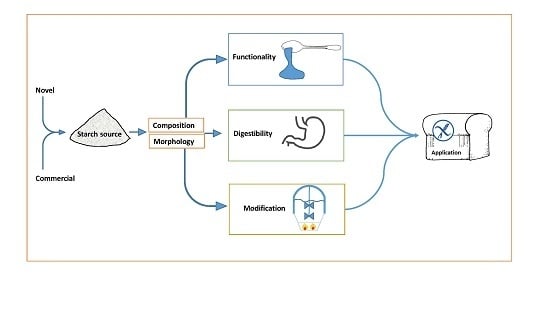
Starch Characteristics Linked to Gluten-Free Products
Abstract
:1. Introduction
2. Starch Production
3. Starch Sources
4. Starch Composition
4.1. Amylose/Amylopectin
4.2. Damaged Starch
4.3. Starch Enzymes
4.4. Lipids
4.5. Protein
5. Starch Morphology
6. Starch Digestibility
7. Starch Functionality
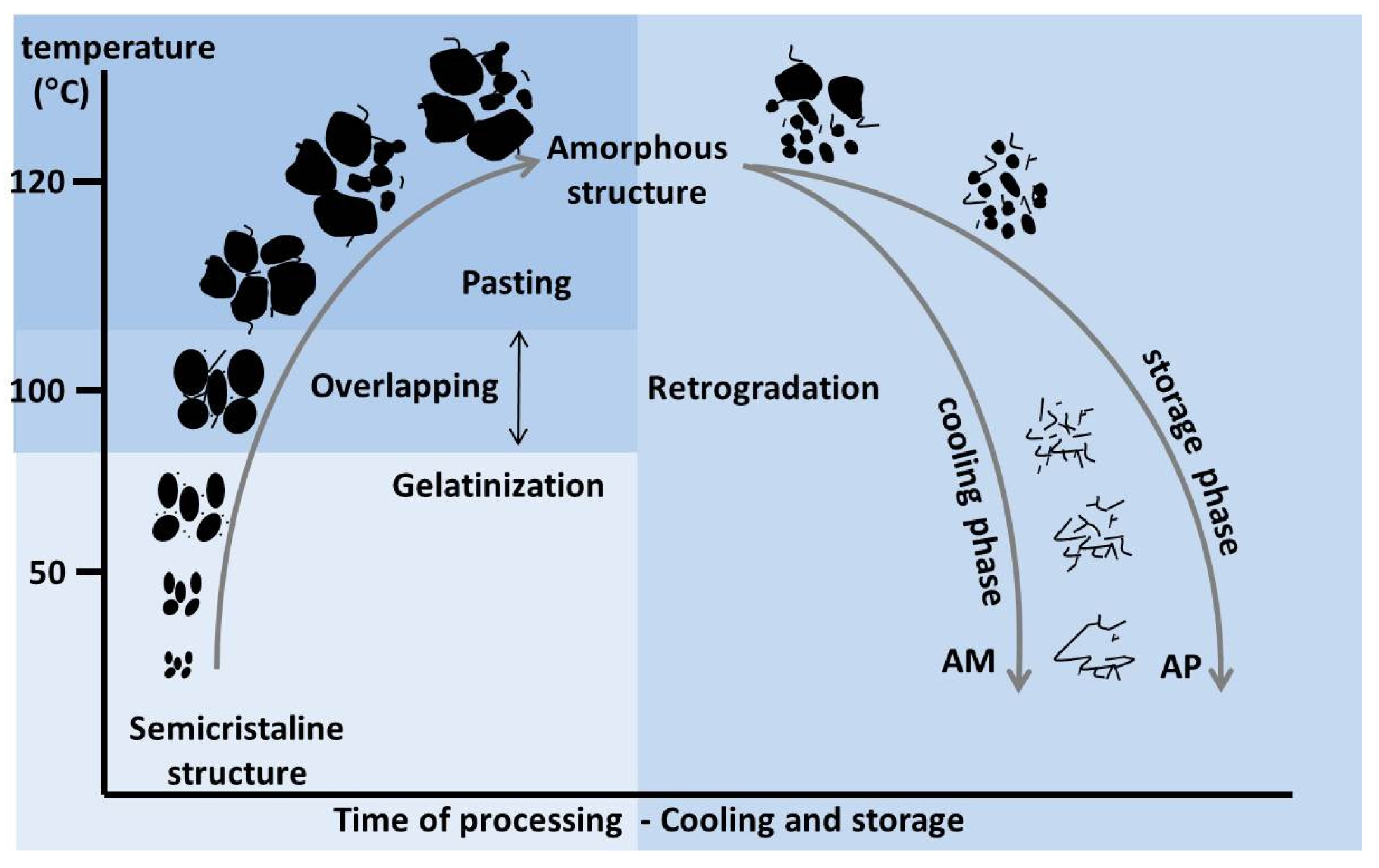
7.1. Gelatinisation
7.2. Pasting
7.3. Retrogradation
8. State of the Art of Starch in Gluten-Free Bread Formulation and Possibilities
9. Starch Modification
9.1. Physical Modifications
9.2. Other Physically Modified Starches
9.3. Innovative Modifications of Starch
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. WJG 2012, 18, 6036. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Renzetti, S.; dal Bello, F. Dough microstructure and textural aspects of gluten-free yeast bread and biscuits. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 107–129. [Google Scholar]
- Koehler, P.; Wieser, H.; Konitzer, K. Celiac Disease and Gluten: Multidisciplinary Challenges and Opportunities; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Thomas, D.; Atwell, W. Starches. In Eagan Press Handbook; American Association of Cereal Chemists: St. Paul, MN, USA, 1999. [Google Scholar]
- Miyazaki, M.R.; Hung, P.V.; Maeda, T.; Morita, N. Recent advances in application of modified starches for breadmaking. Trends Food Sci. Technol. 2006, 17, 591–599. [Google Scholar] [CrossRef]
- Ward, F.M.; Andon, S.A. Hydrocolloids as film formers, adhesives and gelling agents for bakery and cereal products. Cereal Food World 2002, 47, 52–55. [Google Scholar]
- Bloksma, A.W. Dough structure, dough rheology, and baking quality. Cereal Foods World 1990, 35, 237–243. [Google Scholar]
- Eliasson, A.-C.; Larsson, K. Cereals in Breadmaking: A Molecular Colloidal Approach, 1st ed.; CRC Press: New York, NY, USA, 1993. [Google Scholar]
- Hug-Iten, S.; Conde-Petit, B.; Echer, F. Structural properties of starch in bread and bread model systems—Influence of an antistaling α-amylose. Cereal Chem. 2001, 78, 421–428. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M. 11 Functionality of Starches and Hydrocolloids in Gluten-Free Foods. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; p. 200. [Google Scholar]
- Lohiniemi, S.; Maki, M.; Kaukinen, K.; Laippala, P.; Collin, P. Gastrointestinal symptoms rating scale in coeliac disease patients on wheat starch-based gluten-free diets. Scand. J. Gastroenterol. 2000, 35, 947–949. [Google Scholar] [PubMed]
- Sanchez, H.D.; Osella, C.A.; de la Torre, M.A. Optimisation of gluten-free bread prepared from cornstarch, rice flour and cassava starch. J. Food Sci. 2002, 67, 416–419. [Google Scholar] [CrossRef]
- Kobylanski, J.R.; Perez, O.E.; Pilosof, A.M.R. Thermal transitions of gluten-free doughs as affected by water, egg white and hydroxypropylmethylcellulose. Thermochim. Acta 2004, 411, 81–89. [Google Scholar] [CrossRef]
- Moore, M.M.; Schober, T.J.; Dockery, P.; Arendt, E.K. Textural comparisons of gluten-free and wheat-based doughs, batters, and breads. Cereal Chem. 2004, 81, 567–575. [Google Scholar] [CrossRef]
- Gallagher, E.; Polenghi, O.; Gormley, T.R. Novel Rice Starches in Gluten-Free Bread. In Proceedings of the International Association of Cereal Chemists Conference, Budapest, Hungary, 26–29 May 2002. [Google Scholar]
- Sànchez, H.D.; Osella, C.A.; de la Torre, M.A. Desarrollo de una formula para pan sin gluten. Inf. Tecnol. 1996, 7, 35–42. [Google Scholar]
- Mäki, M. Celiac Disease Treatment: Gluten-Free Diet and Beyond. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Wieser, H.; Koehler, P. Production of gluten-free wheat starch by peptidase treatment. J. Cereal Sci. 2014, 60, 202–209. [Google Scholar] [CrossRef]
- Gallagher, E. Gluten-Free Food Science and Technology, 1st ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 107–129. [Google Scholar]
- Zuckerforschung. Available online: http://www.zuckerforschung.at/ (accessed on 16 February 2017).
