Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics
Abstract
:1. Introduction
2. Lethality of Heat on Different Microorganisms
3. Mechanisms of Microbial Inactivation by Heat
3.1. General Considerations
3.2. Effect of Heat on Cellular Targets
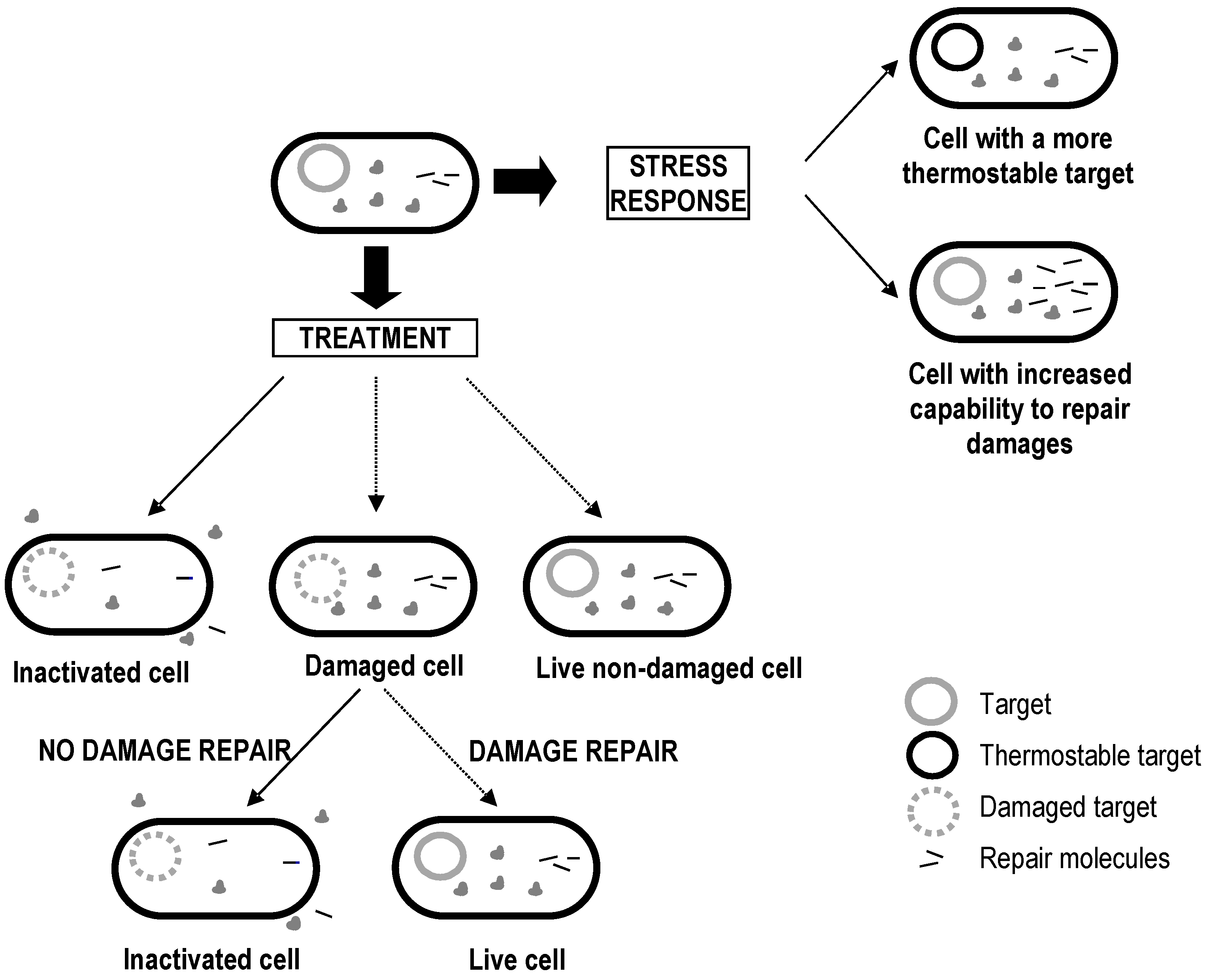
4. Factors Affecting Bacterial Inactivation
4.1. Factors Acting Prior to Treatment
4.2. Factors Acting during Treatment
4.3. Factors Acting after Treatment
5. Biological Basis for the Kinetics of Inactivation by Heat
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gould, G.W. Heat induced injury and inactivation. In Mechanisms of Action of Food Preservation Procedures; Elsevier Applied Science: London, UK, 1989; pp. 11–42. [Google Scholar]
- Splittstoesser, D.F. Fungi of importance in processed fruits. In Handbook of Applied Mycology. Volume 3: Foods and Feeds; Arora, K.D., Mukerji, K.G., Marth, E.H., Eds.; Marcel Dekker: New York, NY, USA, 1991; pp. 201–219. [Google Scholar]
- Shearer, A.E.H.; Mazzotta, A.S.; Chuyate, R.; Gombas, D.E. Heat Resistance of Juice Spoilage Microorganisms. J. Food Prot. 2002, 65, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Bayne, H.G.; Michener, H.D. Heat resistance of Byssochlamys ascospores. Appl. Environ. Microbiol. 1979, 37, 449–453. [Google Scholar] [PubMed]
- Pagán, R.; Mañas, P.; Raso, J.; Condón, S. Bacterial resistance to ultrasonic waves under pressure at non lethal (manosonication), and lethal (manothermosonication) temperatures. Appl. Environ. Microbiol. 1999, 65, 297–300. [Google Scholar] [PubMed]
- Sörqvist, S. Heat resistance in liquids of Enteroccus spp., Listeria spp., Escherichia coli, Yersinia enterocolitica, Salmonella spp. and Campylobacter spp. Acta Vet. Scan. 2003, 44, 1–9. [Google Scholar] [CrossRef]
- Sagarzazu, N.; Cebrián, G.; Pagán, R.; Condón, S.; Mañas, P. Resistance of Campylobacter jejuni to heat and to pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2010, 11, 283–289. [Google Scholar] [CrossRef]
- Cebrián, G.; Sagarzazu, N.; Pagán, R.; Condón, S.; Mañas, P. Heat and pulsed electric field resistance of pigmented and non-pigmented enterotoxigenic strains of Staphylococcus aureus in exponential and stationary-phase of growth. Int. J. Food Microbiol. 2007, 118, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, G.; Sagarzazu, N.; Pagán, N.; Condón, S.; Mañas, P. Resistance of Escherichia coli grown at different temperatures to various environmental stresses. J. Appl. Microbiol. 2008, 105, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, C.; Condón, S.; Pagán, R. Thermobacteriological characterization of Enterobacter sakazakii. Int. J. Food Microbiol. 2009, 136, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Casp, A.; Abril, J. Procesos de Conservación de Los Alimentos, 2nd ed.; Editorial Mundi-Prensa: Madrid, Spain, 2003. [Google Scholar]
- Hassani, M. Estudio de la Cinética de Inactivación Microbiana en Condiciones no Isotérmicas: Aplicación a Los Procesos de Pasteurización. Ph.D. Thesis, University of Zaragoza, Zaragoza, Spain, 2006. [Google Scholar]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods 5: Characteristics of Microbial Pathogens; Blackie Academic & Professional: London, UK, 2006. [Google Scholar]
- Pagán, R.; Condón, S.; Sala, F. Effects of several factors on the heat-shock-induced thermotolerance of Listeria monocytogenes. Appl. Environ. Microbiol. 1997, 63, 3225–3232. [Google Scholar] [PubMed]
- Palop, A. Estudio de la Influencia de Diversos Factores Ambientales Sobre la Termorresistencia de Bacillus subtilis, B. licheniformis, and B. coagulans. Ph.D. Thesis, University of Zaragoza, Zaragoza, Spain, 2005. [Google Scholar]
- Martínez, S.; López, M.; Bernardo, A. Thermal inactivation of Enterococcus faecium: Effect of growth temperature and physiological state of microbial cells. Lett. Appl. Microbiol. 2003, 37, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M. High temperature food preservation, and characteristics of thermophilic microorganisms. In Modern Food Microbiology, 4th ed.; Chapman & Hall: New York, NY, USA, 1992; pp. 335–355. [Google Scholar]
