0 |
| Temperature | 39.92 | 3.53 | 0.0001 |
| Temperature 2 | 17.05 | 5.43 | 0.0257 |
| Power Density | 15.3 | 3.52 | 0.0075 |
| Power Density 2 | 2.67 | 5.43 | 0.6437 |
| Temp × Power Density | 6.34 | 3.32 | 0.2026 |
| Vitamin B5 | |||
| Mean | 58.76 | 1.77 | 0 |
| Temperature | 45.96 | 2.83 | 0.0001 |
| Temperature 2 | 44.15 | 4.34 | 0.0002 |
| Power Density | −4.2 | 2.82 | 0.1965 |
| Power Density 2 | 6.19 | 4.34 | 0.2132 |
| Temp × Power Density | 4.62 | 3.45 | 0.2389 |
| Factor | Effect | Standard Error | p |
|---|---|---|---|
| Vitamin C | |||
| Mean | 96.67 | 3.26 | 0 |
| Temperature | −8.54 | 5.18 | 0.1602 |
| Temperature 2 | 18.15 | 7.97 | 0.0718 |
| Power Density | −5.35 | 5.18 | 0.349 |
| Power Density 2 | −11.55 | 7.97 | 0.2072 |
| Temp × Power Density | −8.54 | 6.34 | 0.2363 |
| Pro-vitamin A | |||
| Mean | 55.87 | 5.23 | 0.0001 |
| Temperature | 6.88 | 8.33 | 0.4468 |
| Temperature 2 | 90.74 | 12.82 | 0.0009 |
| Power Density | 11.13 | 8.34 | 0.2393 |
| Power Density 2 | 3.59 | 12.84 | 0.7907 |
| Temp × Power Density | −12.59 | 10.2 | 0.272 |
| Vitamin E | |||
| Mean | 95.48 | 10.94 | 0.0003 |
| Temperature | −207.21 | 17.42 | 0.0001 |
| Temperature 2 | 113.59 | 26.8 | 0.0082 |
| Power Density | −47.68 | 17.42 | 0.0409 |
| Power Density 2 | −37.93 | 26.81 | 0.2162 |
| Temp × Power Density | 38.18 | 21.33 | 0.1335 |
| Factor | Effect | Standard Error | p |
|---|---|---|---|
| Mean | 0.902 | 0.07 | 0.001 |
| Temperature | 0.01 | 0.076 | 0.9022 |
| Temperature 2 | −0.289 | 0.132 | 0.1157 |
| Power Density | 0.651 | 0.077 | 0.0034 |
| Power Density 2 | −0.437 | 0.131 | 0.0454 |
| Temp × Power Density | 0.025 | 0.093 | 0.8098 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, V.O.; Rodrigues, S.; Fernandes, F.A.N. Improvements on the Stability and Vitamin Content of Acerola Juice Obtained by Ultrasonic Processing. Foods 2018, 7, 68. https://doi.org/10.3390/foods7050068
Santos VO, Rodrigues S, Fernandes FAN. Improvements on the Stability and Vitamin Content of Acerola Juice Obtained by Ultrasonic Processing. Foods. 2018; 7(5):68. https://doi.org/10.3390/foods7050068
Chicago/Turabian StyleSantos, Valéria O., Sueli Rodrigues, and Fabiano A. N. Fernandes. 2018. "Improvements on the Stability and Vitamin Content of Acerola Juice Obtained by Ultrasonic Processing" Foods 7, no. 5: 68. https://doi.org/10.3390/foods7050068