- Avérous, L.R.; Halley, P.J. Starch polymers: From the field to industrial products. In Starch Polymers: From Genetic Engineering to Green Applications, 1st ed.; Halley, P.J., Avérous, L.R., Eds.; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Mintel, Database: Gluten-Free Foods US. Available online: http://www.mintel.com/ (accessed on 11 December 2015).
- Sarawong, C.; Gutiérrez, Z.R.; Berghofer, E.; Schoenlechner, R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int. J. Food Sci. Technol. 2014, 49, 1825–1833. [Google Scholar] [CrossRef]
- Krupa, U.; Rosell, C.M.; Sadowska, J.; Soral-Śmietana, M. Bean starch as ingredient for gluten-free bread. J. Food Process. Preserv. 2010, 34, 501–518. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. The influence of acorn flour on rheological properties of gluten-free dough and physical characteristics of the bread. Eur. Food Res. Technol. 2015, 240, 1135–1143. [Google Scholar] [CrossRef]
- Bertoft, E. On the nature of categories of chains in amylopectin and their connection to the super helix model. Carbohydr. Polym. 2004, 57, 211–224. [Google Scholar] [CrossRef]
- Pasqualone, A.; Caponio, F.; Summo, C.; Paradiso, V.M.; Bottega, G.; Pagani, M.A. Gluten-free bread making trials from cassava (Manihot esculenta Crantz) flour and sensory evaluation of the final product. Int. J. Food Prop. 2010, 13, 562–573. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Manners, D.J. Recent developments in our understanding of amylopectin structure. Carbohydr. Polym. 1989, 11, 87–112. [Google Scholar] [CrossRef]
- Takeda, Y.; Maruta, N.; Hizukuri, S. Examination of the structure of amylose by tritium labelling of the reducing terminal. Carbohydr. Res. 1992, 227, 113–120. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch-Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Jane, J. Structural features of starch granules II. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Schirmer, M.; Höchstötter, A.; Jekle, M.; Arendt, E.; Becker, T. Physicochemical and morphological characterization of different starches with variable amylose/amylopectin ratio. Food Hydrocoll. 2012, 32, 52–63. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Sikora, M.; Tomasik, P.; Krystyjan, M. Characterization of potato starch fractions and their interaction with hydrocolloids. Starch-Stärke 2010, 62, 341–349. [Google Scholar] [CrossRef]
- Schwall, G.P.; Safford, R.; Westcott, R.J.; Jeffcoat, R.; Tayal, A.; Shi, Y.-C.; Gidley, M.J.; Jobling, S.A. Production of very-high-amylose potato starch by inhibition of SBE A and B. Nat. Biotechnol. 2000, 18, 551–554. [Google Scholar] [PubMed]
- Lii, C.-Y.; Tsai, M.-L.; Tseng, K.-H. Effect of amylose content on the rheological property of rice starch. Cereal Chem. 1996, 73, 415–420. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Whistler, R.L.; Johnson, C. Effect of acid hydrolysis on the retrogradation of amylose. Cereal Chem. 1948, 25, 418–424. [Google Scholar]
- Bello-Pérez, L.A.; Pano de Léon, Y.; Agama-Acevedo, E.; Paredes-López, O. Isolation and Partial Characterization of Amaranth and Banana Starches. Starch-Stärke 1998, 50, 409–413. [Google Scholar] [CrossRef]
- Christa, K.; Soral-Smietana, M.; Lewandowicz, G. Buckwheat starch: Structure, functionality and enzyme in vitro susceptibility upon the roasting process. Int. J. Food Sci. Nutr. 2009, 60, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Ratnayake, W.S. Starch characteristics of black bean, chick pea, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem. 2002, 78, 489–498. [Google Scholar] [CrossRef]
- Lorenz, K.; Coulter, L. Quinoa flour in baked products. Plant Foods Hum. Nutr. 1991, 41, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, S.W.; Belz, M.C.; Heitmann, M.; Zannini, E.; Arendt, E.K. Fundamental Study on the Impact of Gluten-Free Starches on the Quality of Gluten-Free Model Breads. Foods 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Bean, S.; Seib, P.A.; Pedersen, J.; Shi, Y.-C. Structure and functional properties of sorghum starches differing in amylose content. J. Agric. Food Chem. 2008, 56, 6680–6685. [Google Scholar] [CrossRef] [PubMed]
- Bultosa, G.; Taylor, J.R.N. Paste and Gel Properties and In Vitro Digestibility of Tef (Eragrostis tef (Zucc.) Trotter) Starch. Starch-Stärke 2004, 56, 20–28. [Google Scholar] [CrossRef]
- Hager, A.-S.; Wolter, A.; Jacob, F.; Zannini, E.; Arendt, E.K. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J. Cereal Sci. 2012, 56, 239–247. [Google Scholar] [CrossRef]
- Seidemann, J.; Ulmann, M. Stärke-Atlas: Grundlagen der Stärke-Mikroskopie und Beschreibung der Wichtigsten Stärkearten; Parey, Berlin & Hamburg: Parey, Germany, 1966. [Google Scholar]
- Soni, P.L.; Sharma, H.; Dun, D.; Gharia, M.M. Physicochemical properties of Quercus leucotrichophora (Oak) starch. Starch-Stärke 1993, 45, 127–130. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.-K. Paticle size distribution, pasting pattern and texture of gel of acorn, mungbean, and buckwheat starches. Korean J. Food Sci. Technol. 2000, 32, 1291–1297. [Google Scholar]
- Stevenson, D.G.; Jane, J.-L.; Inglett, G.E. Physicochemical Properties of Pin Oak (Quercus palustris Muenchh.) Acorn Starch. Starch-Stärke 2006, 58, 553–560. [Google Scholar] [CrossRef]
- Matsuguma, L.S.; Lacerda, L.G.; Schnitzler, E.; Carvalho Filho, M.A.D.S.; Franco, C.M.L.; Demiate, I.M. Characterization of native and oxidized starches of two varieties of Peruvian carrot (Arracacia xanthorrhiza, B.) from two production areas of Paraná state, Brazil. Braz. Arch. Biol. Technol. 2009, 52, 701–713. [Google Scholar] [CrossRef]
- Rocha, T.S.; Cunha, V.A.; Jane, J.-L.; Franco, C.M. Structural characterization of Peruvian carrot (Arracacia xanthorrhiza) starch and the effect of annealing on its semicrystalline structure. J. Agric. Food Chem. 2011, 59, 4208–4216. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.; Lares, M. Chemical Composition, Mineral Profile, and Functional Properties of Canna (Canna edulis) and Arrowroot (Maranta spp.) Starches. Plant Foods Hum. Nutr. 2005, 60, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana starch: Production, physicochemical properties, and digestibility—A review. Carbohydr. Polym. 2005, 59, 443–458. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Olu-Owolabi, B.I.; Olawumi, E.K.; Lawal, O.S. Functional properties of native, physically and chemically modified breadfruit (Artocarpus artilis) starch. Ind. Crops Prod. 2005, 21, 343–351. [Google Scholar] [CrossRef]