- Olson, J.C., Jr.; Nottingham, P.M. Temperature. In Microbial Ecology of Foods. Volume 1: Factors Affecting Life and Death of Microorganisms; Silliker, J.H., Ed.; Academic Press: London, UK, 1980; pp. 1–38. [Google Scholar]
- Palumbo, S.A.; Williams, A.C.; Buchanan, R.L.; Phillips, J.G. Thermal resistance of Aeromonas hydrophila. J. Food Prot. 1987, 50, 761–764. [Google Scholar] [CrossRef]
- Stringer, S.C.; George, S.M.; Peck, M.W. Thermal inactivation of Escherichia coli O157:H7. J. Appl. Microbiol. Symp. Suppl. 2000, 88, 79S–89S. [Google Scholar] [CrossRef]
- Sherry, A.E.; Patterson, M.F.; Madden, R.H. Comparison of 40 Salmonella enterica serovars injured by thermal, high-pressure and irradiation stress. J. Appl. Microbiol. 2004, 96, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.; Bayne, H.G.; Garibaldi, J.A. Heat resistance of Salmonella: The uniqueness of Salmonella seftenberg 775W. Appl. Environ. Microbiol. 1996, 17, 78–82. [Google Scholar]
- Mañas, P.; Pagán, R.; Sala, F.J.; Condón, S. Low molecular weight milk whey components protect Salmonella senftenberg 775W against heat by mechanism involving divalent cations. J. Appl. Microbiol. 2001, 91, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, T.J.; Slater, E.; McAlpine, K.; Rowbury, R.J.; Gilbert, R. Salmonella enteritidis phage type 4 isolates more tolerant of heat, acid or hydrogen peroxide also survive longer on surfaces. Appl. Environ. Microbiol. 1995, 61, 3161–3164. [Google Scholar] [PubMed]
- Mackey, B.M.; Mañas, P. Inactivation of Escherichia coli by high pressure. In High-Pressure Microbiology; Michiels, C., Aertsen, A., Bartlett, D., Yayanos, Y., Eds.; ASM Press: Washington, DC, USA, 2008; pp. 53–85. [Google Scholar]
- Miles, C.A. Relating cell killing to inactivation of critical components. Appl. Environ. Microbiol. 2006, 72, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Niven, G.W.; Miles, C.A.; Mackey, B.M. The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: An in vivo study using differential scanning calorimetry. Microbiology 1999, 145, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Mackey, B.M. Injured bacteria. In The Microbiological Safety, and Quality of Food; Lund, M., Baird-Parker, T.C., Gould, G.W., Eds.; Aspen Publisher: Gaithersburg, MD, USA, 2000; Volume I, pp. 315–341. [Google Scholar]
- Yura, T.; Kanemori, M.; Morita, M.T. The heat shock response: Regulation and function. In Bacterial Stress Responses; Storz, G., Hegge-Aronis, R., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 3–18. [Google Scholar]
- Cebrián, G.; Condón, S.; Mañas, P. Heat-adaptation induced thermotolerance in Staphylococcus aureus: Influence of the alternative factor σB. Int. J. Food Microbiol. 2009, 135, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Mackey, B.M. Changes in antibiotic sensitivity and cell surface hydrophobicity in Escherichia coli injured by heating, freezing, drying or gamma radiation. FEMS Microbiol. Lett. 1983, 20, 395–399. [Google Scholar] [CrossRef]
- Tsuchido, T.; Katsui, N.; Takeuchi, A.; Takano, M.; Shibasaki, I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl. Environ. Microbiol. 1995, 50, 298–303. [Google Scholar]
- Teixeira, P.; Castro, H.; Mohácsi-Farkas, C.; Kirby, R. Identification of sites of injury in Lactobacillus bulgaricus during heat stress. J. Appl. Microbiol. 1997, 83, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, R.I.; Ordal, Z.J. Thermal injury and inactivation in vegetative bacteria. In Inhibition and Inactivation of Vegetative Microbes; Skinner, F.A., Hugo, W.B., Eds.; Academic Press: London, UK, 1976; pp. 153–190. [Google Scholar]
- Hurst, A.; Hughes, A.; Beare-Rogers, J.L.; Collins-Thompson, D.L. Pysiological studies on the recovery of salt tolerance by Staphylococcus aureus after sublethal heating. J. Bacteriol. 1973, 116, 901–907. [Google Scholar] [PubMed]
