Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food
Abstract
:1. Introduction
Determination of the SFE: Theoretical Information
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Surface Tension of the Test Liquids
2.2.2. Validation of the Novel Method
2.2.3. Case Studies
2.2.4. Statistical Evaluation
3. Results
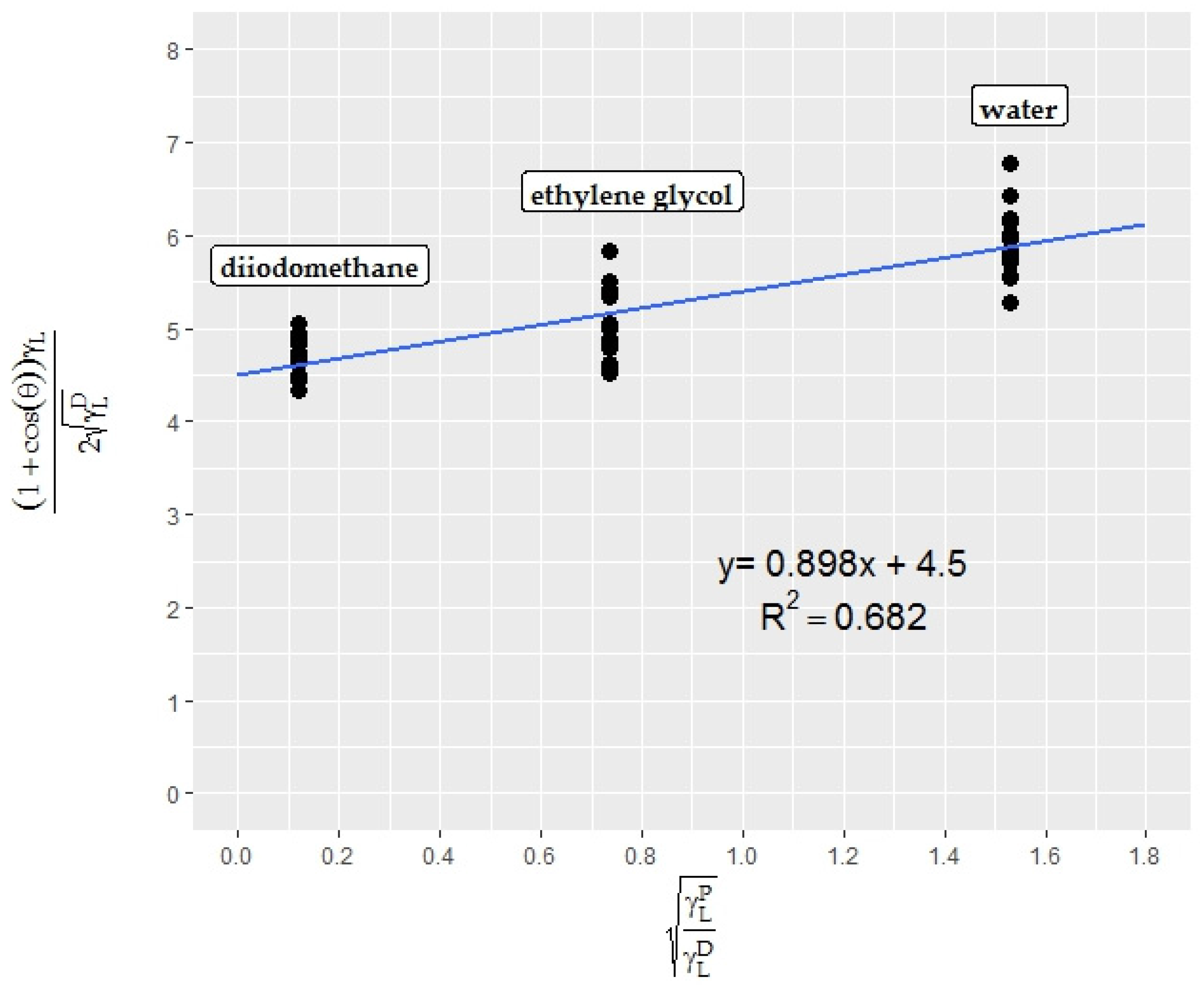
3.1. Surface Tension of the Test Liquids
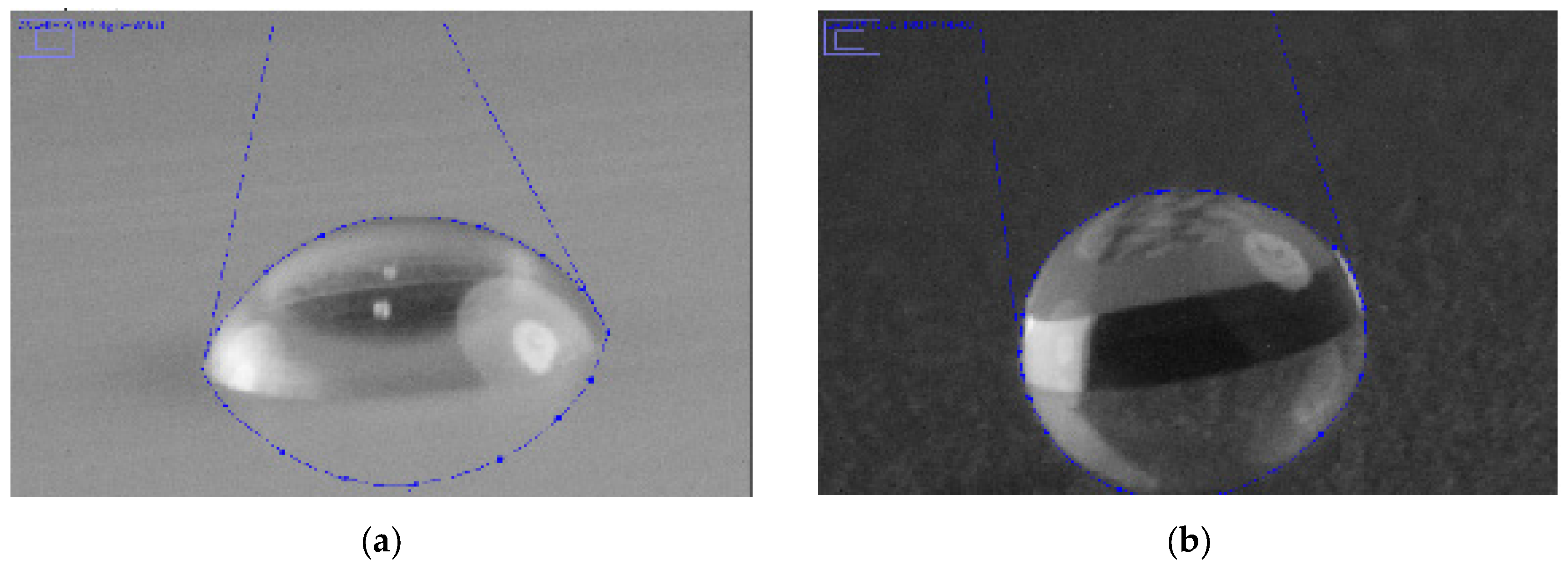
3.2. Validation of the Snake-Based Method
3.2.1. Contact Angle Measurement
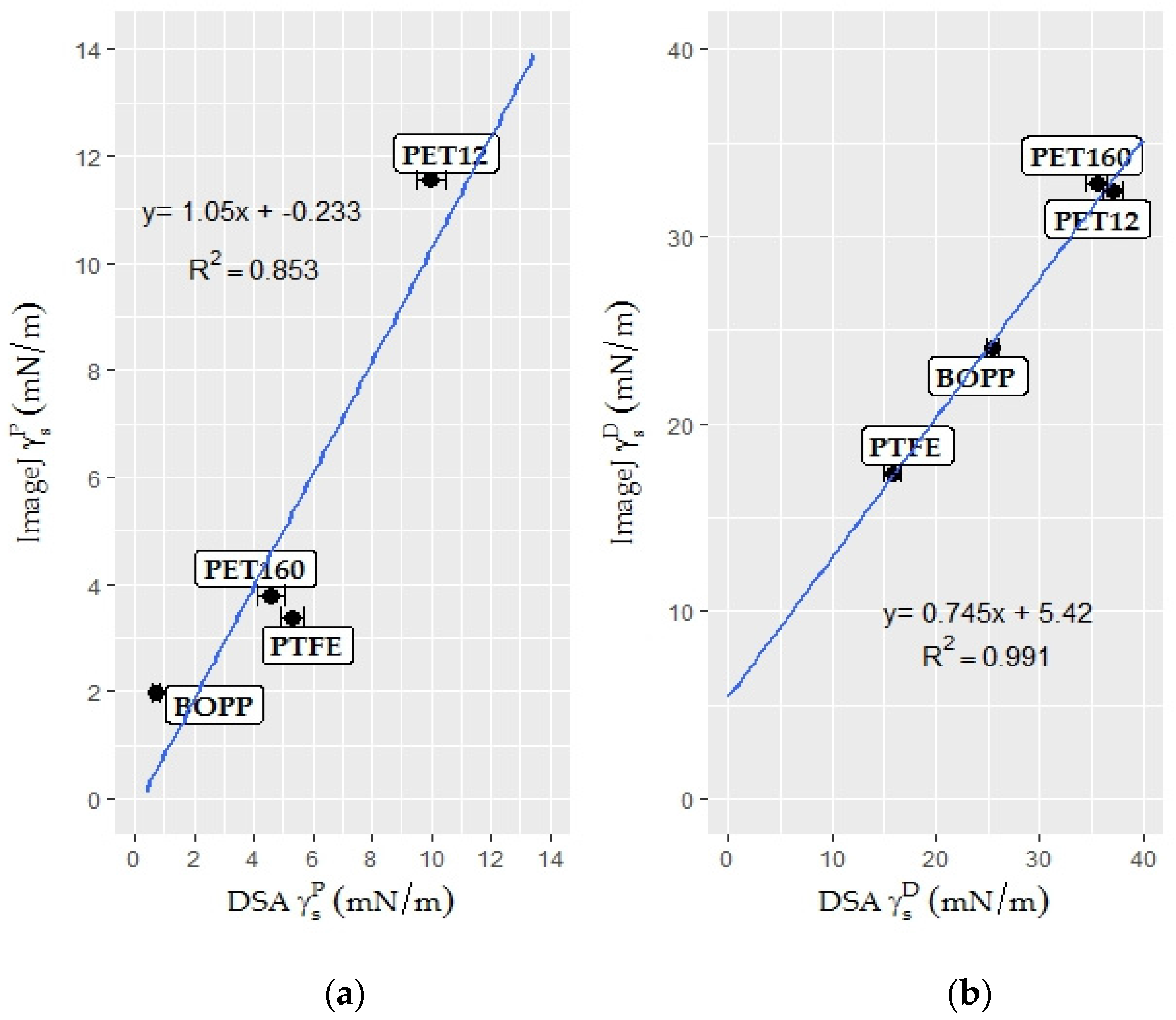
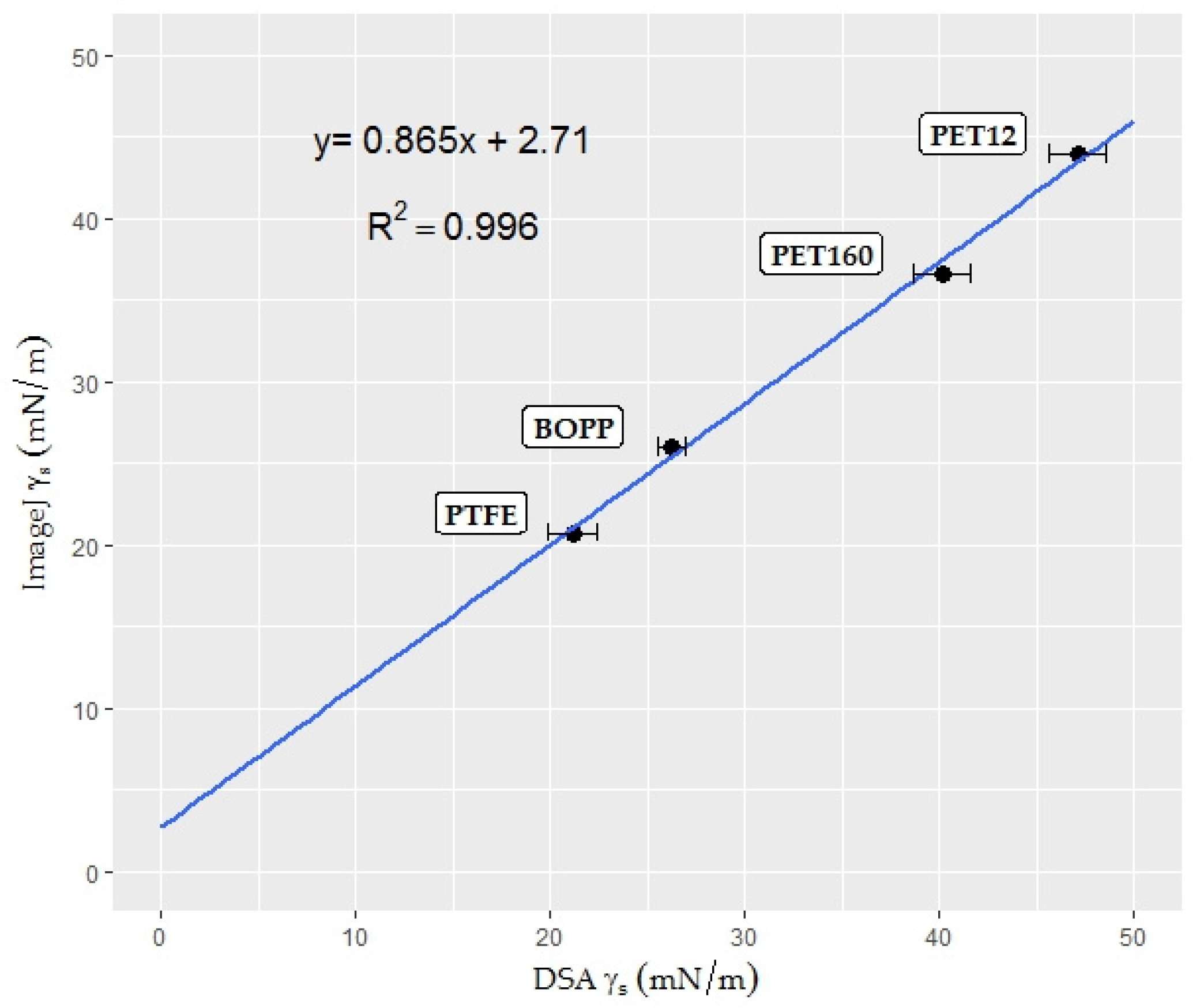
3.2.2. Surface Free Energy Measurement by the Polar—Dispersive Approach
3.3. Case Studies
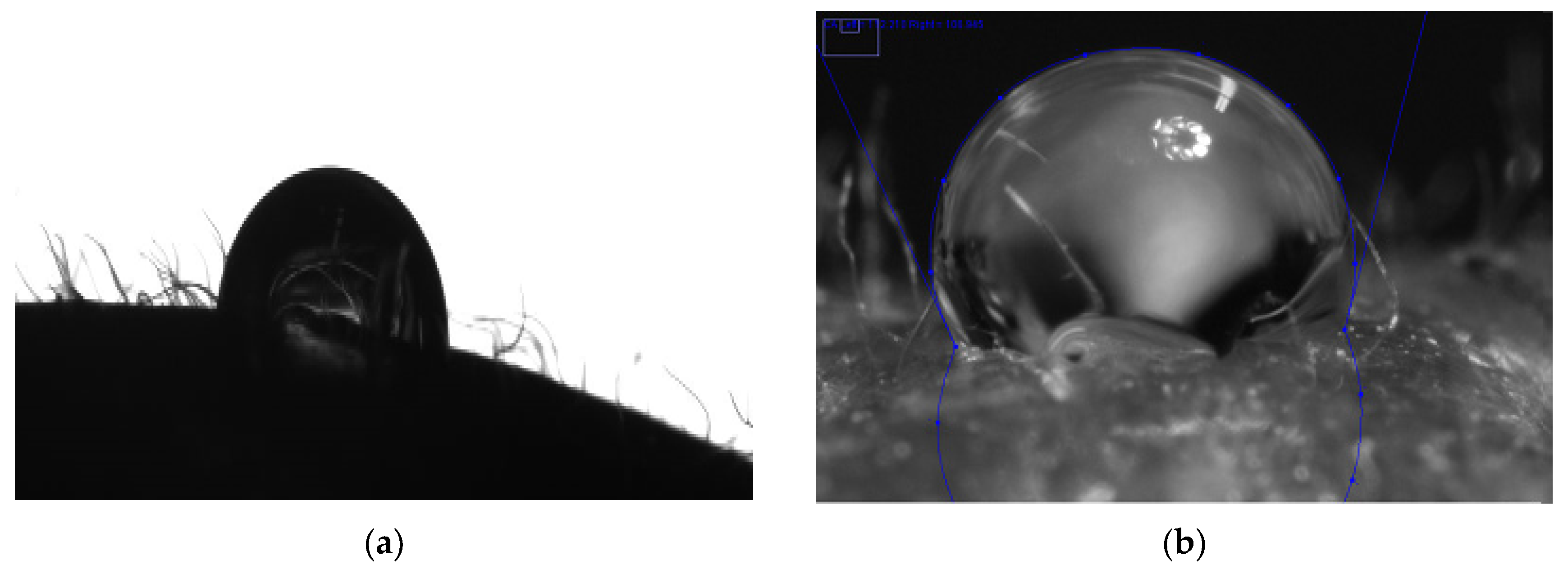
3.3.1. Strawberry
3.3.2. Endive Salad
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Yildirim, I. Surface free energy characterization of powders. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2001. [Google Scholar]