- Nwokocha, L.M.; Williams, P.A. Comparative study of physicochemical properties of breadfruit (Artocarpus altilis) and white yam starches. Carbohydr. Polym. 2011, 85, 294–302. [Google Scholar] [CrossRef]
- Santacruz, S.; Koch, K.; Svensson, E.; Ruales, J.; Eliasson, A.C. Three underutilised sources of starch from the Andean region in Ecuador Part I. Physico-chemical characterisation. Carbohydr. Polym. 2002, 49, 63–70. [Google Scholar] [CrossRef]
- Thitipraphunkul, K.; Uttapap, D.; Piyachomkwan, K.; Takeda, Y. A comparative study of edible canna (Canna edulis) starch from different cultivars. Part I. Chemical composition and physicochemical properties. Carbohydr. Polym. 2003, 53, 317–324. [Google Scholar] [CrossRef]
- Demiate, I.M.; Oetterer, M.; Wosiacki, G. Characterization of chestnut (Castanea sativa, Mill) starch for industrial utilization. Braz. Arch. Biol. Technol. 2001, 44, 69–78. [Google Scholar] [CrossRef]
- Huang, J.; Schols, H.A.; van Soest, J.J.G.; Jin, Z.; Sulmann, E.; Voragen, A.G.J. Physicochemical properties and amylopectin chain profiles of cowpea, chickpea and yellow pea starches. Food Chem. 2007, 101, 1338–1345. [Google Scholar] [CrossRef]
- Agunbiade, S.O.; Longe, O.G. The physico-functional characteristics of starches from cowpea (Vigna unguiculata), pigeon pea (Cajanus cajan) and yambean (Sphenostylis stenocarpa). Food Chem. 1999, 65, 469–474. [Google Scholar] [CrossRef]
- Jayakody, L.; Hoover, R.; Liu, Q.; Weber, E. Studies on tuber and root starches. I. Structure and physicochemical properties of innala (Solenostemon rotundifolius) starches grown in Sri Lanka. Food Res. Int. 2005, 38, 615–629. [Google Scholar] [CrossRef]
- Geng, Z.; Zongdao, C.; Yimin, W. Physicochemical properties of lotus (Nelumbo nucifera Gaertn.) and kudzu (Pueraria hirsute Matsum.) starches. Int. J. Food Sci. Technol. 2007, 42, 1449–1455. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; McKnight, S.; Panozzo, J.F.; Kasapis, S.; Adhikari, R.; Adhikari, B. Physicochemical and functional characteristics of lentil starch. Carbohydr. Polym. 2013, 92, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Lin, C.-L.; Chen, J.-C. Characteristics of mung bean starch isolated by using lactic acid fermentation solution as the steeping liquor. Food Chem. 2006, 99, 794–802. [Google Scholar] [CrossRef]
- Israkarn, K.; Hongsprabhas, P.; Hongsprabhas, P. Influences of granule-associated proteins on physicochemical properties of mungbean and cassava starches. Carbohydr. Polym. 2007, 68, 314–322. [Google Scholar] [CrossRef]
- Hernández-Lauzardo, A.N.; Méndez-Montealvo, G.; Velázquez del Valle, M.G.; Solorza-Feria, J.; Bello-Pérez, L.A. Isolation and Partial Characterization of Mexican Oxalis tuberosa Starch. Starch-Stärke 2004, 56, 357–363. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Williams, P.A.; Doublier, J.-L.; Durand, S.; Buleon, A. Physico-chemical characterisation of sago starch. Carbohydr. Polym. 1999, 38, 361–370. [Google Scholar] [CrossRef]
- Jane, J.; Shen, L.; Chen, J.; Lim, S.; Kasemsuwan, T.; Nip, W. Physical and Chemical Studies of Taro Starches and Flours1 2. Cereal Chem. 1992, 69, 528–535. [Google Scholar]
- Himeda, M.; Njintang, N.; Nguimbou, R.; Gaiani, C.; Scher, J.; Facho, B.; Mbofung, C. Physicochemical, rheological and thermal properties of taro (Colocassia esculenta) starch harvested at different maturity stages. Int. J. Biosci. 2012, 2, 14–27. [Google Scholar]
- Pérez, E.; Schultz, F.S.; de Delahaye, E.P. Characterization of some properties of starches isolated from Xanthosoma sagittifolium (tannia) and Colocassia esculenta (taro). Carbohydr. Polym. 2005, 60, 139–145. [Google Scholar] [CrossRef]
- Jayakody, L.; Hoover, R.; Liu, Q.; Donner, E. Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea sp.) starches grown in Sri Lanka. Carbohydr. Polym. 2007, 69, 148–163. [Google Scholar] [CrossRef]
- Gomand, S.V.; Lamberts, L.; Derde, L.J.; Goesaert, H.; Vandeputte, G.E.; Goderis, B.; Visser, R.G.F.; Delcour, J.A. Structural properties and gelatinisation characteristics of potato and cassava starches and mutants thereof. Food Hydrocoll. 2010, 24, 307–317. [Google Scholar] [CrossRef]
- Alvani, K.; Qi, X.; Tester, R.F.; Snape, C.E. Physico-chemical properties of potato starches. Food Chem. 2011, 125, 958–965. [Google Scholar] [CrossRef]
- Han, X.-Z.; Campanella, O.H.; Mix, N.C.; Hamaker, B.R. Consequence of Starch Damage on Rheological Properties of Maize Starch Pastes 1. Cereal Chem. 2002, 79, 897–901. [Google Scholar] [CrossRef]
- Barrera, G.N.; Bustos, M.C.; Iturriaga, L.; Flores, S.K.; León, A.E.; Ribotta, P.D. Effect of damaged starch on the rheological properties of wheat starch suspensions. J. Food Eng. 2013, 116, 233–239. [Google Scholar] [CrossRef]
- Faridi, H. Application of rheology in the cookie and cracker industry. In Dough Rheology and Baked Product Texture, 1st ed.; Faridi, H., Faubion, J.M., Eds.; Springer: Berlin, Germany, 1990; pp. 363–384. [Google Scholar]
- Devi, A.F.; Fibrianto, K.; Torley, P.J.; Bhandari, B. Physical properties of cryomilled rice starch. J. Cereal Sci. 2009, 49, 278–284. [Google Scholar] [CrossRef]
- Van der Maarel, M.J.; van der Veen, B.; Uitdehaag, J.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef]
- Sluimer, P. Principles of Breadmaking: Functionality of Raw Materials and Process Steps; American Association of Cereal Chemists: St. Paul, MN, USA, 2005. [Google Scholar]
- Giannone, V.; Lauro, M.R.; Spina, A.; Pasqualone, A.; Auditore, L.; Puglisi, I.; Puglisi, G. A novel α-amylase-lipase formulation as anti-staling agent in durum wheat bread. LWT-Food Sci. Technol. 2016, 65, 381–389. [Google Scholar] [CrossRef]