- Kramer, B.; Thielmann, J. Monitoring the live to dead transition of bacteria during thermal stress by a multi-method approach. J. Microbiol. Methods 2016, 123, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Marcén, M.; Ruiz, V.; Serrano, M.J.; Condón, S.; Mañas, P. Oxidative stress in E. coli cells upon exposure to heat treatments. Int. J. Food Microbiol. 2017, 241, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Pierson, M.D.; Tomlins, R.I.; Ordal, Z.J. Biosynthesis during recovery of heat-injured Salmonella typhimurium. J. Bacteriol. 1971, 105, 1234–1236. [Google Scholar] [PubMed]
- Hurst, A.; Hughes, A. Stability of ribosomes of Staphylococcus aureus S6 sublethally heated in different buffers. J. Bacteriol. 1978, 133, 564–568. [Google Scholar] [PubMed]
- Coote, P.J.; Jones, M.V.; Seymour, I.J.; Rowe, D.L.; Ferdinando, D.P.; McArthur, A.J.; Cole, M.B. Activity of the plasma membrane H+-ATPase is a key physiological determinant of thermotolerance in Saccharomyces cerevisiae. Microbiology 1994, 140, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- González, I.; López, M.; Mazas, M.; Bernardo, A.; Martín, R. Effect of pH of the recovery medium on the apparent heat resistance of three strains of Bacillus cereus. Int. J. Food Microbiol. 1996, 31, 341–347. [Google Scholar] [CrossRef]
- Leguerinel, I.; Spegagne, I.; Couvert, O.; Coroller, L.; Mafart, P. Quantifying the effects of heating temperature, and combined effects of heating medium pH and recovery medium pH on the heat resistance of Salmonella typhimurium. Int. J. Food Microbiol. 2007, 116, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackey, B.M.; Miles, C.A.; Parsons, S.E.; Seymour, D.A. Thermal denaturation of whole cells, and cell components of Escherichia coli examined by differential scanning calorimetry. J. Gen. Microbiol. 1991, 137, 2361–2374. [Google Scholar] [CrossRef] [PubMed]
- Zamenhof, S. Gene unstabilization induced by heat and by nitrous acid. J. Bacteriol. 1961, 81, 111–117. [Google Scholar] [PubMed]
- Kadota, H.; Uchida, A.; Sako, Y.; Harada, K. Heat induced DNA injury in spores and vegetative cells of Bacillus subtilis. In Spores VII; Chambliss, G., Vary, J.C., Eds.; ASM Press: Washington, DC, USA, 1978; pp. 27–30. [Google Scholar]
- Sedgwick, S.G.; Bridges, B.A. Evidence for indirect production of DNA strand scissions during mild heating of Escherichia coli. J. Gen. Microbiol. 1972, 71, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.F. Nucleic acid damage in thermal inactivation of vegetative microorganisms. Adv. Biochem. Eng. 1977, 5, 49–67. [Google Scholar] [CrossRef]
- Mackey, B.M.; Seymour, D.A. The effect of catalase on recovery of heat-injured DNA-repair mutants of Escherichia coli. J. Gen. Microbiol. 1987, 133, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Earnshaw, R.G.; Appleyard, J.; Hurst, R.M. Understanding physical inactivation processes: Combining preservation opportunities using heat, ultrasound and pressure. Int. J. Food Microbiol. 1995, 28, 197–219. [Google Scholar] [CrossRef]
- Iandolo, J.J.; Ordal, Z.J. Repair of thermal injury of Staphylococcus aureus. J. Bacteriol. 1966, 91, 134–142. [Google Scholar] [PubMed]
- Mohácsi-Farkas, C.; Farkas, J.; Meszaros, L.; Reichart, O.; Andrassy, E. Thermal denaturation of bacterial cells examined by differential scanning calorimetry. J. Therm. Anal. Calorim. 1999, 57, 409–414. [Google Scholar] [CrossRef]
- Lee, J.; Kaletunç, G. Calorimetric determination of inactivation parameters of microorganisms. J. Appl. Microbiol. 2002, 93, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kaletunç, G. Evaluation of the heat inactivation of Escherichia coli and Lactobacillus plantarum by differential scanning calorimetry. Appl. Environ. Microbiol. 2002, 68, 5379–5386. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Corry, J.E.; Miles, C.A. Heat resistance and mechanism of heat inactivation in thermophilic campylobacters. Appl. Environ. Microbiol. 2006, 72, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Tolker-Nielsen, T.; Molin, S. Role of ribosome degradation in the death of heat-stressed Salmonella typhimurium. FEMS Microbiol. Lett. 1996, 142, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Heden, G.G.; Wyckoff, R.W.G. The electron microscopy of heated bacteria. J. Bacteriol. 1949, 58, 153–160. [Google Scholar] [PubMed]