- Meiron, T.S.; Marmur, A.; Saguy, I.S. Contact angle measurement on rough surfaces. J. Colloid Interface Sci. 2004, 274, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, 3rd ed.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Zenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Lugscheider, E.; Bobzin, K. The influence on surface free energy of PVD-coatings. Surf. Coat. Tech. 2001, 142–144, 755–760. [Google Scholar] [CrossRef]
- Ribeiro, C.; Vicente, A.A.; Teixeira, J.A.; Miranda, C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol. Technol. 2007, 44, 63–70. [Google Scholar] [CrossRef]
- Skurtys, O.; Velásquez, P.; Henriquez, O.; Matiacevich, S.; Enrione, J.; Osorio, F. Wetting behavior of chitosan solutions on blueberry epicarp with or without epicuticular waxes. LWT—Food Sci. Technol. 2011, 44, 1449–1457. [Google Scholar] [CrossRef]
- Aydt, T.P.; Weller, C.L.; Testin, R.F. Mechanical and barrier properties of edible corn and wheat protein films. Am. Soc. Agric. Eng. 1991, 34, 207–211. [Google Scholar] [CrossRef]
- Polymers Seyedi, S.; Koocheki, A.; Mohebbi, M.; Zahedi, Y. Lepidium perfoliatum seed gum: A new source of carbohydrate to make a biodegradable film. Carbohydr. Polym. 2014, 101, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.D.; Hollingsworth, R.G.; Leroux, E.; Salmieri, S.; Lacroix, M. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res. Int. 2011, 44, 198–203. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Orts, W. Edible Films and Coatings: Why, What, and How? In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar]
- Han, J.H. 4—Antimicrobial food packaging A2—Ahvenainen, Raija. In Novel Food Packaging Techniques; Woodhead Publishing: Cambridge, UK, 2003; pp. 50–70. [Google Scholar]