- Antranikian, G.; Winkelmann, G. Microbial degradation of starch. In Microbial Degradation of Natural Products; Winkelmann, G., Ed.; Wiley-Blackwell: Oxford, UK, 1992; pp. 27–56. [Google Scholar]
- Luallen, T.; Eliasson, A. Utilizing starches in product development. In Starch in Food: Structure, Function and Applications, 1st ed.; Eliasson, A., Ed.; CRC Press: New York, NY, USA, 2004; pp. 393–424. [Google Scholar]
- Nimz, O.; Gessler, K.; Usón, I.; Sheldrick, G.M.; Saenger, W. Inclusion complexes of V-amylose with undecanoic acid and dodecanol at atomic resolution: X-ray structures with cycloamylose containing 26 d-glucoses (cyclohexaicosaose) as host. Carbohydr. Res. 2004, 339, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R. Lipids in cereal starches: A review. J. Cereal Sci. 1988, 8, 1–15. [Google Scholar] [CrossRef]
- Pareyt, B.; Finnie, S.M.; Putseys, J.A.; Delcour, J.A. Lipids in bread making: Sources, interactions, and impact on bread quality. J. Cereal Sci. 2011, 54, 266–279. [Google Scholar] [CrossRef]
- Jane, J.-L.; Xu, A.; Radosavljevic, M.; Seib, P. Location of amylose in normal starch granules. I. Susceptibility of amylose and amylopectin to cross-linking reagents. Cereal Chem. 1992, 69, 405–409. [Google Scholar]
- Soulaka, A.B.; Morrison, W.R. The amylose and lipid contents, dimensions, and gelatinisation characteristics of some wheat starches and their A- and B-granule fractions. J. Sci. Food Agric. 1985, 36, 709–718. [Google Scholar] [CrossRef]
- Mishra, S.; Rai, T. Morphology and functional properties of corn, potato and tapioca starches. Food Hydrocoll. 2006, 20, 557–566. [Google Scholar] [CrossRef]
- Atichokudomchai, N.; Shobsngob, S.; Varavinit, S. Morphological Properties of Acid-modified Tapioca Starch. Starch-Stärke 2000, 52, 283–289. [Google Scholar] [CrossRef]
- Jane, J.L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke 1994, 46, 121–129. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Ultrasound-treated starch: SEM and TEM imaging, and functional behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Bechtel, D.B.; Zayas, I.; Kaleikau, L.; Pomeranz, Y. Size-distribution of wheat starch granules during endosperm development. Cereal Chem. 1990, 67, 59–63. [Google Scholar]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends in Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Tester, R.; Karkalas, J.; Qi, X. Starch structure and digestibility enzyme-substrate relationship. World Poult. Sci. J. 2004, 60, 186–195. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Lehmann, U.; Robin, F. Slowly digestible starch–its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266–273. [Google Scholar]
- Hager, A.-S.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K. Starch properties, in vitro digestibility and sensory evaluation of fresh egg pasta produced from oat, teff and wheat flour. J. Cereal Sci. 2013, 58, 156–163. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Arendt, E.K. In vitro starch digestibility and predicted glycaemic indexes of buckwheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. J. Cereal Sci. 2013, 58, 431–436. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Arendt, E.K. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct. 2014, 5, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Flores-Silva, P.C.; Berrios, J.D.J.; Pan, J.; Osorio-Díaz, P.; Bello-Pérez, L.A. Gluten-free spaghetti made with chickpea, unripe plantain and maize flours: Functional and chemical properties and starch digestibility. Int. J. Food Sci. Technol 2014, 49, 1985–1991. [Google Scholar] [CrossRef]
- Segura, M.E.M.; Rosell, C.M. Chemical composition and starch digestibility of different gluten-free breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Gularte, M.A.; Gómez, M.; Rosell, C.M. Impact of legume flours on quality and in vitro digestibility of starch and protein from gluten-free cakes. Food Bioprocess Technol. 2012, 5, 3142–3150. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Capriles, V.; Coelho, K.; Guerra-Matias, A.; Arêas, J. Effects of processing methods on amaranth starch digestibility and predicted glycemic index. J. Food Sci. 2008, 73, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.; Qi, X.; Karkalas, J. Hydrolysis of native starches with amylases. Anim. Feed Sci. Technol. 2006, 130, 39–54. [Google Scholar] [CrossRef]
- Dreher, M.L.; Dreher, C.J.; Berry, J.W.; Fleming, S.E. Starch digestibility of foods: A nutritional perspective. Crit. Rev. Food Sci. Nutr. 1984, 20, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Langworthy, C.; Deuel, H.J., Jr. Digestibility of raw corn, potato, and wheat starches. J. Biol. Chem. 1920, 42, 27–40. [Google Scholar]
- Oates, C.G. Towards an understanding of starch granule structure and hydrolysis. Trends Food Sci. Technol. 1997, 8, 375–382. [Google Scholar] [CrossRef]
- Thorne, M.J.; Thompson, L.; Jenkins, D. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am. J. Clin. Nutr. 1983, 38, 481–488. [Google Scholar] [PubMed]
- Granfeldt, Y.; Drews, A.; Björck, I. Arepas made from high amylose corn flour produce favorably low glucose and insulin responses in healthy humans. J. Nutr. 1995, 125, 459–465. [Google Scholar] [PubMed]
- Frei, M.; Siddhuraju, P.; Becker, K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003, 83, 395–402. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, H.; Duan, Z.; Linlin, Z.; Wu, D. Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J. Cereal Sci. 2004, 40, 231–237. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Ramadan, M.F. Degradability of different phosphorylated starches and thermoplastic films prepared from corn starch phosphomonoesters. Starch Starke 2001, 53, 317–322. [Google Scholar] [CrossRef]
- Anguita, M.; Gasa, J.; Martín-Orúe, S.; Pérez, J. Study of the effect of technological processes on starch hydrolysis, non-starch polysaccharides solubilization and physicochemical properties of different ingredients using a two-step in vitro system. Anim. Feed Sci. Technol. 2006, 129, 99–115. [Google Scholar] [CrossRef]