- Laskowska, E.; Bohdanowicz, J.; Kuczyńska-Wiśnik, D.; Matuszewska, E.; Kędzierska, S.; Taylor, A. Aggregation of heat-shock-denatured, endogenous proteins and distribution of the IbpA/B and Fda marker-proteins in Escherichia coli WT and grpE280 cells. Microbiology 2004, 150, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Govers, S.K.; Gayan, E.; Aertsen, A. Intracellular movement of protein aggregates reveals heterogeneous inactivation and resuscitation dynamics in stressed populations of Escherichia coli. Environ. Microbiol. 2017, 19, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Baatout, S.; De Boever, P.; Mergeay, M. Temperature-induced changes in bacterial physiology as determined by flow cytometry. Ann. Microbiol. 2005, 55, 73–80. [Google Scholar]
- Arku, B.; Fanning, S.; Jordan, K. Flow cytometry to assess biochemical pathways in heat-stressed Cronobacter spp. (formerly Enterobacter sakazakii). J. Appl. Microbiol. 2011, 111, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Mols, M.; Ceragioli, M.; Abee, T. Heat stress leads to superoxide formation in Bacillus cereus detected using the fluorescent probe MitoSOX. Int. J. Food Microbiol. 2011, 151, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Ledward, D.A.; Park, R.W.; Robson, R.L. Factors affecting the heat resistance of Escherichia coli O157:H7. Lett. Appl. Microbiol. 1998, 26, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Huisman, G.W.; Siegele, D.A.; Zambrano, M.M.; Kolter, R. Morphological and physiological changes during stationary phase. In Escherichia coli and Salmonella: Cellular, and Molecular Biology; Neidhardt, F.C., Curtis, R., III, Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; ASM Press: Washington, DC, USA, 1996; pp. 1672–1682. [Google Scholar]
- Price, C.W. Protective function and regulation of the general stress response in Bacillus subtilis and related Gram-positive bacteria. In Bacterial Stress Responses; Storz, G., Hengge-Aronis, R., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 179–189. [Google Scholar]
- Knabel, S.J.; Walker, H.W.; Hartman, P.A.; Mendonca, A.F. Effects of growth temperature, and strictly anaerobic recovery on the survival of Listeria monocytogenes during pasteurization. Appl. Environ. Microbiol. 1990, 56, 370–376. [Google Scholar] [PubMed]
- Beuchat, L.R. Injury and repair of gram negative bacteria, with special consideration of the involvement of the cytoplasmic membrane. Adv. Appl. Microbiol. 1978, 23, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J. Bacteriol. 2003, 185, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Marmer, B.S.; Benedict, R.C. Influence of growth temperature on injury and death of Listeria monocytogenes Scott A during a mild heat treatment. J. Food Prot. 1991, 54, 166–169. [Google Scholar] [CrossRef]
- Schumann, W. Temperature sensors of eubacteria. Adv. Appl. Microbiol. 2009, 67, 213–256. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, G.; Blankschien, M.; Herman, C.; Gross, C.A.; Rhodius, V.A. Regulon, and promoter analysis of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress. Genes Dev. 2006, 20, 1776–1789. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, J.M.; Iskander, I.K.; Amundson, C.H. Relation of the heat resistance of salmonellae to the water activity of the environment. Appl. Microbiol. 1970, 19, 429–433. [Google Scholar] [PubMed]
- Cotterill, O.J.; Glauert, J. Thermal resistance of salmonellae in egg yolk products containing sugar or salt. Poult. Sci. 1969, 48, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Hurst, A.; Hughes, A. The protective effect of some food ingredients on Staphylococcus aureus MF31. J. Appl. Bacteriol. 1983, 55, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Shebuski, J.R.; Vilhelmsson, O.; Miller, K.J. Effects of low water activity on the thermal tolerance of Staphylococcus aureus. J. Food Prot. 2000, 63, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Leyer, G.J.; Johnson, E.A. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl. Environ. Microbiol. 1993, 59, 1842–1847. [Google Scholar] [PubMed]