- Choi, W.Y.; Park, H.J.; Ahn, D.J.; Lee, J.; Lee, C.Y. Wettability of Chitosan Coating Solution on ’Fuji’ Apple Skin. J. Food Sci. 2002, 67, 2668–2672. [Google Scholar] [CrossRef]
- Ramírez, C.; Gallegos, I.; Ihl, M.; Bifani, V. Study of contact angle, wettability and water vapor permeability in carboxymethylcellulose (CMC) based film with murta leaves (Ugni molinae Turcz) extract. J. Food Eng. 2012, 109, 424–429. [Google Scholar] [CrossRef]
- Park, H.J. Development of advanced edible coatings for fruits. Trends Food Sci. Technol. 1999, 10, 254–260. [Google Scholar] [CrossRef]
- Flores, Z.; San Martín, D.; Ricardo, V.C.; Tabilo-Munizaga, G.; Osorio, F.; Javier, L.V. Physicochemical characterization of chitosan-based coating-forming emulsions: Effect of homogenization method and carvacrol content. Food Hydrocoll. 2016, 61, 851–857. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. The Use of Electric Fields for Edible Coatings and Films Development and Production: A Review. Food Eng. Rev. 2010, 2, 244–255. [Google Scholar] [CrossRef]
- Kaelble, D.H. Physical Chemistry of Adhesion; John Wiley: New York, NY, USA, 1971. [Google Scholar]
- Rulison, C. Models for Surface Free Energy Calculation. 1999. Available online: https://www.kruss.de/fileadmin/user_upload/website/literature/kruss-tn306-en.pdf (accessed on 20 April 2017).
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Dos Santos, E.A.; Farina, M.; Soares, G.A.; Anselme, K. Surface energy of hydroxyapatite and β-tricalcium phosphate ceramics driving serum protein adsorption and osteoblast adhesion. J. Mater. Sci. Mater. Med. 2007, 19, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Hejda, F.; Solar, P.; Kousal, J. Surface Free Energy Determination by Contact Angle Measurements—A Comparison of Various Approaches. Available online: https://www.mff.cuni.cz/veda/konference/wds/proc/pdf10/WDS10_304_f4_Hejda.pdf (accessed on 20 April 2017).
- Kruss GmbH. Pendant Drop. Available online: https://www.kruss.de/services/education-theory/glossary/pendant-drop/ (accessed on 8 February 2017).
- ImageJ image processing and analysis in java. Available online: https://imagej.nih.gov/ij/ (accessed on 23 July 2016).
- Stalder, A. Drop Shape Analysis. Available online: http://bigwww.epfl.ch/demo/dropanalysis/ (accessed on 29 July 2016).
- Williams, D.L.; Kuhn, A.T.; Amann, M.A.; Hausinger, M.B.; Konarik, M.M.; Nesselrode, E.I. Computerised Measurement of Contact Angles. Galvanotechnik 2010, 101, 2502–2512. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Auguie, B. gridExtra: Miscellaneous Functions for "Grid" Graphics. Available online: https://rdrr.io/cran/gridExtra/ (accessed on 29 February 2017).