- Rehman, Z.-U.; Shah, W. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Bravo, L.; Siddhuraju, P.; Saura-Calixto, F. Effect of various processing methods on the in vitro starch digestibility and resistant starch content of Indian pulses. J. Agric. Food Chem. 1998, 46, 4667–4674. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Altan, A.; McCarthy, K.; Maskan, M. Effect of Extrusion Cooking on Functional Properties and in vitro Starch Digestibility of Barley-Based Extrudates from Fruit and Vegetable By-Products. J. Food Sci. 2009, 74, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Roopa, S.; Premavalli, K. Effect of processing on starch fractions in different varieties of finger millet. Food Chem. 2008, 106, 875–882. [Google Scholar] [CrossRef]
- Kim, E.H.-J.; Petrie, J.R.; Motoi, L.; Morgenstern, M.P.; Sutton, K.H.; Mishra, S.; Simmons, L.D. Effect of structural and physicochemical characteristics of the protein matrix in pasta on in vitro starch digestibility. Food Biophys. 2008, 3, 229–234. [Google Scholar] [CrossRef]
- Geng, Z.; Zongdao, C.; Toledo, R. Effects of different processing methods on the glycemic index of kudzu starch. Chin. Cereals Oils Assoc. 2003, 5, 12. [Google Scholar]
- Chung, H.J.; Liu, Q. Effect of gamma irradiation on molecular structure and physicochemical properties of corn starch. J. Food Sci. 2009, 74, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Monro, J.; Hedderley, D. Effect of processing on slowly digestible starch and resistant starch in potato. Starch-Stärke 2008, 60, 500–507. [Google Scholar] [CrossRef]
- Mason, W. Starch use in foods. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 745–795. [Google Scholar]
- Schirmer, M.; Jekle, M.; Becker, T. Starch gelatinization and its complexity for analysis. Starch 2015, 67, 30–41. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Halley, P.J. Starch modification to develop novel starch-biopolymer blends: State of art and perspectives. In Starch Polymers: From Genetic Engineering to Green Applications, 1st ed.; Halley, P.J., Avérous, L.R., Eds.; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Atwell, W.; Hood, L.; Lineback, D.; Varriano-Marston, E.; Zobel, H. The terminology and methodology associated with basic starch phenomena. Cereal Foods World (USA) 1988, 33, 306. [Google Scholar]
- Doublier, J.L. Rheological studies on starch—Flow behaviour of wheat starch pastes. Starch-Stärke 1981, 33, 415–420. [Google Scholar] [CrossRef]
- Miles, M.J.; Morris, V.J.; Orford, P.D.; Ring, S.G. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr. Res. 1985, 135, 271–281. [Google Scholar] [CrossRef]
- Swinkels, J.J.M. Sources of starch, its chemistry and physics. In Starch Conversion Technology, 1st ed.; VanBeynum, G.M.A., Roels, J.A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1985; pp. 15–45. [Google Scholar]
- Gray, J.; Bemiller, J. Bread staling: Molecular basis and control. Compr. Rev. Food Sci. Food Saf. 2003, 2, 1–21. [Google Scholar] [CrossRef]
- Bulkin, B.J.; Kwak, Y.; Dea, I. Retrogradation kinetics of waxy-corn and potato starches; a rapid, Raman-spectroscopic study. Carbohydr. Res. 1987, 160, 95–112. [Google Scholar] [CrossRef]
- Schober, T.J. Manufacture of gluten-free specialty breads and confectionery products. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 130–180. [Google Scholar]
- Cooke, D.; Gidley, M.J. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydr. Res. 1992, 227, 103–112. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Donald, A.M. Gelatinisation of starch: A combined SAXS/WAXS/DSC and SANS study. Carbohydr. Res. 1998, 308, 133–147. [Google Scholar] [CrossRef]
- Deffenbaugh, L.; Walker, C. Comparison of starch pasting properties in the Brabender Viscoamylograph and the Rapid Visco-Analyzer. Cereal Chem. 1989, 66, 493–499. [Google Scholar]
- Rosell, C.M.; Matos, M.E. Market and Nutrition Issues of Gluten-Free Foodstuff. In Advances in the Understanding of Gluten related Pathology and the Evolution of Gluten-Free Foods; OmniaScience Monographs: Barcelona, Spain, 2015; pp. 675–713. [Google Scholar]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences Between a Gluten-free Diet and a Diet Containing Equivalent Products with Gluten. Plant Food Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef] [PubMed]
- López, A.C.B.; Pereira, A.J.G.; Junqueira, R.G. Flour mixture of rice flour, corn and cassava starch in the production of gluten-free white bread. Braz. Arch. Biol. Technol. 2004, 47, 63–70. [Google Scholar] [CrossRef]
- Schober, T.J.; Messerschmidt, M.; Bean, S.R.; Park, S.-H.; Arendt, E.K. Gluten-free bread from sorghum: Quality differences among hybrids. Cereal Chem. 2005, 82, 394–404. [Google Scholar] [CrossRef]
- McCarthy, D.; Gallagher, E.; Gormley, T.; Schober, T.; Arendt, E. Application of response surface methodology in the development of gluten-free bread. Cereal Chem. 2005, 82, 609–615. [Google Scholar] [CrossRef]
- Moore, M.M.; Heinbockel, M.; Dockery, P.; Ulmer, H.; Arendt, E.K. Network formation in gluten-free bread with application of transglutaminase. Cereal Chem. 2006, 83, 28–36. [Google Scholar] [CrossRef]
- Korus, J.; Grzelak, K.; Achremowicz, K.; Sabat, R. Influence of prebiotic additions on the quality of gluten-free bread and on the content of inulin and fructooligosaccharides. Food Sci. Technol. Int. 2006, 12, 489–495. [Google Scholar] [CrossRef]
- Schober, T.J.; Bean, S.R.; Boyle, D.L. Gluten-free sorghum bread improved by sourdough fermentation: Biochemical, rheological, and microstructural background. J. Agric. Food Chem. 2007, 55, 5137–5146. [Google Scholar] [CrossRef] [PubMed]
- Gambuś, H.; Sikora, M.; Ziobro, R. The effect of composition of hydrocolloids on properties of gluten-free bread. Acta Sci. Pol. Technol. Aliment. 2007, 6, 61–74. [Google Scholar]
- Schober, T.J.; Bean, S.R.; Boyle, D.L.; Park, S.-H. Improved viscoelastic zein–starch doughs for leavened gluten-free breads: Their rheology and microstructure. J. Cereal Sci. 2008, 48, 755–767. [Google Scholar] [CrossRef]
- Sabanis, D.; Lebesi, D.; Tzia, C. Effect of dietary fibre enrichment on selected properties of gluten-free bread. LWT-Food Sci. Technol. 2009, 42, 1380–1389. [Google Scholar] [CrossRef]