- Ryu, J.H.; Beuchat, L.R. Influence of acid tolerance responses on survival, growth, and cross-protection of Escherichia coli O157:H7 in acidified media and fruit juices. Int. J. Food Microbiol. 1998, 45, 185–193. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Fernández, A.; López, M.; Arenas, R.; Bernardo, A. Modifications in membrane fatty acid composition of Salmonella typhimurium in response to growth conditions and their effect on heat resistance. Int. J. Food Microbiol. 2008, 123, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Abee, T.; Wouters, J.A. Microbial stress response in minimal processing. Int. J. Food Microbiol. 1999, 50, 65–91. [Google Scholar] [CrossRef]
- Lou, Y.; Yousef, A.E. Adaptation to sublethal environmental stresses protects Listeria monocytogenes against lethal preservation factors. Appl. Environ. Microbiol. 1997, 63, 1252–1255. [Google Scholar] [PubMed]
- Aertsen, A.; Vanoirbeek, K.; De Spiegeleer, P.; Sermon, J.; Hauben, K.; Farewell, A.; Nyström, T.; Michiels, C.W. Heat shock protein-mediated resistance to high hydrostatic pressure in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.D.; Chou, C.C. Effect of heat shock on thermal tolerance and susceptibility of Listeria monocytogenes to other environmental stresses. Food Microbiol. 2004, 21, 605–610. [Google Scholar] [CrossRef]
- Lim, B.; Gross, C.A. Cellular response to heat shock and cold shock. In Bacterial Stress Responses, 2nd ed.; Storz, G., Hengge, R., Eds.; ASM Press: Washington, DC, USA, 2001; pp. 93–114. [Google Scholar]
- Yamamori, T.; Yura, T. Genetic control of heat-shock protein synthesis, and its bearing on growth, and thermal resistance in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 1992, 79, 860–864. [Google Scholar] [CrossRef]
- Mackey, B.M.; Derrick, C.M. Elevation of the heat resistance of Salmonella typhimurium by sub-lethal heat shock. J. Appl. Bacteriol. 1986, 61, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Mackey, B.M.; Derrick, C.M. Changes in the heat resistance of Salmonella typhimurium during heating at rising temperatures. Lett. Appl. Microbiol. 1987, 4, 13–16. [Google Scholar] [CrossRef]
- Mackey, B.M.; Derrick, C.M. The effect of prior shock on the thermoresistance of Salmonella thompson in foods. Lett. Appl. Microbiol. 1987, 5, 115–118. [Google Scholar] [CrossRef]
- Linton, R.H.; Pierson, M.D.; Bishop, J.R. Increase in heat resistance of Listeria monocytogenes Scott A by sublethal heat shock. J. Food Prot. 1990, 53, 924–927. [Google Scholar] [CrossRef]
- Whitaker, R.D.; Batt, C.A. Characterization of the heat shock response in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 1991, 57, 1408–1412. [Google Scholar] [PubMed]
- Shenoy, K.; Murano, E.A. Effect of heat shock on the thermotolerance and protein composition of Yersinia enterocolitica in brain heart infusion broth and ground pork. J. Food Prot. 1996, 59, 360–364. [Google Scholar] [CrossRef]
- De Angelis, M.; Di Cagno, R.; Huet, C.; Crecchio, C.; Fox, P.F.; Gobbetti, M. Heat shock response in Lactobacillus plantarum. Appl. Environ. Microbiol. 2004, 70, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Cebrián, G.; Mañas, P.; Condón, S.; Pagán, R. Induced thermotolerance under nonisothermal treatments of a heat sensitive and a resistant strain of Staphylococcus aureus in media of different pH. Lett. Appl. Microbiol. 2006, 43, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Condón, S.; Pagán, R. Predicting microbial heat inactivation under nonisothermal treatments. J. Food Prot. 2007, 70, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Mañas, P.; Condón, S.; Pagán, R. Predicting heat inactivation of Staphylococcus aureus under nonisothermal treatments at different pH. Mol. Nutr. Food Res. 2007, 50, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, G.; Sagarzazu, N.; Pagán, R.; Condón, S.; Mañas, P. Development of stress resistance in Staphylococcus aureus after exposure to sublethal environmental conditions. Int. J. Food Microbiol. 