Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cladodes Harvest and Preparation
2.2. Proximate Composition of Cladodes
2.3. Determination of β-polysaccharides Content
2.4. Extraction and Profiling of Phenolic Compounds from Cladodes
2.5. Assay of DPPH Radical Scavenging Activity
2.6. Assay of FRAP Reducing Antioxidant Power
2.7. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition, Total Phenolics and β-polysaccharides Contents
3.2. In vitro Antioxidant Activity of Cladodes
3.3. Evaluation of Phenolic Profile by UHPLC-ESI-QTOF-MS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Houérou, H.N. Cacti (Opuntia spp.) as a fodder crop for marginal lands in the Mediterranean basin. Proceedings of the 4th International Congress on cactus pear and cochineal. Acta Hortic. 2002, 581, 21–46. [Google Scholar] [CrossRef]
- Fernandez-Lopez, J.A.; Almela, L.; Obón, J.M.; Castellar, M.R. Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, H.R.; Kim, J.; Jang, Y.S. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J. Agric. Food. Chem. 2002, 50, 6490–6496. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.G.; Arias, E. Introduction in: Cactus (Opuntia spp.) as Forage; Mondragon-Jacobo, C., Ed.; Food and Agriculture Organization (FAO): Rome, Italy, 2001; pp. 1–4. [Google Scholar]
- Gutierrez, M.A. Medicinal use of the latin food staple nopales: The rickly pear cactus. Nutr. Bytes 1998, 4, 1–3. [Google Scholar]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and medicinal use of cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Mondello, M.R.; Monforte, M.T.; Galluzzo, M.; Miceli, N.; Tripodo, M.M. Effect of Opuntia ficus-indica (L.) Mill. cladodes in wound-healing process. J. Prof. Assoc. Cactus. 2003, 5, 1–16. [Google Scholar]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Santini, A. Nutraceuticals: An healthy bet for the future. J. Food Res. 2014, 3, 1–2. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Abdelmaksoud, W.; Ennour, M.; Attia, H. Cladodes from Opuntia ficus-indica as a source of dietary fiber. Effect on dough characteristic and cake making. Ind. Crops Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Lovergrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goni, I. By-products of Opuntia ficus-indica as a source of antioxidant dietary fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Knaze, V.; Zamora-Rosa, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care. 2017, 20, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Gallo, M.; Le Grottaglie, L.; Scala, C.; Ferrara, L.; Santini, A. Determination of cholesterol in Italian chicken eggs. Food Chem. 2012, 132, 701–708. [Google Scholar] [CrossRef]
- Del Socorro Santos Díaz, M.; Barba de la Rosa, A.-P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and benefits in chronic diseases. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.R.; Achour, L. Seeds of Opuntia spp. as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Ind. Crops Prod. 2017, 65, 383–389. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profile and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef]