- Onyango, C.; Unbehend, G.; Lindhauer, M.G. Effect of cellulose-derivatives and emulsifiers on creep-recovery and crumb properties of gluten-free bread prepared from sorghum and gelatinised cassava starch. Food Res. Int. 2009, 42, 949–955. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. The impact of resistant starch on characteristics of gluten-free dough and bread. Food Hydrocoll. 2009, 23, 988–995. [Google Scholar] [CrossRef]
- Mezaize, S.; Chevallier, S.; Le Bail, A.; de Lamballerie, M. Optimization of Gluten-Free Formulations for French-Style Breads. J. Food Sci. 2009, 74, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Wronkowsk, M.; Soral-Śmietana, M. Effect of Buckwheat Flour on Microelements and Proteins. Czech J. Food Sci. 2011, 29, 103–108. [Google Scholar]
- Van Riemsdijk, L.E.; van der Goot, A.J.; Hamer, R.J.; Boom, R.M. Preparation of gluten-free bread using a meso-structured whey protein particle system. J. Cereal Sci. 2011, 53, 355–361. [Google Scholar] [CrossRef]
- Milde, L.B.; Ramallo, L.A.; Puppo, M.C. Gluten-free bread based on tapioca starch: Texture and sensory studies. Food Bioprocess Technol. 2012, 5, 888–896. [Google Scholar] [CrossRef]
- Miñarro, B.; Albanell, E.; Aguilar, N.; Guamis, B.; Capellas, M. Effect of legume flours on baking characteristics of gluten-free bread. J. Cereal Sci. 2012, 56, 476–481. [Google Scholar] [CrossRef]
- Ziobro, R.; Korus, J.; Witczak, M.; Juszczak, L. Influence of modified starches on properties of gluten-free dough and bread. Part II: Quality and staling of gluten-free bread. Food Hydrocoll. 2012, 29, 68–74. [Google Scholar] [CrossRef]
- Pongjaruvat, W.; Methacanon, P.; Seetapan, N.; Fuongfuchat, A.; Gamonpilas, C. Influence of pregelatinised tapioca starch and transglutaminase on dough rheology and quality of gluten-free jasmine rice breads. Food Hydrocoll. 2014, 36, 143–150. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Merino, C.; Martínez, M.M.; Gómez, M. Mixture design of rice flour, maize starch and wheat starch for optimization of gluten free bread quality. J. Food Sci. Technol. 2015, 52, 6323–6333. [Google Scholar] [CrossRef] [PubMed]
- Korus, J.; Witczak, T.; Ziobro, R.; Juszczak, L. Linseed (Linum usitatissimum L.) mucilage as a novel structure forming agent in gluten-free bread. LWT-Food Sci. Technol. 2015, 62, 257–264. [Google Scholar] [CrossRef]
- O’Shea, N.; Rößle, C.; Arendt, E.; Gallagher, E. Modelling the effects of orange pomace using response surface design for gluten-free bread baking. Food Chem. 2015, 166, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chickpea and tiger nut flours as alternatives to emulsifier and shortening in gluten-free bread. LWT-Food Sci. Technol. 2015, 62, 225–232. [Google Scholar] [CrossRef]
- Delcour, J.; Hoseney, R.C. Principles of Cereal Science and Technology, 3rd ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2010. [Google Scholar]
- Dar, Y.L. Starches as Food Texturizing Systems. In Functionalizing Carbohydrates for Food Applications: Texturizing and Bioactive/Flavor Delivery System, 1st ed.; DesTech Publications: Toronto, ON, Canada, 2014; p. 41. [Google Scholar]
- Chiu, C.-W.; Solarek, D. Modification of starches. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Davidson, V.; Paton, D.; Diosady, L.; Larocque, G. Degradation of wheat starch in a single screw extruder: Characteristics of extruded starch polymers. J. Food Sci. 1984, 49, 453–458. [Google Scholar] [CrossRef]
- Steeneken, P.A.M.; Woortman, A.J.J. Superheated starch: A novel approach towards spreadable particle gels. Food Hydrocoll. 2009, 23, 394–405. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Bustamante, M.; Churruca, I.; Simon, E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Szymońska, J.; Krok, F.; Komorowska-Czepirska, E.; Rębilas, K. Modification of granular potato starch by multiple deep-freezing and thawing. Carbohydr. Polym. 2003, 52, 1–10. [Google Scholar] [CrossRef]
- Szymońska, J.; Wodnicka, K. Effect of multiple freezing and thawing on the surface and functional properties of granular potato starch. Food Hydrocoll. 2005, 19, 753–760. [Google Scholar] [CrossRef]
- Zarguili, I.; Maache-Rezzoug, Z.; Loisel, C.; Doublier, J.L. Influence of DIC hydrothermal process conditions on the gelatinization properties of standard maize starch. J. Food Eng. 2006, 77, 454–461. [Google Scholar] [CrossRef]
- Maache-Rezzoug, Z.; Maugard, T.; Zarguili, I.; Bezzine, E.; El Marzouki, M.N.; Loisel, C. Effect of instantaneous controlled pressure drop (DIC) on physicochemical properties of wheat, waxy and standard maize starches. J. Cereal Sci. 2009, 49, 346–353. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch-Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]




| Common Starch | Botanical Name | Composition | Granule | Reference | |||||||
| Starch (%) | Damage Starch (%) | Moisture (%) | Amylose (%) on s.b | Protein (%) | Lipid (%) | Ash (%) | Size (µm) | Shape | |||
| Amaranth | Amaranthus | 96.2 | - | 5.2 | 7.8 | 0.9 | 0.2 | 0.12 | 1.0–1.3 | polygonal | [40] |
| Buckwheat | Fagopyrum esculentum | 82.5–90.2 | - | - | 12.4–17.1 | 1.15–3.96 | - | 0.23–0.23 | 2.0–9.0 | polygonal irregular, spherical | [41] |
| Corn | Zea mays | 96.3 | 1.3 | 12.6 | 22.7 | 0.37 | 0.21 | 0.07 | 5.0–30.0 | round, polygonal | [33] |
| Waxy | 94.3 | 1.91 | 12.8 | 2.5 | 0.2 | 0.12 | 0.07 | 5.0–30.0 | round, polygonal | [33] | |
| High amylose | 92.2 | 1.74 | 12.8 | 71 | 0.56 | 0.21 | 0.13 | 5.0–30.0 | round, polygonal | [33] | |
| Oat | Avena sativa | - | 2.0–2.8 | - | 19.6–24.5 | 0.02–0.09 | 0.85–1.31 | 0.13–0.20 | 3.8–10.5 | compound granule, polyhedral, irregular | [42] |
| Potato | Solanum tuberosum | 93.4 | 0.46 | 14.6 | 20.9 | 0.08 | 0.19 | 0.33 | 15–100 | oval, round | [34] |
| Quinoa | Chenopodium quinoa | - | - | - | 10.0–21.0 | - | - | - | 1–2.5 | compound granule, polygonal | [43] |
| Rice | Oryza sativa | 82.4 | 7.4 | 12.5 | 46.4 | 0.04 | 0.7 | - | 3.0–8.0 | compound, polygonal | [44] |
| Sorghum | Sorghum bicolor (L.) | 81–5 | - | - | 14.0–23.7 | 0.25–0.28 | - | 0.10–0.14 | 16.0–20.0 | round, polygonal | [45] |