2010, 140, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mackey, B.M.; Derrick, C.M. Heat shock synthesis, and thermotolerance in Salmonella typhimurium. J. Appl. Bacteriol. 1990, 69, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ziemienowicz, A.; Skowyra, D.; Zeilstra-Ryalls, J.; Fayet, O.; Georgopoulos, C.; Zylicz, M. Both the Escherichia coli chaperone systems, GroEL/GroES and DnaK/DnaJ/GrpE, can reactivate heat-treated RNA polymerase. Different mechanisms for the same activity. J. Biol. Chem. 1993, 268, 25425–25431. [Google Scholar] [PubMed]
- Jobin, M.P.; Delmas, F.; Garmyn, D.; Diviès, C.; Guzzo, J. Molecular characterization of the gene encoding an 18-kilodalton small heat shock protein associated with the membrane of Leuconostoc oenos. Appl. Environ. Microbiol. 1997, 63, 609–614. [Google Scholar] [PubMed]
- Bochkareva, E.S.; Solovieva, M.E.; Girshovich, A.S. Targeting of GroEL to SecA on the cytoplasmic membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA 1998, 95, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Kornitzer, D.; Teff, D.; Altuvia, S.; Oppenheim, A.B. Isolation, characterization, and sequence of an Escherichia coli heat shock gene, htpX. J. Bacteriol. 1991, 173, 2944–2953. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.E.; Blattner, F.R. Characterization of twenty-six new heat-shock genes of Escherichia coli. J. Bacteriol. 1993, 175, 5242–5252. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.C.; Feng, G.; Winkler, M.E. Transcription of the mutL repair, miaA tRNA modification, hfq pleiotropic regulator, and hflA region protease genes of Escherichia coli K-12 from clustered Esigma32-specific promoters during heat shock. J. Bacteriol. 1996, 178, 5719–5731. [Google Scholar] [CrossRef] [PubMed]
- Török, Z.; Horváth, I.; Goloubinoff, P.; Kovács, E.; Glatz, A.; Balogh, G.; Vigh, L. Evidence for a lipochaperonin: Association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc. Natl. Acad. Sci. USA 1997, 94, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.; Brandt, W.; Rumbak, E.; Lindsey, G. The LEA-like protein HSP 12 in Saccharomyces cerevisiae has a plasma membrane location and protects membranes against desiccation and ethanol-induced stress. Biochim. Biophys. Acta 2000, 1463, 267–278. [Google Scholar] [CrossRef]
- Shigapova, N.; Török, Z.; Balogh, G.; Goloubinoff, P.; Vígh, L.; Horváth, I. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2005, 328, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, N.M.; Horváth, I.; Török, Z.; Wolkers, W.F.; Balogi, Z.; Shigapova, N.; Crowe, L.M.; Tablin, F.; Vierling, E.; Crowe, J.H.; et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. USA 2002, 99, 13504–13509. [Google Scholar] [CrossRef] [PubMed]
- Coucheney, F.; Gal, L.; Beney, L.; Lherminier, J.; Gervais, P.; Guzzo, J. A small HSP, Lo18, interacts with the cell membrane and modulates lipid physical state under heat shock conditions in a lactic acid bacterium. Biochim. Biophys. Acta 2005, 1720, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.G.; Link, T.M. Hfq structure, function and ligand binding. Curr. Opin. Microbiol. 2007, 10, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, S.; Hain, T.; Wouters, J.A.; Hossain, H.; de Vos, W.M.; Abee, T.; Chakraborty, T.; Wells-Bennik, M.H.J. The heat-shock response of Listeria monocytogenes comprises genes involved in heat shock, cell division, cell wall synthesis, and the SOS response. Microbiology 2007, 153, 3593–3607. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, S.; Hartke, A.; Giard, J.C.; Benachour, A.; Boutibonnes, P.; Auffray, Y. Relationship between stress response toward bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol. Lett. 1996, 138, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, S.; Hartke, A.; Giard, J.C.; Auffray, Y. Alkaline stress response in Enterococcus faecalis: Adaptation, cross-protection and changes in protein synthesis. Appl. Environ. Microbiol. 1997, 63, 812–814. [Google Scholar] [PubMed]