- Slowikowski, K. ggrepel: Repulsive Text and Label Geoms for ‘ggplot2’. Available online: https://rdrr.io/cran/ggrepel/ (accessed on 24 November 2016).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Navarro, D. Learning Statistics with R: A Tutorial for Psychology Students and Other Beginners, (Version 0.5); University of Adelaide: Adelaide, Australia, 2015. [Google Scholar]
- Nguyen, T.; Johns, W. E. Polar and dispersion force contributions to the total surface free energy of wood. Wood Sci. Technol. 1978, 12, 63–74. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Influence of Surface Roughness on Superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef] [PubMed]
- Guimond, S.; Radu, I.; Czeremuszkin, G.; Carlsson, D.J.; Wertheimer, M.R. Biaxially Oriented Polypropylene (BOPP) Surface Modification by Nitrogen Atmospheric Pressure Glow Discharge (APGD) and by Air Corona. Plasmas Polym. 2002, 7, 71–88. [Google Scholar] [CrossRef]
- Wang, J.; Huang, N.; Yang, P.; Leng, Y.X.; Sun, H.; Liu, Z.Y.; Chu, P.K. The effects of amorphous carbon films deposited on polyethylene terephthalate on bacterial adhesion. Biomaterials 2004, 25, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.; Qunji, X. Surface modification of PTFE by 60Co γ-ray irradiation. J. Appl. Polym. Sci. 1998, 69, 435–441. [Google Scholar] [CrossRef]
- Kako, T.; Nakajima, A.; Irie, H.; Kato, Z.; Uematsu, K.; Watanabe, T.; Hashimoto, K. Adhesion and sliding of wet snow on a super-hydrophobic surface with hydrophilic channels. J. Mater. Sci. 2004, 39, 547–555. [Google Scholar] [CrossRef]
- Velásquez, P.; Skurtys, O.; Enrione, J.; Osorio, F. Evaluation of Surface Free Energy of Various Fruit Epicarps Using Acid–Base and Zisman Approaches. Food Biophys. 2011, 6, 349–358. [Google Scholar] [CrossRef]
- Hoffman, A.S. Lecture on Contact Angles. 2005. Available online: https://www.uweb.engr.washington.edu/education/pdf/ASHsurfscontact%20angles05.pdf (accessed on 15 February 2017).
- Cerqueira, M.A.; Lima, Á.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. Suitability of novel galactomannans as edible coatings for tropical fruits. J. Food Eng. 2009, 94, 372–378. [Google Scholar] [CrossRef]




| Liquids | Surface Tension ( | Polar Forces ( | Dispersive Forces ( |
|---|---|---|---|
| water | 72.8 | 51.0 | 21.8 |
| ethylene glycol | 47.7 | 16.8 | 30.9 |
| diiodomethane | 50.8 | 0.7 | 50.1 |
| glycerol | 63.4 | 26.4 | 37.0 |
| Superficial Properties | Novel Method (mN/m) | Linear Regression Models | DSA (mN/m) |
|---|---|---|---|
| Surface free energy | 21.10 | 21.26 | |
| Polar component | 0.81 | 0.99 | |
| Dispersive component | 20.29 | 19.96 |
| Superficial Properties | Novel Method (mN/m) | Linear Regression Models | DSA (mN/m) |
|---|---|---|---|
| Surface free energy | 43.11 | 46.71 | |
| Polar component | 23.39 | 22.50 | |
| Dispersive component | 19.72 | 19.19 |
| Study | Plastic Film | Polar Component (γSP) | Dispersive Component (γSD) | SFE (γS) |
|---|---|---|---|---|
| Present study | BOPP | 0.71 | 24.03 | 26.23 |
| Guimond et al. [35] | BOPP | ~0.5 1 | ~24.5 1 | ~25 1 |
| Present study | 12 µm PET | 9.98 | 37.12 | 47.10 |
| Present study | 160 µm PET | 4.58 | 35.55 | 40.13 |
| Wang et al. [36] | 10 µm PET | - | - | 45.6 1 |
| Present study | PTFE | 5.31 | 15.82 | 21.13 |
| Jun and Qunji et al. [37] | PTFE | 3.93 | 28.42 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senturk Parreidt, T.; Schmid, M.; Hauser, C. Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food. Foods 2017, 6, 31. https://doi.org/10.3390/foods6040031
Senturk Parreidt T, Schmid M, Hauser C. Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food. Foods. 2017; 6(4):31. https://doi.org/10.3390/foods6040031
Chicago/Turabian StyleSenturk Parreidt, Tugce, Markus Schmid, and Carolin Hauser. 2017. "Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food" Foods 6, no. 4: 31. https://doi.org/10.3390/foods6040031