| Tapioca | Manihot esculenta | 95.2 | 0.7 | 13.7 | 36 | 0.03 | n/a | 5.0–35 | compound, truncated oval | [44] | |
| Teff | Eragrostis tef | - | 28.3 | 0.19 | 0.89 | 0.13 | 1.0–2.0 | compound, polyhedral | [46] | ||
| Wheat | Triticum | 84.6 | 1.97 | 12.8 | - | 0.19 | 0.14 | 0.16 | 1.0–45 | round, lenticular | [47] |
| Uncommon Starch | Composition | Granule | Reference | ||||||||
| Starch (%) | Damage Starch (%) | Moisture (%) | Amylose (%) on s.b | Protein (%) | Lipid (%) | Ash (%) | Size (µm) | Shape | |||
| Acorn | Quercus | 90–91 | 21%–28% | - | 43–56 | 1.9–2.4 | 1.5–1.65 | - | 3–59 | oval, irregularly round, and ovoid with diameters ranging | [48,49,50,51] |
| Arracacha | Arracacia xanthorrhiza | 97.6–98.7 | - | 6.9–8.7 | 17.4–19.4 | 0.14–0.26 | 0.39–0.42 | 0.01–0.31 | 22–55 | round and irregular shaped granules | [52,53] |
| Arrowroot | Maranta arundinacea | - | - | 15.3 | 15.21 | 0.5 | 0.18 | 0.21 | 22–26 | - | [54] |
| Banana | Musa | 98.1 | - | 9.9 | 9.1–17.2 | 0.87–1.08 | 0.27–0.41 | 6.0–80.0 | irregular in shape, elongated ovals with ridges | [55] | |
| Black bean | Phaseolus vulgaris | - | 1.5–2.0 | - | 27.2–29.5 | 0.04–0.07 | 0.20–0.40 | 0.63–0.65 | 7.0–30.0 | round, irregular, elliptical, oval | [42] |
| Breadfruit | Artocarpus altilis | 78.5–84.5 | - | 12.2–19.3 | - | 1.33–1.61 | 0.29–0.51 | 0.20–0.75 | 2.0–8.0 | Small and mostly indented | [56,57] |
| Cana | Cana edulis | - | - | 9.4–10.0 | 23.4–24.2 | 0.07–0.08 | 0.014–0.019 | 0.25–0.33 | 10–100 | rounded and oval-shaped granules with smooth surfaces | [58,59] |
| Chestnut | Castanea | 96.1 | - | - | 21.5 | 0.83 | 1.51 | 0.51 | - | oval, irregularly round, and ovoid with diameters ranging | [60] |
| Chickpea | Cicer arietinum L. | 94 | 1.6–2.1 | 11.9 | 23.3–27.2 | 0.57 | 0.1 | 0.05 | 9.0–31.0 | round, irregular, elliptical, oval | [42,61] |
| Cow pea | Vigna unguiculata L. | 93.1 | - | 11.5 | 25.8 | 0.49 | 0.15 | - | 16.3–22.6 | morphologically irregular, oval and kidney-shaped | [61,62] |
| Faba bean | Vicia faba L. | 90.2–90.8 | - | 3.0–3.6 | 33.7–33.9 | 3.88–5.37 | - | 0.82–1.13 | 6.0–25 | round, elliptical, smooth surface | [61] |
| Innala | Solenostemon rotundifolius | - | 0.1 | 8.9- 9.7 | 18.7 - 25.2 | 0.05 -0.07 | 0.25 -0.28 | 0.09 - 0.1 | 5.0–25.0 | dome-shaped and hemispherical | [63] |
| Kudzu | Pueraria hirsute Matsum | 98.6 | - | 12.4 | 22.91 | 0.58 | - | 0.52 | 24.08 | spherical hemispherical and polygonal shaped | [64] |
| Lentil | Lens culinaris, M. | - | 1.5–1.6 | - | 23.5–24.7 | 0.05–0.06 | 0.30–0.40 | 0.03–0.04 | 7.0–28.0 | round, irregular, elliptical, oval | [42,65] |
| Lotus | Nelumbo nucifera Gaertn. | 99.2 | - | 15.3 | 30.61 | 0.16 | - | 0.54 | 50.3 | small, rounded. val shape with smooth surface | [64] |
| Mung-Bean | V. radiate | 88.3 | - | 11.4 | 30.9–31.1 | 0.07–0.16 | 0.16–0.20 | 0.08 | 0.4–48.0 | irregular, oval, round, kidney | [66,67] |
| Navy bean | Phaseolus vulgaris | - | 1.5–1.8 | - | 28.2–28.6 | 0.07–0.08 | 0.3 | 0.60–0.65 | 8.0–32.0 | round, irregular, elliptical, oval | [42] |
| Oca | Oxalis tuberosa | 90.5 | - | - | 33 | 0.34 | 0.52 | 0.52 | 25–50 | oval and elliptical shapes | [68] |
| Pinto bean | Phaseolus vulgaris | - | 1.5–1.6 | - | 35.0–35.5 | 0.06–0.07 | 0.50–0.55 | 0.26–0.27 | 6.0–32.0 | round, irregular, elliptical, oval | [42] |
| Sago | Metroxylon sagu | - | - | 10.6–20.0 | 24–30 | 0.13–0.25 | 0.10–0.13 | 0.06–0.43 | 20–40 | oval granules | [69] |
| Taro | Colocasia esculenta | 98.9–99.0 | - | 7.8–7.9 | 27.6–35.9 | 0.62–0.69 | 0.06–0.07 | 0.31–0.35 | 1.0–12.0 | polygonal, irregular shape | [70,71] |
| Tania | Xanthosoma sagittifolium | 99.1 | - | 13.4 | 35.3 | 0.56 | 0.1 | 0.2 | 2.0–12.5 | small, round, large, truncated ellipsoidal-shaped | [72] |
| White yam | Dioscorea alata | - | - | 11.4 | - | 0.69 | 0.29 | 0.15 | 19–30 | large, polyhedral and smooth | [57] |
| Yam | Dioscorea esculenta | - | - | 8.3–11.0 | 20.0–31.0 | 0.01–0.03 | 0.2–0.44 | 0.13–0.32 | 3.0–45.0 | polygonal/truncated oval | [73] |
| Yellow pea | Pisum sativum | 92.3 | - | 11.3 | 31.2 | 0.52 | 0.07 | - | 7.0–3.2 | round, elliptical, smooth surface | [61] |
| Factors | Results | Reference |
|---|---|---|
| Granule size | Small granules are faster digested than bigger ones | [31,107,108] |
| Small granule specific area may decrease extent of enzyme binding and result in less hydrolysis | [109] | |
| Granule Surface | Pinholes and equatorial grooves or furrows result in faster digestion. Cereal starch faster digestible than tuber and legume starch | [110] |
| Smooth surface of potato starch has high resistance to enzymatic hydrolysis | [109,111] | |
| Granule surface proteins and lipids block adsorption sites resulting in less enzyme binding | [112] | |
| Composition | Native starches containing high amylose contents digest slower | [113,114,115,116] |
| Amylose—lipid complexes favour restrictions towards hydrolysis | [117] |
| Processes | Effect | RDS * Content | Reference |
|---|---|---|---|
| Grinding | Decrease of particle size; increase in surface area; increased hydrolysis; faster digestion | Increase | [118] |
| Cooking | Gelatinisation of starch; Easier available for enzymatic attack; increased hydrolysis; faster digestion | Increase | [119,120] |
| Extrusion Cooking | Starch loses structural integrity due to shearing and kneading, making it more susceptible towards enzymatic attacks; increased hydrolysis; faster digestion | Increase | [121,122] |
| Dehulling | Removal of the α-amylase inhabitants such as phytic acid, tannins, polyphenols leaving starch structure fragile and more susceptible to enzymatic degradation | Increase | [95,119] |
| Soaking | |||
| Germination | |||
| Autoclaving | Gelatinisation behaviour | Increase | [123] |
| Puffing | Gelatinisation behaviour | Increase | [123] |
| Baking | Gelatinisation behaviour | Decrease | [123] |
| Frying | Gelatinisation behaviour | Decrease | [123] |
| Roasting | Gelatinisation behaviour | Increase | [123] |
| Sheeting of pasta | Reduction in cohesiveness between starch and protein of dough increase amylase accessibility | Increase | [124] |