- Mattick, K.L.; Jorgensen, F.; Legan, J.D.; Lappin-Scott, H.M.; Humphrey, T.J. Habituation of Salmonella spp. at reduced water activity and its effect on heat tolerance. Appl. Environ. Microbiol. 2000, 66, 4921–4925. [Google Scholar] [CrossRef] [PubMed]
- Boutibonnes, P.; Bisson, V.; Thammavongs, B.; Hartke, A.; Panoff, J.M.; Benachour, A.; Auffray, Y. Induction of thermotolerance by chemical agents in Lactococcus lactis subsp. lactis IL1403. Int. J. Food Microbiol. 1995, 25, 93–94. [Google Scholar] [CrossRef]
- Van Houdt, R.; Michiels, C. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Mañas, P.; Pagán, R.; Raso, J.; Condón, S. Predicting thermal inactivation in media of different pH of Salmonella grown at different temperatures. Int. J. Food Microbiol. 2003, 87, 45–53. [Google Scholar] [CrossRef]
- Hansen, N.H.; Riemann, H. Factors affecting the heat resistance of nonsporing organisms. J. Appl. Bacteriol. 1963, 26, 314–333. [Google Scholar] [CrossRef]
- Fernández, A.; Álvarez-Ordoñez, A.; López, M.; Bernardo, A. Effect of organic acids on thermal inactivation of acid and cold stressed Enterococcus faecium. Food Microbiol. 2009, 26, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Baird-Parker, A.C.; Boothroyd, M.; Jones, E. The effect of water activity on the heat resistance of heat sensitive and heat resistant strains of Salmonellae. J. Appl. Bacteriol. 1970, 33, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I. Resistencia al Calor y a Los Ultrasonidos Bajo Presión de Salmonella enteritidis y Salmonella senftenberg en Medios de Distinta Actividad de Agua. Master’s Thesis, University of Zaragoza, Zaragoza, Spain, 2000. [Google Scholar]
- Summer, S.S.; Sandros, T.M.; Harmon, M.C.; Scott, V.N.; Bernard, D.T. Heat resistance of Salmonella typhimurium, and Listeria monocytogenes in sucrose solutions of various water activities. J. Food Sci. 1996, 56, 1741–1743. [Google Scholar] [CrossRef]
- Smith, J.L.; Benedict, R.C.; Haas, M.; Palumbo, S.A. Heat injury in Staphylococcus aureus 196E: Protection by metabolizable and non-metabolizable sugars and polyols. Appl. Environ. Microbiol. 1983, 46, 1417–1419. [Google Scholar] [PubMed]
- Crowe, J.H.; Crowe, L.M. Stabilization of dry liposomes by carbohydrates. Dev. Biol. Stand. 1991, 74, 285–294. [Google Scholar]
- Lee, A.C.; Goepfert, J.M. Influence of selected solutes on thermally induced death and injury of Salmonella typhimurium. J. Milk Food Technol. 1975, 38, 195–200. [Google Scholar] [CrossRef]
- Esty, J.R.; Meyer, K.F. The heat resistance of the spores of B. Botulinus and allied anaerobes. XI. J. Infect. Dis. 1922, 31, 650–663. [Google Scholar] [CrossRef]
- Mañas, P.; Pagán, R.; Leguérinel, I.; Condón, S.; Mafart, P.; Sala, F.J. Effect of sodium chloride concentration on the heat resistance and recovery of Salmonella typhimurium. Int. J. Food Microbiol. 2001, 63, 209–216. [Google Scholar] [CrossRef]
- Casadei, M.A.; Ingram, R.; Hitchings, E.; Archer, J.; Gaze, J.E. Heat resistance of Bacillus cereus, Salmonella typhimurium and Lactobacillus delbrueckii in relation to pH and ethanol. Int. J. Food Microbiol. 2001, 63, 125–134. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Humpheson, L.; Adams, M.R. Effect of nisin on heat injury and inactivation of Salmonella enteritidis PT4. Int. J. Food Microbiol. 1998, 18, 7–13. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Ouazzou, A.A.; Condón, S.; García-Gonzalo, D.; Pagán, R. Inactivation of Escherichia coli O157:H7 in fruit juices by combined treatments of citrus fruit essential oils and heat. Int. J. Food Microbiol. 2012, 159, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Lechowich, R.V. Effect of salt concentration in the recovery medium on heat-injured Streptococcus faecalis. Appl. Microbiol. 1968, 16, 772–776. [Google Scholar] [PubMed]