- Dok-Go, H.; Lee, K.H.; Kim, H.J.; Lee, E.H.; Lee, J.; Song, Y.S.; Lee, Y.H.; Jin, C.; Lee, Y.S.; Cho, J. Neuroprotective effects of antioxidative flavonoids quercetin, β-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. Saboten. Brain Res. 2003, 965, 130–136. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Diary Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Eberendu, A.R.; Luta, G.; Edwards, J.A.; Mcanalley, B.H.; Davis, B.; Rodriguez, S.; Ray Henry, C. Quantitative colorimetric analysis of Aloe polysaccharides as a measure of Aloe Vera quanlity in commercial products. J. AOAC Int. 2005, 88, 684–691. [Google Scholar] [PubMed]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical constituents and in vitro eadical scavening activity of different Aloe species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Masoero, F.; Trevisan, M.; Lucini, L. Evaluation of phenolic profile and antioxidant capacity in gluten-free flours. Food Chem. 2017, 228, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M'Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- De Santiago, E.; Domínguez-Fernández, M.; Cid, C.; De Pena, M.-P. Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus-indica). Food Chem. 2018, 240, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.W.; De León-Rodríguez, A.; Fomsgaard, I.S.; Barba de la Rosa, A.P. Proximate composition, penolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Comp. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Skerget, M.; Kotnik, P.; Hadolin, M.; Hras, A.; Simonic, M.; Knez, Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Montesano, D.; Gennari, O.; Seccia, S.; Albrizio, S. A simple and selective analytical procedure for the extraction and quantification of lutein from tomato by-products by HPLC-DAD. Food Anal. Methods. 2012, 5, 710–715. [Google Scholar] [CrossRef]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. J. Food Comp. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Santos-Zea, L.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Comparative analyses of total phenols, antioxidant activity, and flavonol glycoside profile of cladode flours from different varieties of Opuntia spp. J. Agric. Food Chem. 2011, 59, 7054–7061. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, E.; Córdoba-Díaz, D.; de Cortes Sánchez-Mata, M.; Díez-Marqués, C.; Honi, I. Effect of boiling on nutritional, antioxidant and physicochemical characteristics in cladodes (Opuntia ficus-indica). LWT Food Sci. Technol. 2013, 51, 296–302. [Google Scholar] [CrossRef]
- Reyes-Agüero, J.A.; Aguirre-Rivera, J.R.; Hernández, H.M. Systematic notes and a detailed description of Opuntia ficus-indica (L.) Mill. (CACTACEAE). Agrociencia. 2005, 39, 395–408. [Google Scholar]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- McClearly, B.V.; Prosky, L. Advanced Dietary Fiber Technology; Blackwell Science: Ames, IW, USA, 2001. [Google Scholar]
- Cardarelli, M.; Rouphael, Y.; Rea, E.; Lucini, L.; Pellizzoni, M.; Colla, G. Effects of fertilization, arbuscolar myxorrhiza, and salinity on growth, yield, and bioactive compounds of two Alow species. HortScience 2013, 48, 568–575. [Google Scholar]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Astello-García, M.; Cervantes, I.; Nair, V.; del Socorro Santos-Díaz, M.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Comp. Anal. 2015, 43, 119–130. [Google Scholar]