| Microwave cooking | penetration through microwaves increases hydrolysis | Increase | [125] |
| Irradiation | degradation and cross-linking of starch chains occur simultaneously during irradiation, leading to an increase in RS | Decrease | [126] |
| Cooling cooked food | Retrograded amylose is highly resistant to hydrolysis | Decrease | [115,116,127] |
| Starch Type | Formulation | References |
|---|---|---|
| Corn starch | Corn starch, rice flour, cassava starch, soy flour | [142] |
| Cassava starch, Corn starch | Cassava starch, corn starch, rice flour, maize flour, dried milk powder, sugar, salt margarine, dried egg, baking powder, water | [143] |
| Corn starch | Sorghum flour, corn starch, water, salt, sugar, and dried yeast | [144] |
| Potato starch | Rice flour, potato starch, and skim milk powder, HPMC | [145] |
| Potato starch | White rice flour, potato starch, corn flour, xanthan gum, skim milk powder, soya flour, and egg powder | [146] |
| Corn starch, Potato starch | Corn starch, potato starch, guar gum, pectin, freeze-dried yeasts, sugar, salt, vegetable oil, water | [147] |
| Potato starch | HPMC, water, sorghum flour, potato starch | [148] |
| Potato starch, Corn starch | Potato starch, corn starch, corn meal, pectin, guar gum, xanthan gum, yeast, sugar, salt, oil, l-lysine, l-threonine, water | [149] |
| Corn starch | Zein, maize starch, HPMC, sugar, salt, active dry yeast | [150] |
| Corn starch | Corn starch, rice flour, HPMC, water, dried yeast, sunflower oil, sucrose, salt. | [151] |
| Cassava starch | Cassava starch, sorghum flour, water, egg white | [152] |
| Potato starch, Corn starch, Tapioca resistant starch, Corn resistant starch | Freeze dried yeast, oil, sucrose, salt, guar gum, pectin, potato starch, corn starch, tapioca resistant starch, corn resistant starch | [153] |
| Corn starch, Potato starch | Rice flour, corn flour, corn starch, potato starch, buckwheat flour, whole egg powder, whey protein, CMC, guar gum, HPMC, xanthan gum, salt, yeast, sunflower oil, water | [154] |
| Corn starch/Potato starch/Bean starch | Corn starch, potato starch, bean starch, premix | [24] |
| Corn starch/Potato starch | Corn starch, potato starch, buckwheat flour, premix | [155] |
| Wheat starch | Wheat starch, whey protein, locust bean gum, salt, dried active bakery yeast, d-glucose | [156] |
| Tapioca starch | Tapioca starch, corn flour, Salt, sugar, yeast, vegetable fat, egg, soybean flour, water | [157] |
| Corn starch | Corn starch, chickpea flour, pea isolate, soy flour, carob germ flour, sugar, baking powder, shortening, baker’s yeast, salt, xanthan gum, emulsifier, water | [158] |
| Corn starch/Potato starch | Maize starch, potato starch, guar gum, pectin, freeze dried yeasts, sucrose, salt, plant oil, water. | [159] |
| GF Wheat starch | Rice flour, gf wheat starch, egg albumen, fat, yeast, emulsifier mixture (DATEM, DMG), HPMC, salt, water | [23] |
| Pregel. Tapioca starch | Jasmine rice flour, pregel. tapioca starch, yeast, sugar, salt, shortening, water | [160] |
| Corn starch/Potato starch | Corn starch, potato starch, pectin, guar gum, yeasts, sugar, salt, oil, water | [25] |
| Corn starch/Wheat starch | Rice flour, corn starch, wheat starch, yeast, salt, oil, HPMC, white sugar | [161] |
| Corn Starch/Potato starch | Corn starch, potato starch, pectin, guar gum, yeast, sucrose, salt, plant oil, water | [162] |
| Potato starch | Rice flour, potato starch, sunflower oil, methylcellulose (MC), salt, castor sugar, dried yeast | [163] |
| Corn starch | Corn starch, tigernut flour, chickpea flour, shortening, sugar, baking powder, emulsifier, xanthan gum, dry yeast, salt | [164] |
| Potato starch, Corn starch, Wheat starch, Rice starch, Tapioca starch | Potato starch, corn starch, rice starch, gf-wheat starch, tapioca starch, water, HPMC, salt, sugar, yeast | [44] |
| Product (27) | Company | Whole Grain Maize | Maize | Rice | Potato | Tapioca | Millet | Buck-Wheat | Gluten-Free Wheat |
|---|---|---|---|---|---|---|---|---|---|
| White Sliced Loaf | Super Value Free From | F + S | F + S | S | S | ||||
| Soft White Sandwich Loaf | Genius | S | F + S | S | S | ||||
| White Loaf | Embrace | F + S | F + S | F + S | S | S | |||
| Cinnamon Raisin Loaf | Dr. Schar | S | F | ||||||
| Multigrain Bread | Dr. Schar | S | S | S | F | ||||
| Deli-Style Bread | Dr. Schar | S | F + S | F | |||||
| Classic White bread | Dr. Schar | S | S | S | F | ||||
| Frozen Hearthy White bread | Dr. Schar | S | S | S | F | ||||
| White Sourdough Artisan Cob | Warburtons | F + S | S | S | S | ||||
| White Artisan Loaf | Warburtons | F + S | S | S | S | ||||
| White Farmhouse Loaf | Warburtons | F + S | S | S | S | ||||
| Seeded Farmhouse Loaf | Warburtons | F + S | S | S | S | ||||
| Farmhouse Loaf | Warburtons | F + S | S | S | S | ||||
| White Bread | Kelkin | F + S | F | S | S | ||||
| Multiseed Bread | Kelkin | F + S | F | S | S | ||||
| Sourdough Bread | Kelkin | S | S | F | |||||
| Multiseed Sourdough Bread | Kelkin | S | F | S | F | ||||
| Brown Seeded Sandwich Loaf | Bfree | S | F | F | S | F | |||
| Soft White Loaf | Bfree | S | F | F | S | F | |||
| Brown Bloomer Slices | M&S | F | F | S | F | ||||
| Brown Seeded Loaf | M&S | F | F | S | F | ||||
| Multigrain Farmhouse Loaf | PureBred | F + S | S | F + S | S | F + S | |||
| White Farmhouse Loaf | Purebred | F + S | S | F + S | S | ||||
| Gluten Free Multigrain Loaf | Has No | F + S | S | F + S | S | S | |||
| Gluten Free White Loaf | Has No | F + S | S | F + S | S | S | |||
| Gluten Free Fibre Sliced Loaf | Juvela | S | |||||||
| Gluten Free White Sliced Loaf | Juvela | S |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horstmann, S.W.; Lynch, K.M.; Arendt, E.K. Starch Characteristics Linked to Gluten-Free Products. Foods 2017, 6, 29. https://doi.org/10.3390/foods6040029
Horstmann SW, Lynch KM, Arendt EK. Starch Characteristics Linked to Gluten-Free Products. Foods. 2017; 6(4):29. https://doi.org/10.3390/foods6040029
Chicago/Turabian StyleHorstmann, Stefan W., Kieran M. Lynch, and Elke K. Arendt. 2017. "Starch Characteristics Linked to Gluten-Free Products" Foods 6, no. 4: 29. https://doi.org/10.3390/foods6040029