- Mackey, B.M.; Boogard, E.; Hayes, C.M.; Baranyi, J. Recovery of heat-injured Listeria monocytogenes. Int. J. Food Microbiol. 1994, 2, 227–237. [Google Scholar] [CrossRef]
- Busch, S.V.; Donnelly, C.W. Development of a repair-enrichment broth for resuscitation of heat-injured Listeria monocytogenes and Listeria innocua. Appl. Environ. Microbiol. 1992, 58, 14–20. [Google Scholar] [PubMed]
- Stephens, P.J.; Druggan, P.; Nebe-von Caron, G. Stressed Salmonella are exposed to reactive oxygen species from two independent sources during recovery in conventional culture media. Int. J. Food Microbiol. 2000, 60, 269–285. [Google Scholar] [CrossRef]
- Esteban, M.D.; Aznar, A.; Fernández, P.S.; Palop, A. Combined effect of nisin, carvacrol and a previous thermal treatment on the growth of Salmonella enteritidis and Salmonella senftenberg. Food Sci. Technol. Int. 2013, 19, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Smolka, L.R.; Nelson, F.E.; Kelley, L.M. Interaction of pH and NaCl on enumeration of heat-stressed Staphylococcus aureus. Appl. Microbiol. 1974, 27, 443–447. [Google Scholar] [PubMed]
- George, S.M.; Richardson, L.C.C.; Pol, I.E.; Peck, M.W. Effect of oxygen concentration and redox potential on recovery of sublethally heat-damaged cells of Escherichia coli O157:H7, Salmonella enteritidis and Listeria monocytogenes. J. Appl. Microbiol. 1998, 84, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, W.D.; Esty, J.R. The thermal death point in relation to time of typical thermophilic organisms. J. Infect. Dis. 1920, 27, 602–617. [Google Scholar] [CrossRef]
- Peleg, M. Advanced Quantitative Microbiology for Food and Biosystems: Models for Predicting Growth and Inactivation; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Gibson, A.M.; Bratchell, N.; Roberts, T.A. Predicting microbial growth: Growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int. J. Food Microbiol. 1988, 6, 155–178. [Google Scholar] [CrossRef]
- Den Besten, H.M.W.; Mataragas, M.; Moezelaar, R.; Abee, T.; Zwietering, M.H. Quantification of the Effects of Salt Stress, and Physiological State on Thermotolerance of Bacillus cereus ATCC 10987 and ATCC 14579. Appl. Environ. Microbiol. 2006, 72, 5884–5894. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Cappuyns, A.M.; Valdramidis, V.P.; Van Impe, J.F. Modeling microbial inactivation kinetics: Primary models. In Progress on Quantitative Approaches of Thermal Food Processing; Valdramidis, V.P., Van Impe, J.F., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 99–120. [Google Scholar]
- Smelt, J.P.; Brul, S. Thermal inactivation of microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. On the use of theWeibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef]


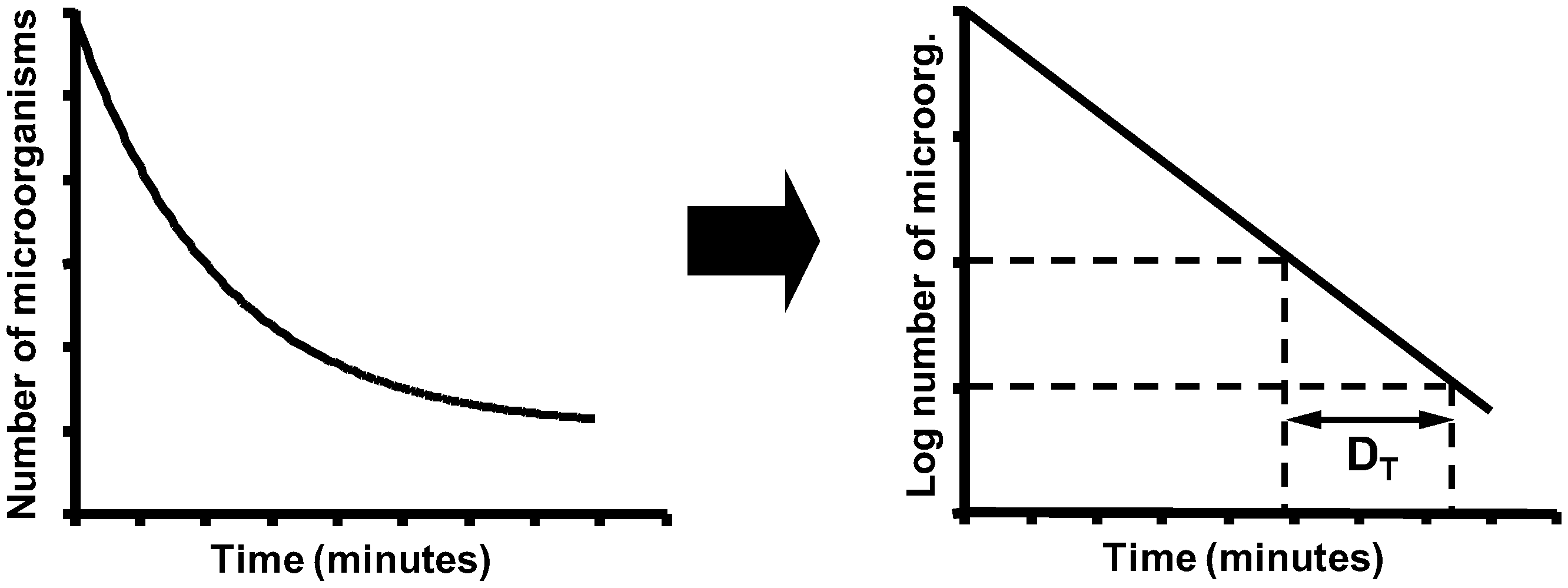


| Bacterial Species | Temperature (°C) | DT (Minutes) | z (°C) | |
|---|---|---|---|---|
| Vegetative cells | Aeromonas hydrophila | 60 | <0.02 | 5.2–7.7 |
| Campylobacter spp. | 60 | <0.01–0.11 | 4.1–4.7 | |
| Yersinia enterocolitica | 60 | 0.07–0.8 | 4.0–5.8 | |
| Salmonella enterica | 60 | 0.1–3.3 | 3.8–6.3 | |
| Cronobacter sakazakii | 60 | 0.05–2.0 | 4.1–6.2 | |
| Escherichia coli | 60 | 0.7–2.7 | 3.2–5.2 | |
| Staphylococcus aureus | 60 | 0.2–6.0 | 3.6–8.5 | |
| Listeria monocytogenes | 60 | 0.5–15 | 5.2–5.8 | |
| Enterococcus faecium | 60 | 5.0–30 | 4.3–8.0 | |
| Spores | Bacillus subtilis | 100 | 3.31–>100 | 6.7–10.1 |
| Clostridium botulinum (proteolytic) | 121 | <0.01–0.22 | 7.6–12.1 | |
| Geobacillus stearothermophilus | 121 | 0.1–5.0 | 7.3–12.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebrián, G.; Condón, S.; Mañas, P. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods 2017, 6, 107. https://doi.org/10.3390/foods6120107
Cebrián G, Condón S, Mañas P. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods. 2017; 6(12):107. https://doi.org/10.3390/foods6120107
Chicago/Turabian StyleCebrián, Guillermo, Santiago Condón, and Pilar Mañas. 2017. "Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics" Foods 6, no. 12: 107. https://doi.org/10.3390/foods6120107