- Ghisoni, S.; Chiodelli, G.; Rocchetti, G.; Kane, D.; Lucini, L. UHPLC-ESI-QTOF-MS screening of lignans and other phenolics in dry seeds for human consumption. J. Funct. Foods. 2017, 34, 229–236. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanism: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
| Parameters | San Cono cladodes |
|---|---|
| Moisture (g water/100 g cladodes) | 92.33 ± 1.36 |
| Protein (%) | 0.58 ± 0.02 |
| Ash (%) | 0.50 ± 0.01 |
| Lipid (%) | 0.12 ± 0.02 |
| Carbohydrates (%) * | 3.05 |
| NDF (%) | 3.42 ± 0.63 |
| ADF (%) | 0.83 ± 0.14 |
| ADL (%) | 0.12 ± 0.02 |
| β-Polysaccharides | TPC | DPPH | FRAP | |
|---|---|---|---|---|
| (mg β-Glucan Equivalents/kg FW) | (mg GAE/kg FW) | (mg GAE/kg FW) | (mg GAE/kg FW) | |
| Cladodes | 2617.39 ± 225.58 | 2633.10 ± 214.78 | 1040.03 ± 112.42 | 1638.17 ± 41.30 |
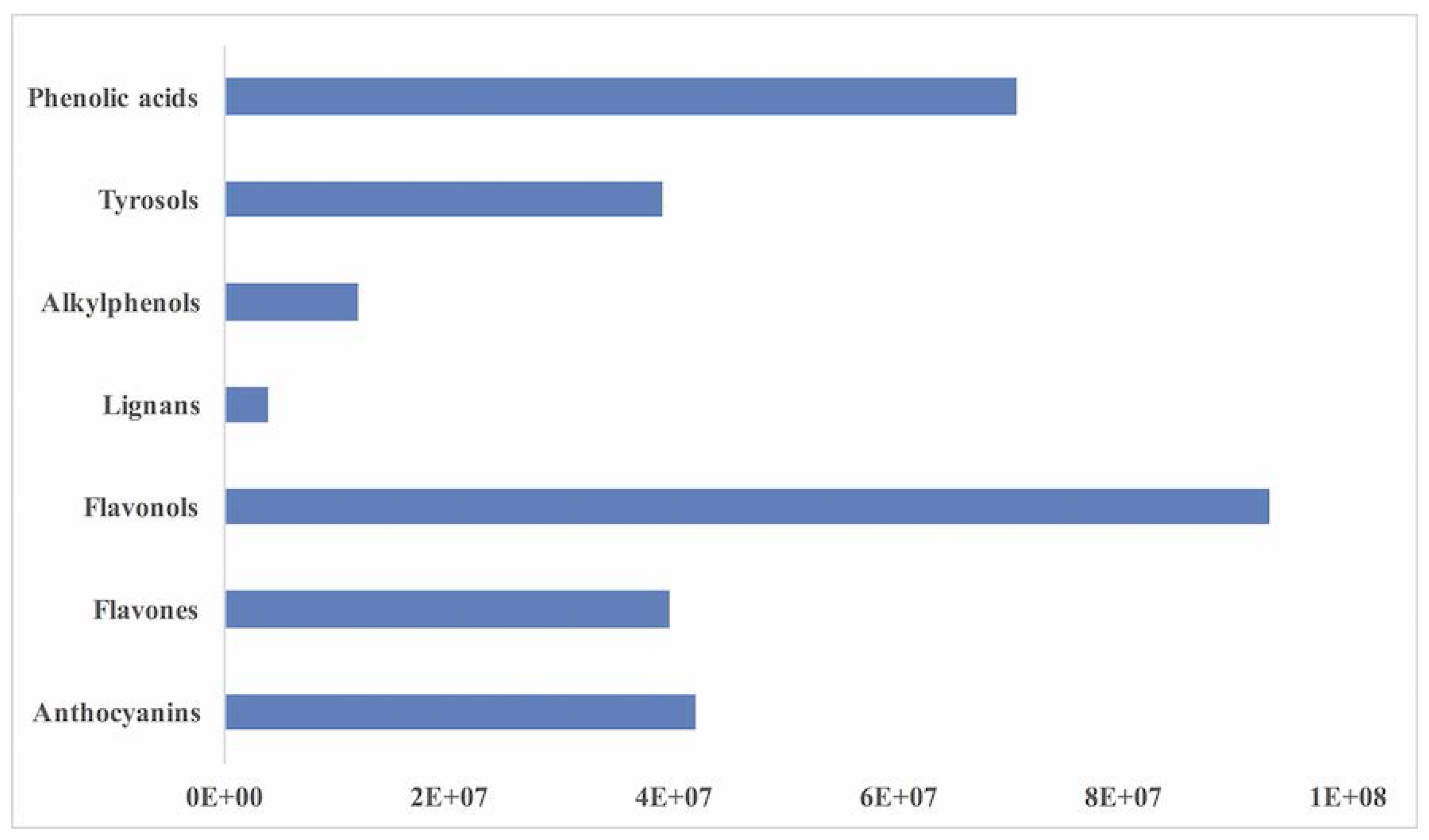
| Phenolic Class | Phenolic Derivatives | mg/kg Equivalents |
|---|---|---|
| Flavonoids—Anthocyanins | Cyanidin-Glu | 1058.55 ± 10.49 |
| Pelargonidin-Glu | 187.97 ± 10.42 | |
| Petunidin-Glu | 186.55 ± 3.11 | |
| Delphinidin-Glu | 2.81 ± 0.31 | |
| Malvidin-Glu | 4.31 ± 0.08 | |
| Flavonoids—Flavones | Luteolin-Glu | 5.14 ± 1.59 |
| Apigenin-Glu | 40.69 ± 0.58 | |
| Isoflavonoids | 6.81 ± 0.52 | |
| Flavonoids—Flavonols | Myricetin-Glu | 8.52 ± 0.55 |
| Quercetin-Glu | 8.97 ± 0.69 | |
| Kaempferol-Glu | 241.68 ± 3.39 | |
| Isorhamnetin-Glu | 98.42 ± 8.52 | |
| Lignans | Furofurans | 6.52 ± 2.24 |
| Dibenzylbutyrolactone | 3.47 ± 0.02 | |
| Other phenolics | Alkylphenols | 65.04 ± 10.43 |
| Hydroxybenzaldehydes | 0.43 ± 0.06 | |
| Hydroxycoumarins | 7.81 ± 0.52 | |
| Tyrosols | 12.89 ± 1.02 | |
| Phenolic acids | Hydroxybenzoics | 114.01 ± 3.34 |
| Hydroxyphenylpropanoics | 91.58 ± 5.98 | |
| Hydroxycinnamics | 1248.24 ± 103.15 |
| DPPH | FRAP | TPC | Cyanidin Eq. | Luteolin Eq. | Catechin Eq. | Ferulate Eq. | Matairesinol Eq. | Tyrosol Eq. | Cardol Eq. | |
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | 1 | n.s. | 1 ** | 0.84 * | 0.95 ** | 0.92 * | 0.96 ** | n.s. | n.s. | 0.59 |
| FRAP | n.s. | 1 | n.s. | −0.74 | n.s. | n.s. | n.s. | 0.90 | −0.84 | −0.61 |
| TPC | 1 ** | n.s. | 1 | −0.84 | 0.95 | −0.92 | 0.96 ** | n.s. | n.s. | 0.59 |
| Cyanidin Eq. | 0.84 * | −0.74 | −0.84 | 1 | −0.64 | 0.56 | −0.65 | n.s. | n.s. | n.s. |
| Luteolin Eq. | 0.95 ** | n.s. | 0.95 | −0.64 | 1 | −0.99 * | 1 * | −0.45 | 0.56 | 0.80 |
| Catechin Eq. | 0.92 * | n.s. | −0.92 | 0.56 | −0.99 * | 1 | −0.99 | 0.54 | −0.64 | −0.86 |
| Ferulate Eq. | 0.96 ** | n.s. | 0.96 ** | −0.65 | 1 * | −0.99 | 1 | −0.44 | 0.54 | 0.79 |
| Matairesinol Eq. | n.s. | 0.90 | n.s. | n.s. | −0.45 | 0.54 | −0.44 | 1 | −0.99 | −0.89 |
| Tyrosol Eq. | n.s. | −0.84 | n.s. | n.s. | 0.56 | −0.64 | 0.54 | −0.99 | 1 | 0.94 |
| Cardol Eq. | 0.59 | −0.61 | 0.59 | n.s. | 0.80 | −0.86 | 0.79 | −0.89 | 0.94 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods 2018, 7, 24. https://doi.org/10.3390/foods7020024
Rocchetti G, Pellizzoni M, Montesano D, Lucini L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods. 2018; 7(2):24. https://doi.org/10.3390/foods7020024
Chicago/Turabian StyleRocchetti, Gabriele, Marco Pellizzoni, Domenico Montesano, and Luigi Lucini. 2018. "Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties" Foods 7, no. 2: 24. https://doi.org/10.3390/foods7020024








